COVID-19 Triage and Test Center: Safety, Feasibility, and Outcomes of Low-Threshold Testing
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Methods of Measurement
2.3. Outcomes
2.4. Statistical Analysis
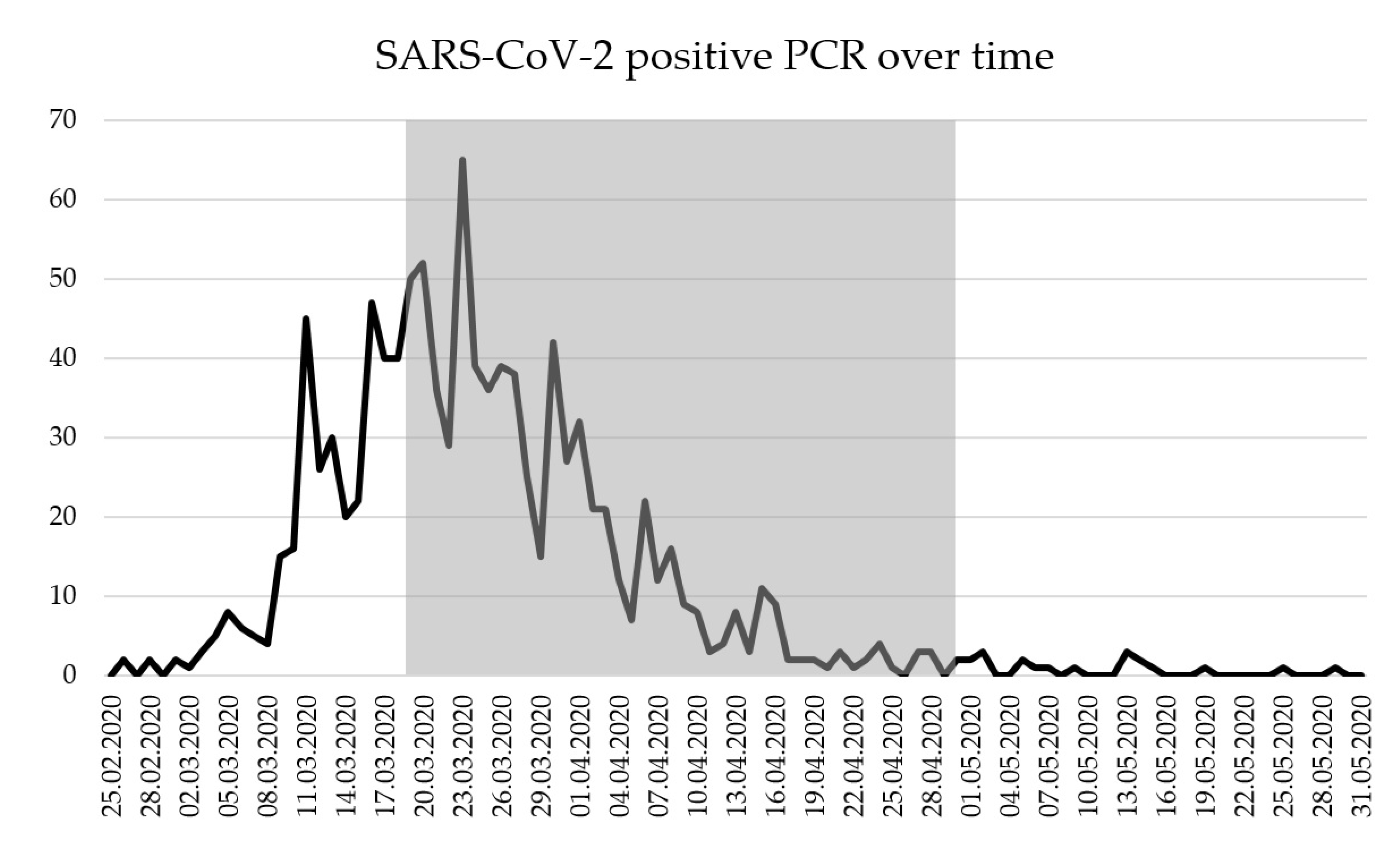
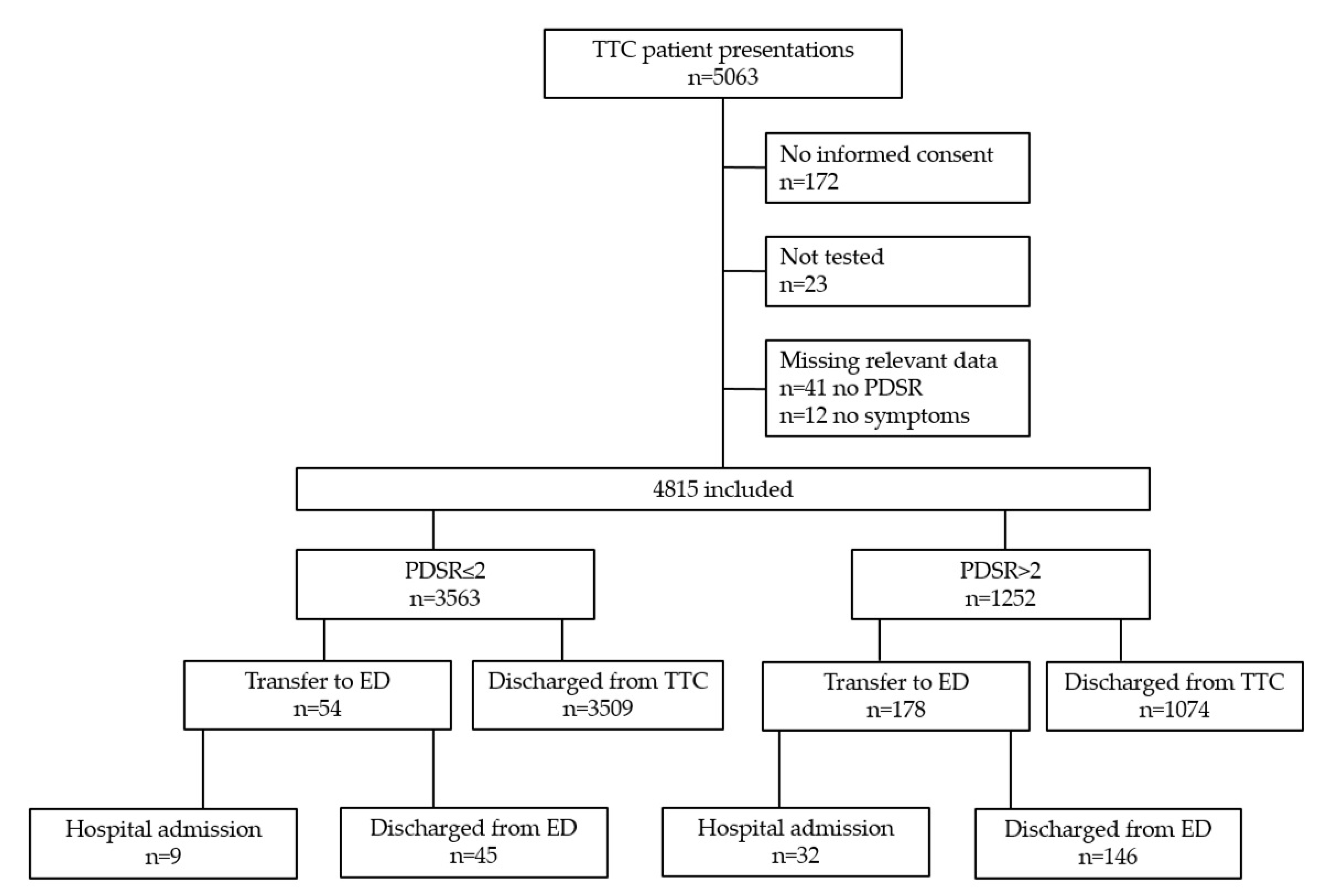
3. Results
3.1. Characteristics of Study Subjects
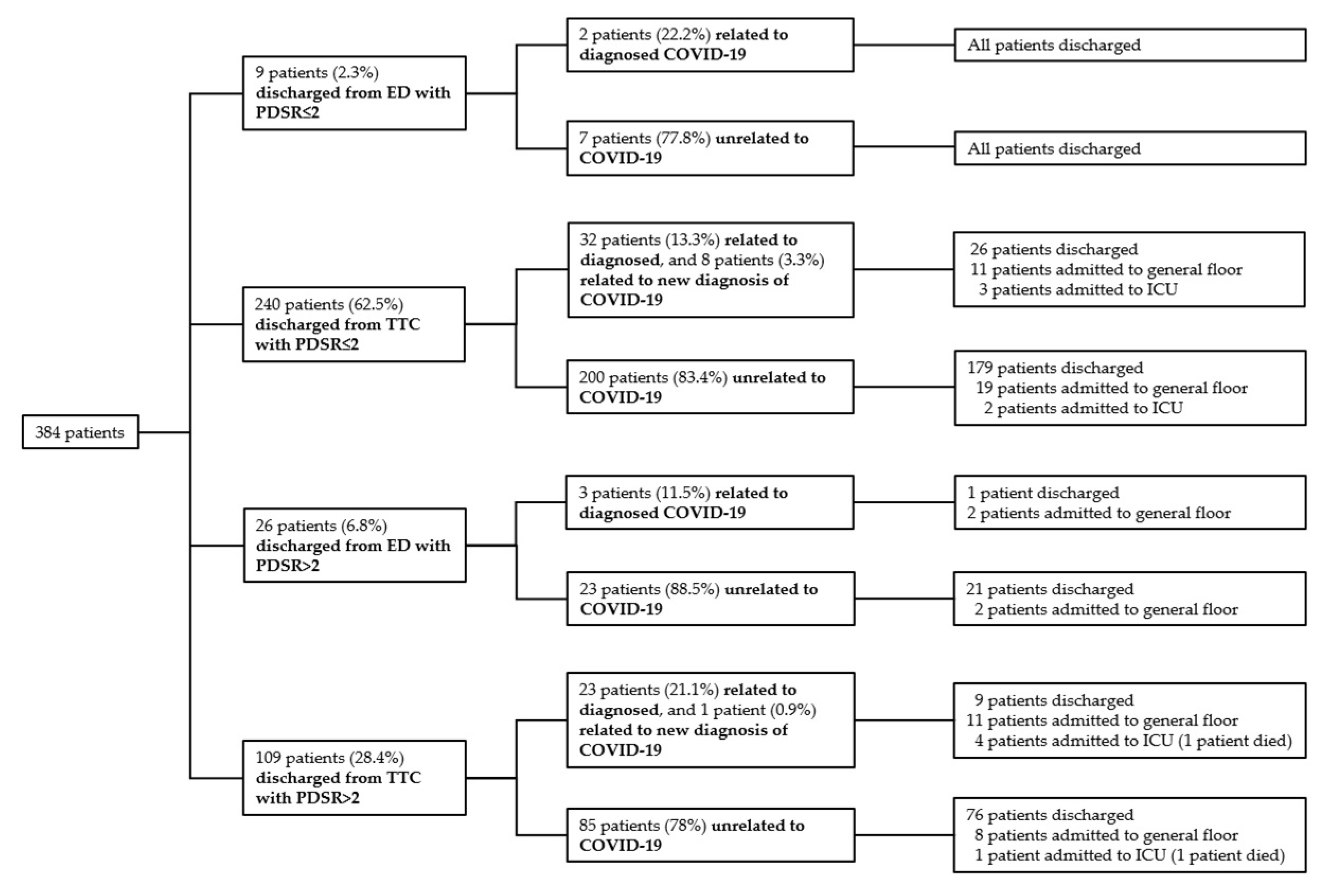
3.2. Safety Outcomes
3.3. Feasibility of the Triage and Test Center (TTC)
3.4. Clinical Predictors for Positive Nasopharyngeal SARS-CoV-2 Swab Result
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| PDSR | Total Presentations | Vitals Measured | Vitals not Neasured |
|---|---|---|---|
| PDSR 1 | 2041 | 223 1 | 1818 2 |
| PDSR 2 | 1522 | 346 3 | 1176 4 |
| PDSR ≤2 | 3563 | 569 (16%) | 2994 (84%) |
| PDSR 3 | 1055 | 1037 5 | 18 6 |
| PDSR 4 | 155 | 151 7 | 4 8 |
| PDSR 5 | 36 | 35 9 | 1 10 |
| PDSR 6 | 6 | 6 11 | 0 |
| PDSR >2 | 1252 | 1229 (98.2%) | 23 (1.8%) |
| All (n = 4815) | Discharged (n = 4774) | Hospitalized (n = 41) | OR [95% CI] | p-Value | |
|---|---|---|---|---|---|
| Age (years, median [min;max]) | 41.6 [13.4;93.3] | 41.4 [13.4;93.3] | 62.1 [21.2;90.7] | 1.08 [1.06;1.10] | <0.001 |
| Male | 2188 (45.4%) | 2162 (45.3%) | 26 (63.4%) | Ref. | Ref. |
| Female | 2627 (54.6%) | 2612 (54.7%) | 15 (36.6%) | 0.48 [0.25;0.90] | 0.022 |
| PDSR≤2 PDSR>2 | 3563 (74%) 1252 (26%) | 3554 (74.4%) 1220 (25.6%) | 9 (22.0%) 32 (78%) | Ref. 10.2; [5.05;23.00] | Ref. <0.001 |
| Symptoms | |||||
| Duration of symptoms (days, median [min;max]) 1 | 4 [1;119] | 4 [1;119] | 6.5 [1;27] | 1.01 [0.98;1.04] | 0.671 |
| Measured/reported fever | 2012 (41.8%) | 1989 (41.7%) | 23 (56.1%) | 1.79 [0.96;3.37] | 0.067 |
| Chills 2 | 1025 (23.8%) | 1008 (23.6%) | 17 (41.5%) | 2.30 [1.20;4.28] | 0.012 |
| Myalgia | 1859 (38.6%) | 1840 (38.5%) | 19 (46.3%) | 1.38 [0.73;2.56] | 0.313 |
| Lymphadenopathy | 491 (10.2%) | 484 (10.1%) | 7 (17.1%) | 1.86 [0.74;3.98] | 0.170 |
| Headache | 2693 (55.9%) | 2671 (55.9%) | 22 (53.7%) | 0.91 [0.49;1.71] | 0.768 |
| Seizure | 86 (1.79%) | 52 (1.7%) | 4 (9.8%) | 6.39 [1.84;16.5] | 0.007 |
| Confusion | 509 (10.6%) | 499 (10.5%) | 10 (24.4%) | 2.79 [1.28;5.55] | 0.011 |
| Nausea | 668 (13.9%) | 650 (13.6%) | 18 (43.9%) | 4.97 [2.62;9.27] | <0.001 |
| Conjunctivitis | 469 (9.7%) | 465 (9.7%) | 4 (9.8%) | 1.04 [0.30;2.61] | 0.946 |
| Exanthema | 244 (5.1%) | 239 (5%) | 5 (12.2%) | 2.71 [0.91;6.38] | 0.070 |
| Coryza | 2575 (53.5%) | 2557 (53.6%) | 18 (43.9%) | 0.68 [0.36;1.26] | 0.223 |
| Otalgia | 749 (15.6%) | 745 (15.6%) | 4 (9.8%) | 0.61 [0.18;1.52] | 0.313 |
| Sore throat | 2694 (56%) | 2679 (56.1%) | 15 (36.6%) | 0.45 [0.23;0.85] | 0.013 |
| Dyspnea | 1158 (24%) | 1139 (23.9%) | 19 (46.3%) | 2.76 [1.47;5.14] | 0.002 |
