Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure—The Austrian LAAC Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment and Indications
2.2. Procedure
2.3. Antithrombotic Treatment
2.4. Follow-Up
2.5. Data Collection
2.6. Endpoints
2.7. Statistical Analysis
3. Results
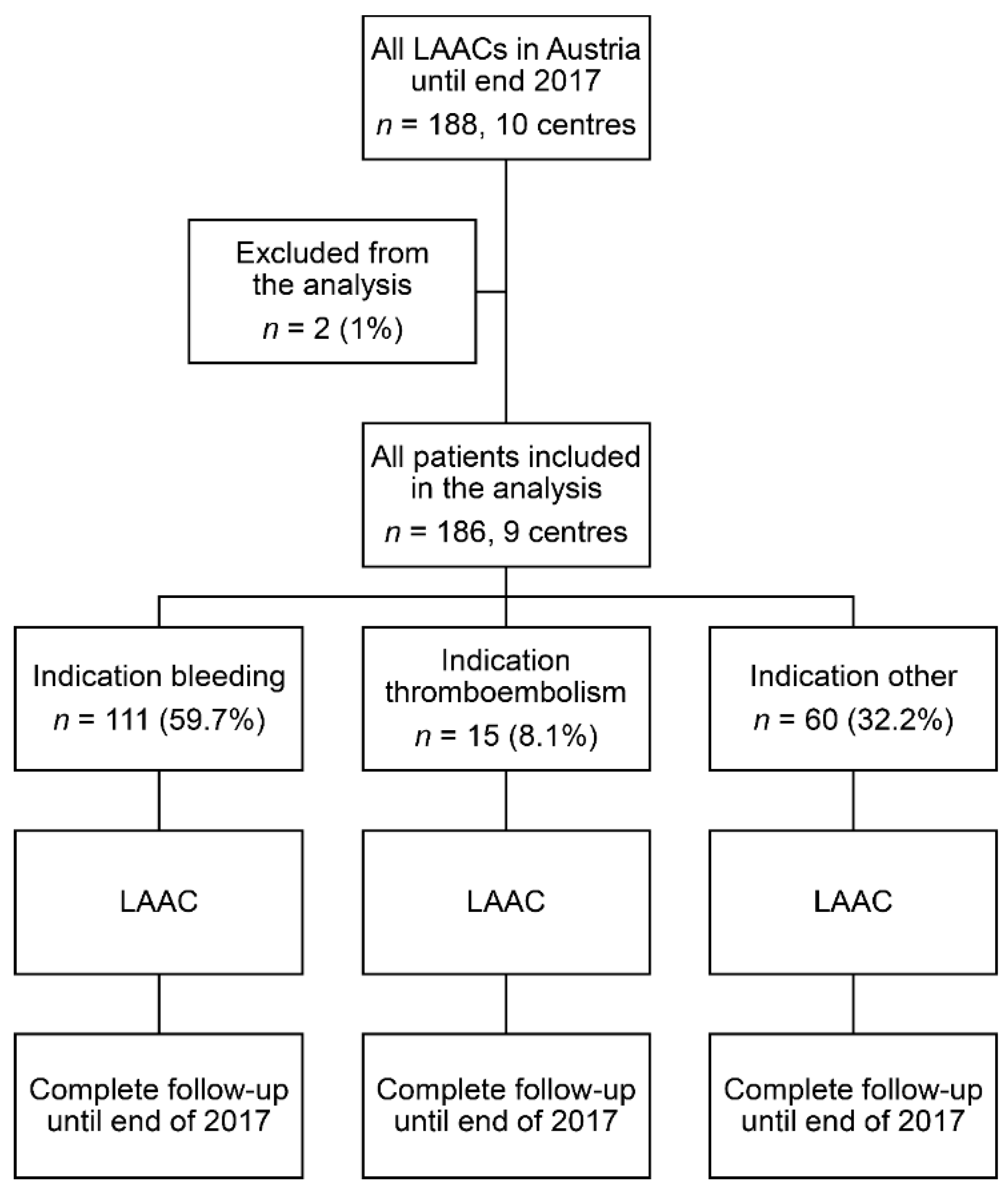
3.1. Indications
3.2. Basic Risk Profile
3.3. Antithrombotic Treatment before LAAC
3.4. Procedure
3.5. Procedural Outcome
3.6. Antithrombotic Treatment after LAAC
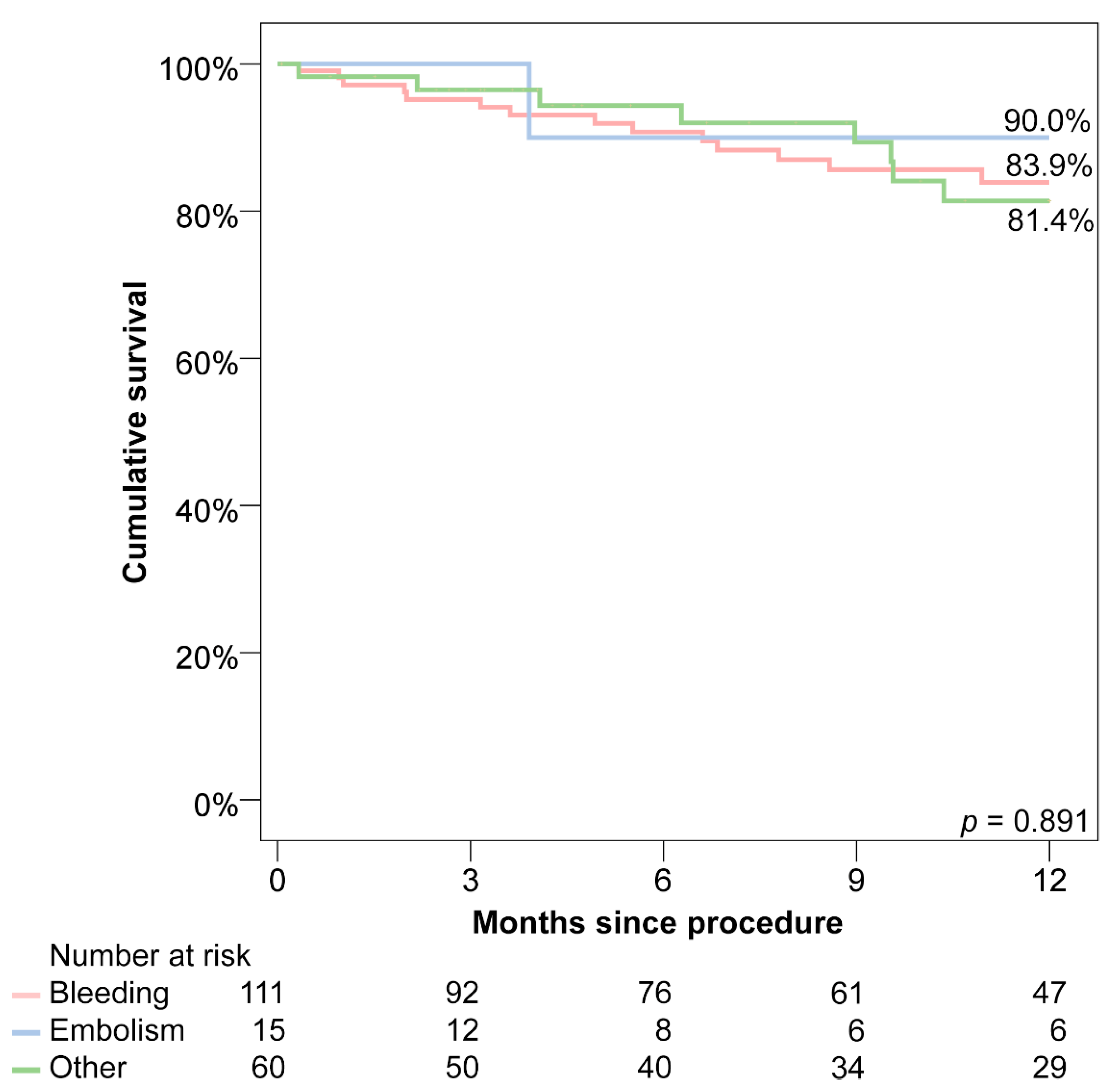
3.7. Follow-Up
3.8. Predicted vs. Observed Events
4. Discussion
4.1. Indications for LAAC
4.2. Procedural Outcome
4.3. Post-Procedural Antithrombotic Treatment
4.4. Long-Term Outcome
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef]
- Meschia, J.F.; Bushnell, C.; Boden-Albala, B.; Braun, L.T.; Bravata, D.M.; Chaturvedi, S.; Creager, M.A.; Eckel, R.H.; Elkind, M.S.; Fornage, M.; et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 3754–3832. [Google Scholar] [CrossRef]
- Boersma, L.V.; Schmidt, B.; Betts, T.R.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; Stein, K.M.; et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri-procedural outcomes from the EWOLUTION registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. Europace 2020, 22, 184. [Google Scholar] [CrossRef]
- Lebhart, G.; Neustädter, C.; Kytir, J. The new Population Register at Statistics Austria: Conceptualization and Methodology for Register-based Flow and Stock Statistics. Austrian J. Stat. 2007, 36, 277–289. [Google Scholar] [CrossRef]
- Olesen, J.B.; Lip, G.Y.; Hansen, M.L.; Hansen, P.R.; Tolstrup, J.S.; Lindhardsen, J.; Selmer, C.; Ahlehoff, O.; Olsen, A.M.; Gislason, G.H.; et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ 2011, 342, d124. [Google Scholar] [CrossRef]
- Olesen, J.B.; Lip, G.Y.; Hansen, P.R.; Lindhardsen, J.; Ahlehoff, O.; Andersson, C.; Weeke, P.; Hansen, M.L.; Gislason, G.H.; Torp-Pedersen, C. Bleeding risk in ‘real world’ patients with atrial fibrillation: Comparison of two established bleeding prediction schemes in a nationwide cohort. J. Thromb. Haemost. 2011, 9, 1460–1467. [Google Scholar] [CrossRef]
- LaHaye, S.A.; Gibbens, S.L.; Ball, D.G.; Day, A.G.; Olesen, J.B.; Skanes, A.C. A clinical decision aid for the selection of antithrombotic therapy for the prevention of stroke due to atrial fibrillation. Eur. Heart J. 2012, 33, 2163–2171. [Google Scholar] [CrossRef]
