Trabeculated Myocardium in Hypertrophic Cardiomyopathy: Clinical Consequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Trabecular Quantification
2.3. Statistical Analysis
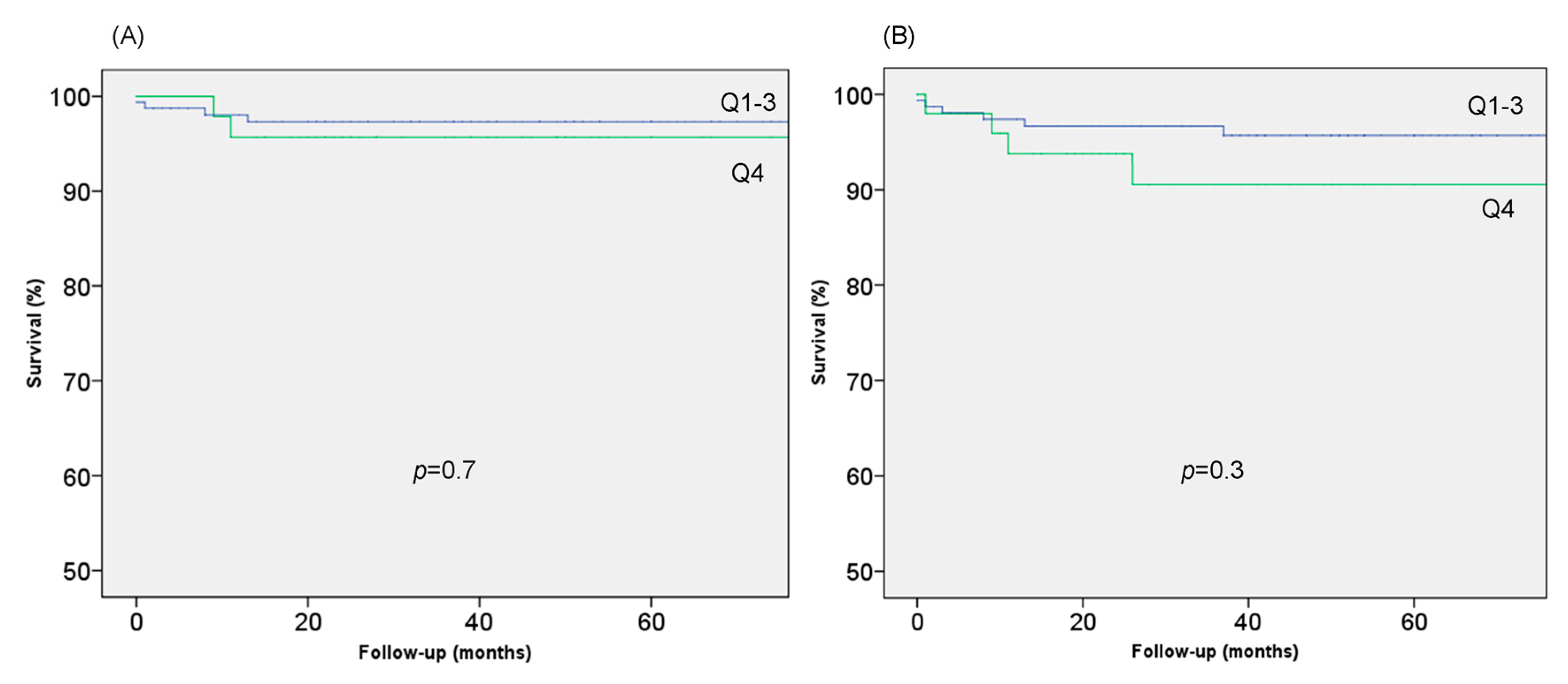
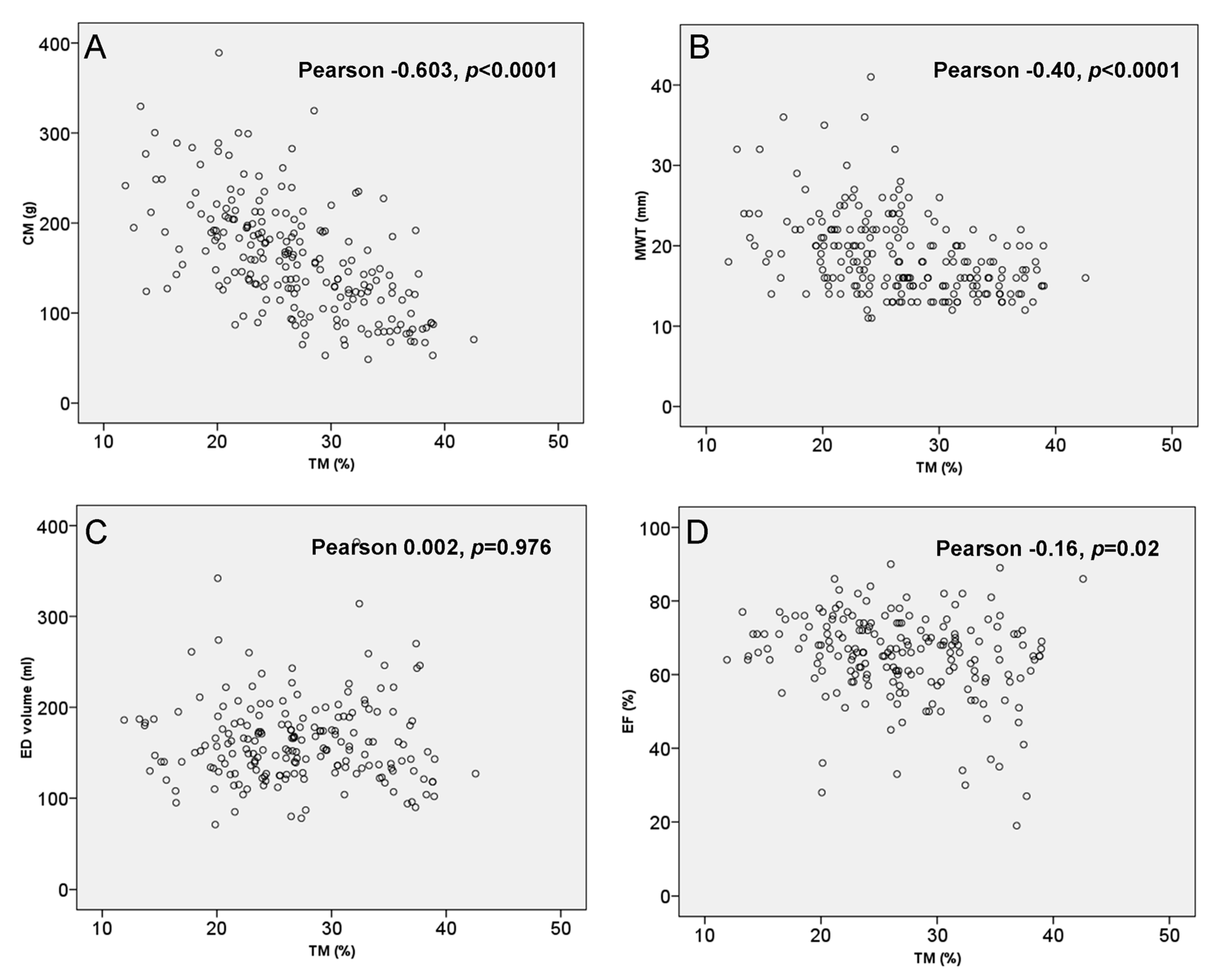
3. Results
3.1. Echocardiography
3.2. LV Trabeculation and Demographics
3.3. LV Trabeculation and Morphological Findings
3.4. LV Trabeculation and Clinical Findings
3.5. Multivariable Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Hypertrophic cardiomyopathy: A systematic review. JAMA 2002, 287, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Seidman, C.E.; McKenna, W.J.; Maron, B.J. The management of hypertrophic cardiomyopathy. N. Engl. J. Med. 1997, 336, 775–785. [Google Scholar] [CrossRef]
- Elliott, P.; McKenna, W.J. Hypertrophic cardiomyopathy. Lancet 2004, 363, 1881–1891. [Google Scholar] [CrossRef]
- Charron, P.; Carrier, L.; Dubourg, O.; Tesson, F.; Desnos, M.; Richard, P.; Bonne, G.; Guicheney, P.; Hainque, B.; Bouhour, J.B.; et al. Penetrance of familial hypertrophic cardiomyopathy. Genet. Couns. 1997, 8, 107–114. [Google Scholar]
- Seidman, J.G.; Seidman, C. The genetic basis for cardiomyopathy: From mutation identification to mechanistic paradigms. Cell 2001, 104, 557–567. [Google Scholar] [CrossRef]
- Gómez, J.; Rqguero, J.R.; Coto, E. The ups and downs of genetic diagnosis of hypertrophic cardiomyopathy. Rev. Esp. Cardiol. 2016, 69, 61–68. [Google Scholar] [CrossRef]
- Arbustini, E.; Favalli, V.; Narula, N.; Serio, A.; Grasso, M. Left ventricular non-compaction. A distinct genetic cardiomyopapthy? J. Am. Coll. Cardiol. 2016, 68, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.; Jenni, R. Left ventricular noncompaction. From physiologic remodeling to noncompaction cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, 723–726. [Google Scholar] [CrossRef]
- Finsterer, J.; Stöllberger, C.; Towbin, J.A. Left ventricular noncompaction cardiomyopathy: Cardiac, neuromuscular, and genetic factors. Nat. Rev. Cardiol. 2017, 14, 224–237. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of the left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef]