| Wheezing | 675 (14%) | 664 (13.9%) | 11 (26.8%) | 2.29 [1.09;4.48] | 0.031 |
| Cough | 3132 (65%) | 3106 (65.1%) | 26 (63.4%) | 0.93 [0.49;1.80] | 0.817 |
| Productive cough | 1119 (23.2%) | 1104 (23.1%) | 15 (36.6%) | 1.93 [0.99;3.62] | 0.054 |
| Hemoptysis | 53 (1.1%) | 52 (1.1%) | 1 (2.4%) | 2.58 [0.11;12.1] | 0.440 |
| Chest pain | 1335 (27.7%) | 1324 (27.7%) | 11 (26.8%) | 0.96 [0.46;1.88] | 0.919 |
| Abdominal pain | 664 (13.8%) | 653 (13.7%) | 11 (26.8%) | 2.33 [1.11;4.56] | 0.027 |
| Diarrhea | 842 (17.5%) | 833 (17.4%) | 9 (22%) | 1.35 [0.60;2.73] | 0.448 |
| Dysuria | 184 (3.8%) | 180 (3.8%) | 4 (9.8%) | 2.85 [0.83;7.24] | 0.089 |
| Exhausted | 2131 (44.3%) | 2105 (44.1%) | 26 (63.4%) | 2.19 [1.17;4.26] | 0.014 |
| Weakness 2 | 1901 (44.1%) | 1873 (43.9%) | 28 (68.3%) | 2.73 [1.44;5.49] | 0.002 |
| Comorbidities | |||||
| Regular medication | 1695 (35.2%) | 1673 (35%) | 22 (53.7%) | 2.14 [1.15;4.02] | 0.016 |
| Neurologic disease | 112 (2.3%) | 109 (2.3%) | 3 (7.3%) | 3.54 [0.82;10.0] | 0.084 |
| Hypertension | 648 (13.5%) | 638 (13.4%) | 10 (24.4%) | 2.11 [0.97;4.20] | 0.058 |
| Diabetes | 152 (3.2%) | 142 (3%) | 10 (24.4%) | 10.6 [4.83;21.4] | <0.001 |
| Overweight | 705 (14.6%) | 693 (14.5%) | 12 (29.3%) | 2.46 [1.19;4.73] | 0.016 |
| Heart disease | 232 (4.8%) | 228 (4.8%) | 4 (9.8%) | 2.23 [0.65;5.64] | 0.178 |
| Pulmonary disease | 221 (4.6%) | 218 (4.6%) | 3 (7.3%) | 1.73 [0.40;4.84] | 0.407 |
| Asthma | 607 (12.6%) | 604 (12.7%) | 3 (7.3%) | 0.57 [0.13;1.59] | 0.317 |
| Chronic obstructive pulmonary disease | 67 (1.4%) | 67 (1.4%) | 0 (0%) | ||
| Renal disease | 81 (1.7%) | 74 (1.6%) | 7 (17.1%) | 13.3 [5.20;29.4] | <0.001 |
| Liver disease | 86 (1.8%) | 84 (1.8%) | 2 (4.9%) | 3.07 [0.46;10.2] | 0.020 |
| Hematological disease | 39 (0.81%) | 37 (0.8%) | 2 (4.9%) | 7.01 [1.03;24.2] | 0.047 |
| HIV infection | 31 (0.6%) | 31 (0.6%) | 0 (0%) | ||
| Rheumatological disease | 231 (4.8%) | 229 (4.8%) | 2 (4.9%) | 1.09 [0.16;3.59] | 0.907 |
| Cancer | 118 (2.5%) | 113 (2.4%) | 5 (12.2%) | 5.88 [1.96;14.0] | 0.003 |
| Vital signs | |||||
| Systolic blood pressure (mmHg, median [min;max]) 3 | 141 [95;222] | 141 [95;222] | 137 [102;165] | 1.01 [0.99;1.03] | 0.151 |
| Diastolic blood pressure (mmHg, median [min;max])3 | 82 [43;130] | 82 [43;130] | 76.5 [47;106] | 1.04 [1.01;1.07] | 0.008 |
| Heart rate (beats per minute, median [min;max]) 4 | 83 [44;141] | 83 [44;141] | 93 [65;136] | 1.05 [1.02;1.07] | <0.001 |
| Respiratory rate (breath per minute, median [min;max]) 5 | 17 [6;33] | 17 [6;33] | 20 [12;26] | 1.23 [1.13;1.34] | <0.001 |
| Oxygen saturation (median [min;max]) 6 | 98 [90;100] | 98 [90;100] | 96 [92;99] | 1.89 [1.58;2.26] | <0.001 |