- Urena, M.; Rodes-Cabau, J.; Freixa, X.; Saw, J.; Webb, J.G.; Freeman, M.; Horlick, E.; Osten, M.; Chan, A.; Marquis, J.F.; et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J. Am. Coll. Cardiol. 2013, 62, 96–102. [Google Scholar] [CrossRef]
- Hutt, E.; Wazni, O.M.; Kaur, S.; Saliba, W.I.; Tarakji, K.G.; Kapadia, S.; Aguilera, J.; Barakat, A.F.; Abdallah, M.; Jaber, W.; et al. Left Atrial Appendage Closure Device Implantation in Patients at Very High Risk for Stroke. Heart Rhythm 2019. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Mobius-Winkler, S.; Miller, M.A.; Neuzil, P.; Schuler, G.; Wiebe, J.; Sick, P.; Sievert, H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: The ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J. Am. Coll. Cardiol. 2013, 61, 2551–2556. [Google Scholar] [CrossRef]
- Kefer, J.; Vermeersch, P.; Budts, W.; Depotter, T.; Aminian, A.; Benit, E.; Stammen, F. Transcatheter left atrial appendage closure for stroke prevention in atrial fibrillation with Amplatzer cardiac plug: The Belgian Registry. Acta Cardiol. 2013, 68, 551–558. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.R.; Nogales-Asensio, J.M.; Infante De Oliveira, E.; De Gama Ribeiro, V.; Ruiz-Salmeron, R.; Arzamendi-Aizpurua, D.; Costa, M.; Gutierrez-Garcia, H.; Fernandez-Diaz, J.A.; Martin-Yuste, V.; et al. Long-term Event Reduction After Left Atrial Appendage Closure. Results of the Iberian Registry II. Rev. Esp. Cardiol. 2019, 72, 449–455. [Google Scholar] [CrossRef]
- Santoro, G.; Meucci, F.; Stolcova, M.; Rezzaghi, M.; Mori, F.; Palmieri, C.; Paradossi, U.; Pastormerlo, L.E.; Rosso, G.; Berti, S. Percutaneous left atrial appendage occlusion in patients with non-valvular atrial fibrillation: Implantation and up to four years follow-up of the AMPLATZER Cardiac Plug. EuroIntervention 2016, 11, 1188–1194. [Google Scholar] [CrossRef]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: Multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016, 11, 1170–1179. [Google Scholar] [CrossRef]
- Landmesser, U.; Tondo, C.; Camm, J.; Diener, H.C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Hildick-Smith, D. Left atrial appendage occlusion with the AMPLATZER Amulet device: One-year follow-up from the prospective global Amulet observational registry. EuroIntervention 2018, 14, e590–e597. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P.; Investigators, P.A. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Holmes, D.; Doshi, S.K.; Neuzil, P.; Kar, S. Safety of percutaneous left atrial appendage closure: Results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation 2011, 123, 417–424. [Google Scholar] [CrossRef]