- Oechslin, E.N.; Attenhofer Jost, C.H.; Rojas, J.R.; Kaufmann, P.A.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef]
- Dedaghat-Hamedani, F.; Haas, J.; Zhu, F.; Geier, C.; Kayvanpour, E.; Liss, M.; Lai, A.; Frese, K.; Pribe-Wolferts, R.; Amr, A.; et al. Clinical genetics and outcome of left ventricular non-compaction cardiomyopathy. Eur. Heart J. 2017, 38, 3449–3460. [Google Scholar] [CrossRef]
- Hussein, A.; Karimianpour, A.; Collier, P.; Krasuski, R.A. Isolated noncompaction of the ventricle in adults. J. Am. Coll. Cardiol. 2005, 66, 578–585. [Google Scholar] [CrossRef] [PubMed]
- van Waning, J.I.; Caliskan, K.; Hoedemaekers, Y.M.; van Spaendonck-Zwarts, K.Y.; Baas, A.F.; Boekholdt, S.M.; van Melle, J.P.; Teske, A.J.; Asselbergs, F.W.; Backx, A.P.C.M.; et al. Genetics, clinical features, and long-term outcome of noncompaction cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, 711–722. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spieling, W.; Agabiti, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Geske, J.B.; MartijnBos, J.; Gersh, B.J.; Ommen, S.R.; Eidem, B.W.; Ackerman, M.J. Deformation patterns in genotyped patients with hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, G.; Cuenca, J.; de Teruel, P.E.L.; Giménez, D.; González-Carrillo, J. A software tool for the automatic quantification of the LV myocardium hypertrabeculation degree. Procedia Comput. Sci. 2015, 51, 610–619. [Google Scholar] [CrossRef]
- Thaman, R.; Gimeno, J.R.; Murphy, R.T.; Kubo, T.; Sachdev, B.; Mogensen, J.; Elliott, P.M.; McKenna, W.J. Prevalence and clinical significance of systolic impairment in hypertrophic Cardiomyopathy. Heart 2005, 91, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gubert, J. Measurement of trabeculated LV mass using CMR imaging in the diagnosis of LVNC. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Papavasilliu, T.; Kuhl, H.P.; Schroder, M.; Susalbeck, Y.; Bondareu, K.O.; Bohn, C.K.; Beek, A.; Hofman, M.M.B.; van Rossum, A.C. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology 2005, 236, 57–64. [Google Scholar] [CrossRef]