| Temperature (°C, median [min;max]) 6 | 36.8 [35;39.5] | 36.7 [35;39.5] | 37.2 [36;39.5] | 3.73 [2.44;5.71] | <0.001 |

References
- Prather, K.A.; Wang, C.C.; Schooley, R.T. Reducing transmission of SARS-CoV-2. Science 2020, 368, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Salathé, M.; Althaus, C.L.; Neher, R.; Stringhini, S.; Hodcroft, E.; Fellay, J.; Zwahlen, M.; Senti, G.; Battegay, M.; Wilder-Smith, A.; et al. COVID-19 epidemic in Switzerland: On the importance of testing, contact tracing and isolation. Swiss Med. Wkly. 2020, 150, w20225. [Google Scholar] [CrossRef] [PubMed]
- Bingisser, R.; Baerlocher, S.M.; Kuster, T.; Ortega, R.N.; Nickel, C.H. Physicians’ Disease Severity Ratings are Non-Inferior to the Emergency Severity Index. J. Clin. Med. 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Nickel, C.H.; Kellett, J.; Ortega, R.N.; Lyngholm, L.; Hanson, S.; Cooksley, T.; Bingisser, R.; Brabrand, M. A simple prognostic score predicts one-year mortality of alert and calm emergency department patients: A prospective two-center observational study. Int. J. Clin. Pr. 2020, 74, e13481. [Google Scholar] [CrossRef] [PubMed]
- Kellett, J.; Nickel, C.H.; Skyttberg, N.; Brabrand, M. Is it possible to quickly identify acutely unwell patients who can be safely managed as outpatients? The need for a "Universal Safe to Discharge Score". Eur. J. Intern. Med. 2019, 67, e13–e15. [Google Scholar] [CrossRef]
- Nickel, C.H.; Kellett, J.; Ortega, R.N.; Lyngholm, L.; Wasingya-Kasereka, L.; Brabrand, M.; Lyngholm, H. Mobility Identifies Acutely Ill Patients at Low Risk of In-Hospital Mortality: A Prospective Multicenter Study. Chest 2019, 156, 316–322. [Google Scholar] [CrossRef]
- Brabrand, M.; Kellett, J.; Opio, M.; Cooksley, T.; Nickel, C.H. Should impaired mobility on presentation be a vital sign? Acta Anaesthesiol. Scand. 2018, 62, 945–952. [Google Scholar] [CrossRef]
- Shah, A.; Challener, D.; Tande, A.J.; Mahmood, M.; O’Horo, J.C.; Berbari, E.; Crane, S.J. Drive-Through Testing. Mayo Clin. Proc. 2020, 95, 1420–1425. [Google Scholar] [CrossRef]
- Klasen, J.M.; Meienberg, A.; Nickel, C.; Bingisser, R. SWAB team instead of SWAT team: Medical students as a frontline force during the COVID-19 pandemic. Med. Educ. 2020, 54, 860. [Google Scholar] [CrossRef]
- Leuzinger, K.; Roloff, T.; Gosert, R.; Sogaard, K.; Naegele, K.; Rentsch, K.; Bingisser, R.; Nickel, C.H.; Pargger, H.; Bassetti, S.; et al. Epidemiology of SARS-CoV-2 Emergence Amidst Community-Acquired Respiratory Viruses. J. Infect. Dis. 2020, 222, 1270–1279. [Google Scholar] [CrossRef]