- Fastner, C.; Nienaber, C.A.; Park, J.W.; Brachmann, J.; Zeymer, U.; Goedde, M.; Sievert, H.; Geist, V.; Lewalter, T.; Krapivsky, A.; et al. Impact of left atrial appendage morphology on indication and procedural outcome after interventional occlusion: Results from the prospective multicentre German LAARGE registry. EuroIntervention 2018, 14, 151–157. [Google Scholar] [CrossRef]
- Berti, S.; Pastormerlo, L.E.; Santoro, G.; Brscic, E.; Montorfano, M.; Vignali, L.; Danna, P.; Tondo, C.; Rezzaghi, M.; D’Amico, G.; et al. Intracardiac Versus Transesophageal Echocardiographic Guidance for Left Atrial Appendage Occlusion: The LAAO Italian Multicenter Registry. JACC Cardiovasc. Interv. 2018, 11, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Nielsen-Kudsk, J.E.; Kefer, J.; Aminian, A.; Berti, S.; Santoro, G.; et al. Incidence and Clinical Impact of Device-Associated Thrombus and Peri-Device Leak Following Left Atrial Appendage Closure With the Amplatzer Cardiac Plug. JACC Cardiovasc. Interv. 2017, 10, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, M.; Attinger-Toller, A.; Shakir, S.; Gloekler, S.; Seifert, B.; Moschovitis, A.; Khattab, A.; Maisano, F.; Meier, B.; Nietlispach, F. Percutaneous left atrial appendage occlusion: Effect of device positioning on outcome. Catheter. Cardiovasc. Interv. 2016, 88, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Berti, S.; Santoro, G.; Brscic, E.; Montorfano, M.; Vignali, L.; Danna, P.; Tondo, C.; D’Amico, G.; Stabile, A.; Sacca, S.; et al. Left atrial appendage closure using AMPLATZER devices: A large, multicenter, Italian registry. Int. J. Cardiol. 2017, 248, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Teiger, E.; Thambo, J.B.; Defaye, P.; Hermida, J.S.; Abbey, S.; Klug, D.; Juliard, J.M.; Pasquie, J.L.; Rioufol, G.; Lepillier, A.; et al. Percutaneous Left Atrial Appendage Closure Is a Reasonable Option for Patients With Atrial Fibrillation at High Risk for Cerebrovascular Events. Circ. Cardiovasc. Interv. 2018, 11, e005841. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.V.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; Betts, T.; et al. Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology. Circ. Arrhythm. Electrophysiol. 2019, 12, e006841. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Indication for LAAC | p-Value | |||
|---|---|---|---|---|---|
| Bleeding | Thromboembolism | Other | Overall | Post-Hoc | |
| Number of patients | 111 | 15 | 60 | N/A | |
| Female | 38.7% | 20.0% | 40.0% | 0.355 | |
| Age (years) | 74 (70–78) | 74 (62–76) | 77 (72–81) | 0.029 | § |
| Body mass index, kg m−2 | 26 (24–30) | 25 (22–30) | 27 (24–31) | 0.409 | |
| CHA2DS2-VASc score | 4.6 ± 1.4 | 4.8 ± 1.2 | 4.2 ± 1.5 | 0.150 | |
| CHADS2 score | 2.8 ± 1.2 | 3.8 ± 0.7 | 2.5 ± 1.2 | <0.001 | **,‡‡ |
| HAS-BLED score | 3.6 ± 0.7 | 2.9 ± 0.6 | 2.9 ± 0.9 | <0.001 | **,†† |
| Congestive heart failure | 27.9% | 6.7% | 11.7% | 0.017 | § |
| Arterial hypertension | 86.5% | 86.7% | 90.0% | 0.780 | |
| Diabetes mellitus | 25.2% | 40.0% | 28.3% | 0.462 | |
| Transitory ischemic attack, stroke or thromboembolism | 47.7% | 100.0% | 28.3% | <0.001 | **,†,‡‡ |
| Vascular disease | 42.3% | 20.0% | 38.3% | 0.270 | |
| Uncontrolled hypertension | 9.0% | 0% | 8.3% | 0.762 | |
| Abnormal renal function | 15.3% | 6.7% | 13.3% | 0.803 | |
| Abnormal hepatic function | 1.8% | 0% | 6.8% | 0.240 | |
| Stroke | 40.5% | 93.3% | 18.3% | <0.001 | **,†,‡‡ |
| History of bleeding | 100.0% | 26.7% | 45.0% | <0.001 | **,†† |
| Labile International Normalised Ratio values | 2.7% | 6.7% | 1.7% | 0.453 | |
| Alcohol abuse | 2.7% | 0% | 3.2% | 0.655 | |
| Coronary artery disease | 45.0% | 6.7% | 50.0% | 0.006 | *,‡‡ |
| Cerebral artery disease | 15.0% | 13.3% | 10.9% | 0.837 | |
| Periphery artery disease | 7.2% | 13.3% | 5.0% | 0.389 | |
| History of percutaneous intervention | 22.5% | 0% | 31.7% | 0.021 | ‡ |
| History of coronary artery bypass grafting | 13.5% | 6.7% | 5.0% | 0.200 | |
| Chronic obstructive pulmonary disease | 18.2% | 6.7% | 7.3% | 0.133 | |
| Dialysis | 1.0% | 0% | 0% | 1.000 | |
| Hyperlipoproteinemia | 35.4% | 64.3% | 29.1% | 0.059 | |
| Paroxysmal AF | 27.6% | 26.7% | 42.6% | 0.165 | |
| Parameter | Indication for LAAC | p-Value | ||
|---|---|---|---|---|
| Bleeding | Thromboembolism | Other | Overall | |
| Follow-up duration (days) | 474 ± 449 | 268 ± 203 | 535 ± 525 | 0.178 |
| Combined endpoint (1 year death, stroke, bleeding or LAAC-associated hospitalisation) | 16.1% | 10.0% | 18.6% | 0.891 |
| Death | 9.0% | 6.7% | 16.7% | 0.253 |