- Leuzinger, K.; Gosert, R.; Sogaard, K.; Naegele, K.; Bielicki, J.; Roloff, T.; Bingisser, R.; Nickel, C.H.; Khanna, N.; Tschudin, S.; et al. Epidemiology and precision of SARS-CoV-2 detection following lockdown and relaxation measures. medRxiv. (under review).
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Heesterbeek, H.; Klinkenberg, D.; Hollingsworth, T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020, 395, 931–934. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019-Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. SSRN Electron. J. 2020, 395, 10223. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K.; Gupta, A. Encephalopathy in patients with COVID-19: A review. J. Med. Virol. 2020, 1–17. [Google Scholar] [CrossRef]
- Bingisser, R.; Dietrich, M.; Ortega, R.N.; Malinovska, A.; Bosia, T.; Nickel, C. Systematically assessed symptoms as outcome predictors in emergency patients. Eur. J. Intern. Med. 2017, 45, 8–12. [Google Scholar] [CrossRef]
- Caterino, J.M.; Kline, D.M.; Leininger, R.; Southerland, L.T.; Carpenter, C.R.; Baugh, C.W.; Pallin, D.J.; Hunold, K.M.; Stevenson, K.B. Nonspecific Symptoms Lack Diagnostic Accuracy for Infection in Older Patients in the Emergency Department. J. Am. Geriatr. Soc. 2018, 67, 484–492. [Google Scholar] [CrossRef]
- Karakoumis, J.; Nickel, C.H.; Kirsch, M.; Rohacek, M.; Geigy, N.; Müller, B.; Ackermann, S.; Bingisser, R. Emergency Presentations With Nonspecific Complaints—The Burden of Morbidity and the Spectrum of Underlying Disease. Medicine 2015, 94, e840. [Google Scholar] [CrossRef]
- Nickel, C.H.; Bingisser, R. Mimics and chameleons of COVID-19. Swiss Med. Wkly. 2020, 150, w20231. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, A.C.; Hardwick, E.H.; Pius, R.; Norman, L.; Holden, A.K.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Xiong, L.; Cai, K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Med. Virol. 2020, 92, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern. Med. 2020, 180, 1081. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, Y.; Wang, Y.; Duan, G.; Yang, H. Dyspnea rather than fever is a risk factor for predicting mortality in patients with COVID-19. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Ciceri, F.; Castagna, A.; Rovere-Querini, P.; De Cobelli, F.; Ruggeri, A.; Galli, L.; Conte, C.; De Lorenzo, R.; Poli, A.; Ambrosio, A.; et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020, 217, 108509. [Google Scholar] [CrossRef]
- Pinto, L.C.; Bertoluci, M.C. Type 2 diabetes as a major risk factor for COVID-19 severity: A meta-analysis. Arch. Endocrinol. Metab. 2020, 64, 199–200. [Google Scholar] [CrossRef]
- Klang, E.; Kassim, G.; Soffer, S.; Freeman, R.; Levin, M.A.; Reich, D.L. Severe Obesity as an Independent Risk Factor for COVID-19 Mortality in Hospitalized Patients Younger than. Obesity 2020, 28, 1595–1599. [Google Scholar] [CrossRef]
- Drager, L.F.; Pio-Abreu, A.; Lopes, R.D.; Bortolotto, L.A. Is Hypertension a Real Risk Factor for Poor Prognosis in the COVID-19 Pandemic? Curr. Hypertens. Rep. 2020, 22, 43. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, W.; Guo, E.; Liu, J.; Yang, B.; Wu, P.; Lin, S.; Peng, T.; Fu, Y.; Li, F.; et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: A propensity score-matched analysis. J. Hematol. Oncol. 2020, 13, 75. [Google Scholar] [CrossRef]
- Russo, V.; Bottino, R.; Carbone, A.; Rago, A.; Papa, A.A.; Golino, P.; Nigro, G. COVID-19 and Heart: From Clinical Features to Pharmacological Implications. J. Clin. Med. 2020, 9, 1944. [Google Scholar] [CrossRef]
- Pallotto, C.; Suardi, L.R.; Esperti, S.; Tarquini, R.; Grifoni, E.; Simone, M.; Valoriani, A.; di Martino, S.; Cei, F.; Sisti, E.; et al. Increased CD4/CD8 ratio as a risk factor for critical illness in coronavirus disease 2019 (COVID-19): A retrospective multicentre study. Infect Dis. 2020, 52, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Ahn, H.; Ryu, H.S.; Kim, B.-J.; Jang, J.; Jung, M.; Kim, J.; Jeong, S.H. Clinical Characteristics and Disease Progression in Early-Stage COVID-19 Patients in South Korea: The Retrospective Cohort Study. SSRN Electron. J. 2020, 9, 1959. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Mudd, P.; West, C.P.; Wilber, E.; Wilber, S.T. Diagnosing COVID-19 in the Emergency Department: A Scoping Review of Clinical Exam, Labs, Imaging Accuracy and Biases. Acad. Emerg. Med. 2020, 27, 653–670. [Google Scholar] [CrossRef]
- Bénézit, F.; Le Turnier, P.; Declerck, C.; Paillé, C.; Revest, M.; Dubée, V.; Tattevin, P.; Arvieux, C.; Baldeyrou, M.; Chapplain, J.-M.; et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020, 20, 1014–1015. [Google Scholar] [CrossRef]
- Watson, J.; Whiting, P.F.; E Brush, J. Interpreting a covid-19 test result. BMJ 2020, 369, m1808. [Google Scholar]
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction–Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [Google Scholar] [CrossRef]
- Bundesamt für Gesundheit BAG, D.Ö.G. 7.5.2020, Zwischenbilanz zum neuen Coronavirus in der Schweiz und im Fürstentum Liechtenstein. 2020 07.05.2020 Coronavirus Krankheit 2019 (COVID-19). Available online: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/k-und-i/aktuelle-ausbrueche-pandemien/2019-nCoV/covid-19-zwischenbilanz-mai-2020.pdf.download.pdf/BAG_Epidemiologische_Zwischenbilanz_zum_neuen_Coronavirus.pdf (accessed on 5 July 2020).

| All (n = 4815) | Positive Swab (n = 572) | Negative Swab (n = 4243) | OR [95% CI] | p-Value | |
|---|---|---|---|---|---|