| Bleeding | 7.2% | 0.0% | 8.3% | 0.668 |
| Stroke, transient ischaemic attack or thromboembolism | 4.5% | 0.0% | 6.7% | 0.762 |
| Ischemic stroke | 1.8% | 0.0% | 1.7% | 1.000 |
| Transient ischaemic attack | 0.9% | 0.0% | 0.0% | 1.000 |
| Thromboembolism | 1.8% | 0.0% | 5.0% | 0.572 |
| Hospitalisation due to LAAC | 0.9% | 6.7% | 1.7% | 0.256 |
| Any hospitalisation | 28.8% | 33.3% | 30.0% | 0.879 |
| Parameter | All Patients | Indication for LAAC | ||
|---|---|---|---|---|
| Bleeding | Thromboembolism | Other | ||
| Annual embolic events | ||||
| Predicted | 8.6% | 8.9% | 9.6% | 7.8% |
| Observed | 3.7% | 3.5% | 0.0% | 4.5% |
| Relative reduction | −57.0% | −61.0% | −100.0% | −42.0% |
| p-Value | 0.035 * | 0.083 | 1.000 | 0.529 |
| Annual bleeding events | ||||
| Predicted | 7.7% | 8.3% | 6.6% | 6.7% |
| Observed | 5.3% | 5.5% | 0.0% | 5.7% |
| Relative reduction | −30.2% | −33.2% | −100.0% | −15.6% |
| p-Value | 0.454 | 0.483 | 1.000 | 1.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zweiker, D.; Sieghartsleitner, R.; Fiedler, L.; Toth, G.G.; Luha, O.; Stix, G.; Gabriel, H.; Vock, P.; Lileg, B.; Strouhal, A.; et al. Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure—The Austrian LAAC Registry. J. Clin. Med. 2020, 9, 3274. https://doi.org/10.3390/jcm9103274
Zweiker D, Sieghartsleitner R, Fiedler L, Toth GG, Luha O, Stix G, Gabriel H, Vock P, Lileg B, Strouhal A, et al. Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure—The Austrian LAAC Registry. Journal of Clinical Medicine. 2020; 9(10):3274. https://doi.org/10.3390/jcm9103274
Chicago/Turabian StyleZweiker, David, Raphael Sieghartsleitner, Lukas Fiedler, Gabor G. Toth, Olev Luha, Guenter Stix, Harald Gabriel, Paul Vock, Brigitte Lileg, Andreas Strouhal, and et al. 2020. "Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure—The Austrian LAAC Registry" Journal of Clinical Medicine 9, no. 10: 3274. https://doi.org/10.3390/jcm9103274
APA StyleZweiker, D., Sieghartsleitner, R., Fiedler, L., Toth, G. G., Luha, O., Stix, G., Gabriel, H., Vock, P., Lileg, B., Strouhal, A., Delle-Karth, G., Pfeffer, M., Aichinger, J., Tkalec, W., Steinwender, C., Sihorsch, K., Binder, R. K., Rammer, M., Barbieri, F., ... Scherr, D. (2020). Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure—The Austrian LAAC Registry. Journal of Clinical Medicine, 9(10), 3274. https://doi.org/10.3390/jcm9103274