| Age (years, median [min/max]) | 41.6 [13.4;93.3] | 45.7 [16.5; 86.8] | 41.2 [13.4;93.3] | 1.31 [1.15;1.50] | <0.001 |
| Male | 2188 (45.4%) | 289 (50.5%) | 1899 (44.8%) | Ref. | Ref. |
| Female | 2627 (54.6%) | 283 (49.5%) | 2344 (55.2%) | 0.79 [0.67;0.94] | 0.009 |
| PDSR ≤2 PDSR >2 | 3563 (74%) 1252 (26%) | 399 (69.8%) 173 (30.2%) | 3164 (74.6%) 1079 (25.4%) | Ref. 1.18 [0.97;1.43] | Ref. 0.104 |
| Symptoms | |||||
| Duration of symptoms (days, median [min/max]) 1 | 4 [1;119] | 5 [1;55] | 4 [1;119] | 0.94 [0.89;1.00] | 0.052 |
| Measured/reported fever | 2012 (41.8%) | 323 (56.5%) | 1689 (39.8%) | 2.03 [1.70;2.42] | <0.001 |
| Chills 2 | 1025 (23.8%) | 165 (33.3%) | 860 (22.6%) | 1.77 [1.44;2.16] | <0.001 |
| Myalgia | 1859 (38.6%) | 301 (52.6%) | 1558 (36.7%) | 1.94 [1.63;2.31] | <0.001 |
| Lymphadenopathy | 491 (10.2%) | 33 (5.8%) | 458 (10.8%) | 0.54 [0.37;0.76] | <0.001 |
| Headache | 2693 (55.9%) | 366 (64%) | 2327 (54.8%) | 1.61 [1.34;1.94] | <0.001 |
| Seizure | 86 (1.79%) | 9 (1.57%) | 77 (1.81%) | 0.83 [0.38;1.57] | 0.592 |
| Confusion | 509 (10.6%) | 76 (13.3%) | 433 (10.2%) | 1.39 [1.06;1.80] | 0.014 |
| Nausea | 668 (13.9%) | 82 (14.3%) | 586 (13.8%) | 1.11 [0.86;1.43] | 0.403 |
| Conjunctivitis | 469 (9.7%) | 51 (8.9%) | 418 (9.9%) | 0.87 [0.64;1.17] | 0.377 |
| Exanthema | 244 (5.1%) | 20 (3.5%) | 224 (5.3%) | 0.66 [0.40;1.03] | 0.081 |
| Coryza | 2575 (53.5%) | 314 (54.9%) | 2261 (53.3%) | 1.10 [0.92;1.32] | 0.279 |
| Otalgia | 749 (15.6%) | 70 (12.2%) | 679 (16%) | 0.77 [0.59;1.00] | 0.055 |
| Sore throat | 2694 (56%) | 247 (43.2%) | 2447 (57.7%) | 0.59 [0.49;0.70] | <0.001 |
| Dyspnea | 1158 (24%) | 101 (17.7%) | 1057 (24.9%) | 0.65 [0.52;0.82] | <0.001 |
| Wheezing | 675 (14%) | 42 (7.3%) | 633 (14.9%) | 0.44 [0.32;0.61] | <0.001 |
| Cough | 3132 (65%) | 415 (72.6%) | 2717 (64%) | 1.50 [1.24;1.83] | <0.001 |
| Productive cough | 1119 (23.2%) | 132 (23.1%) | 987 (23.3%) | 0.97 [0.78;1.19] | 0.743 |
| Hemoptysis | 53 (1.1%) | 4 (0.7%) | 49 (1.2%) | 0.61 [0.18;1.51] | 0.345 |
| Chest pain | 1335 (27.7%) | 139 (24.3%) | 1196 (28.2%) | 0.83 [0.68;1.02] | 0.075 |
| Abdominal pain | 664 (13.8%) | 63 (11%) | 601 (14.2%) | 0.79 [0.59;1.03] | 0.087 |
| Diarrhea | 842 (17.5%) | 112 (19.6%) | 730 (17.2%) | 1.21 [0.97;1.51] | 0.093 |
| Dysuria | 184 (3.8%) | 22 (3.9%) | 162 (3.8%) | 0.99 [0.61;1.53] | 0.981 |
| Exhausted | 2131 (44.3%) | 271 (47.4%) | 1860 (43.8%) | 1.18 [0.98;1.40] | 0.073 |
| Weakness 2 | 1901 (44.1%) | 255 (51.4%) | 1646 (43.2%) | 1.46 [1.21;1.76] | <0.001 |
| Comorbidities | |||||
| Regular medication | 1695 (35.2%) | 190 (33.2%) | 1505 (35.5%) | 0.81 [0.66;0.98] | 0.030 |
| Neurologic disease | 112 (2.3%) | 7 (1.2%) | 105 (2.5%) | 0.45 [0.19;0.90] | 0.040 |
| Hypertension | 648 (13.5%) | 84 (14.7%) | 564 (13.3%) | 0.91 [0.70;1.19] | 0.501 |
| Diabetes | 152 (3.2%) | 31 (5.4%) | 121 (2.9%) | 1.64 [1.07;2.45] | 0.019 |
| Overweight | 705 (14.6%) | 82 (14.3%) | 623 (14.7%) | 0.90 [0.70;1.16] | 0.428 |
| Heart disease | 232 (4.8%) | 32 (5.6%) | 200 (4.7%) | 0.97 [0.65;1.43] | 0.898 |
| Pulmonary disease | 221 (4.6%) | 9 (1.6%) | 212 (5%) | 0.27 [0.13;0.50] | <0.001 |
| Asthma | 607 (12.6%) | 51 (8.9%) | 556 (13.1%) | 0.66 [0.48;0.89] | 0.007 |
| Chronic obstructive pulmonary disease | 67 (1.4%) | 4 (0.7%) | 63 (1.5%) | 0.38 [0.11;0.93] | 0.062 |
| Renal disease | 81 (1.7%) | 15 (2.6%) | 66 (1.6%) | 1.54 [0.84;2.66] | 0.138 |
| Liver disease | 86 (1.8%) | 10 (1.8%) | 76 (1.8%) | 0.85 [0.41;1.58] | 0.627 |
| Hematological disease | 39 (0.81%) | 5 (0.9%) | 34 (0.8%) | 1.03 [0.35;2.42] | 0.958 |
| HIV infection | 31 (0.6%) | 5 (0.9%) | 26 (0.6%) | 1.27 [0.43;3.06] | 0.631 |
| Rheumatological disease | 231 (4.8%) | 26 (4.6%) | 205 (4.8%) | 0.82 [0.53;1.24] | 0.373 |
| Cancer | 118 (2.5%) | 16 (2.8%) | 102 (2.4%) | 0.97 [0.54;1.62] | 0.910 |
| Vital signs | |||||
| Systolic blood pressure (mmHg, median [min/max]) 3 | 141 [95;222] | 139 [97;197] | 142 [95;222] | 1.38 [1.12;1.70] | 0.002 |
| Diastolic blood pressure (mmHg, median [min/max]) 3 | 82 [43;130] | 81 [46;130] | 82 [43;120] | 1.21 [1.01;1.44] | 0.036 |
| Heart rate (beats per minute, median [min/max]) 4 | 83 [44;141] | 85 [44;130] | 83 [47;141] | 1.24 [1.03;1.48] | 0.019 |
| Respiratory rate (breath per minute, median [min/max]) 5 | 17 [6;33] | 17 [7;32] | 16 [6;33] | 1.42 [1.17;1.71] | <0.001 |
| Oxygen saturation (median [min/max]) 6 | 98 [90;100] | 97 [92;100] | 98 [90;100] | 1.06 [0.96;1.17] | 0.275 |
| Temperature (°C, median [min/max]) 6 | 36.8 [35;39.5] | 36.9 [35.2;39.1] | 36.7 [35;39.5] | 1.52 [1.30;1.78] | <0.001 |
| Diagnoses of COVID-19 in Presentations discharged from TTC (n = 4583) | |
| Discharged with PDSR ≤2 (n = 3509) | 392 positive testing for SARS-CoV-2 |
| Discharged with PDSR >2 (n = 1074) | 146 positive testing for SARS-CoV-2 |
| Diagnoses of hospitalized patients with PDSR ≤2 (n = 9) | |
| Number of patients | Diagnosis |
| 2 | COVID-19 1 |
| 1 | Anorectal abscess |
| 1 | Behavioral disorder |
| 1 | Pneumonia |
| 1 | Hepatitis |
| 1 | Atraumatic Pneumomediastinum |
| 1 | Sinusitis |
| 1 | Screening before planned hospital admission for vocal cord biopsy |
| Diagnoses of patients with PDSR ≤2 discharged from ED (n = 45) | |
| Number of patients | Diagnosis |
| 6 | COVID-19 |
| 24 | Presumed viral infection tested negative for SARS-CoV-2 |
| 5 | Chest pain of unclear origin |
| 2 | Asthma |
| 2 | Dyspnea of unclear origin |
| 1 | Hypertensive urgency |
| 1 | Diarrhea |
| 1 | Urinary tract infection |
| 1 | Abdominal pain of unclear origin |
| 1 | Cocaine intoxication |
| 1 | Behavioral disorder |
| Diagnoses of hospitalized patients with PDSR >2 (n = 32) | |
| Number of patients | Diagnosis |
| 12 | COVID-19 2 |
| 6 | Pneumonia |
| 2 | Coronary heart disease |
| 2 | Presumed viral infection tested negative for SARS-CoV-2 |
| 1 | Decompensated heart failure |
| 1 | Perimyocarditis |
| 1 | Pulmonary embolism |
| 1 | Asthma |
| 1 | Angina |
| 1 | Appendicitis |
| 1 | Urinary tract infection |
| 1 | Pyelonephritis |
| 1 | Wound infection |
| 1 | Behavioral disorder |
| Diagnoses of patients with PDSR >2 discharged from ED (n = 146) | |
| Number of patients | Diagnosis |
| 17 | COVID-19 |
| 78 | Presumed viral infection tested negative for SARS-CoV-2 |
| 14 | Chest pain of unclear origin |
| 12 | Asthma |
| 5 | Angina |
| 3 | Hypertensive urgency |
| 3 | Urinary tract infection |
| 2 | Abdominal pain of unclear origin |
| 2 | Pneumonia |
| 2 | Dyspnea of unclear origin |
| 1 | Pulmonary embolism |
| 1 | Chronic cough of unclear origin |
| 1 | Exacerbation of chronic obstructive pulmonary disease |
| 1 | Laryngitis |
| 1 | Dyspepsia |
| 1 | Pyelonephritis |
| 1 | Traumatic subarachnoid hemorrhage |
| 1 | Behavioral disorder |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansella, G.; Rueegg, M.; Widmer, A.F.; Tschudin-Sutter, S.; Battegay, M.; Hoff, J.; Søgaard, K.K.; Egli, A.; Stieltjes, B.; Leuzinger, K.; et al. COVID-19 Triage and Test Center: Safety, Feasibility, and Outcomes of Low-Threshold Testing. J. Clin. Med. 2020, 9, 3217. https://doi.org/10.3390/jcm9103217
Mansella G, Rueegg M, Widmer AF, Tschudin-Sutter S, Battegay M, Hoff J, Søgaard KK, Egli A, Stieltjes B, Leuzinger K, et al. COVID-19 Triage and Test Center: Safety, Feasibility, and Outcomes of Low-Threshold Testing. Journal of Clinical Medicine. 2020; 9(10):3217. https://doi.org/10.3390/jcm9103217
Chicago/Turabian StyleMansella, Gregory, Marco Rueegg, Andreas F. Widmer, Sarah Tschudin-Sutter, Manuel Battegay, Julia Hoff, Kirstine K. Søgaard, Adrian Egli, Bram Stieltjes, Karoline Leuzinger, and et al. 2020. "COVID-19 Triage and Test Center: Safety, Feasibility, and Outcomes of Low-Threshold Testing" Journal of Clinical Medicine 9, no. 10: 3217. https://doi.org/10.3390/jcm9103217
APA StyleMansella, G., Rueegg, M., Widmer, A. F., Tschudin-Sutter, S., Battegay, M., Hoff, J., Søgaard, K. K., Egli, A., Stieltjes, B., Leuzinger, K., Hirsch, H. H., Meienberg, A., Burkard, T., Mayr, M., Bingisser, R., & Nickel, C. H. (2020). COVID-19 Triage and Test Center: Safety, Feasibility, and Outcomes of Low-Threshold Testing. Journal of Clinical Medicine, 9(10), 3217. https://doi.org/10.3390/jcm9103217