- Bernabé, G.; González-Carrillo, J.; Cuenca, J.; Rodríguez Sánchez, D.; Saura-Espín, D.; Gimeno, J.R. Performance of a new software tool for automatic quantification of left ventricular trabeculations. Rev. Esp. Cardiol. 2017, 70, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Captur, G.; Lopes, L.R.; Patel, V.; Li, C.; Bassett, P.; Syrris, P. Abnormal cardiac formation in hypertrophic cardiomyopathy—Fractal analysis of trabeculae and preclinical gene expression. Circ. Cardiovasc. Genet. 2014, 7, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, G.; Casanova, J.D.; Cuenca, J.; González-Carrillo, J. A self-optimized software tool for quantifying the degree of left ventricle hyper-trabeculation. J. Supercomput. 2019, 75, 1625. [Google Scholar] [CrossRef]
- Captur, G.; Ho, C.Y.; Schlossarek, S.; Kerwin, J.; Mirabel, M.; Wilson, R.; Rosmini, S.; Obianyo, C.; Reant, P.; Bassett, P.; et al. The embryological basis of subclinical hypertrophic cardiomyopathy. Sci. Rep. 2016, 21, 27714. [Google Scholar] [CrossRef] [PubMed]
- Gati, S.; Chandra, N.; Bennett, R.L.; Reed, M.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Sheikh, N.; Zaidi, A.; Wilson, M.; et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013, 99, 401–408. [Google Scholar] [CrossRef]
- Gati, S.; Papadakis, M.; Papamichael, N.D.; Zaidi, A.; Sheikh, N.; Reed, M.; Sharma, R.; Thilaganathan, B.; Sharma, S. Reversible de novo left ventricular trabeculations in pregnant women: Implications for the diagnosis of left ventricular non-compaction in low-risk populations. Circulation 2014, 130, 475–483. [Google Scholar] [CrossRef]
- Murphy, R.T.; Thaman, R.; Blanes, J.G.; Ward, D.; Sevdalis, E.; Papra, E.; Kiotsekoglou, A.; Tome, M.T.; Pellerin, D.; McKenna, W.J.; et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur. Heart J. 2005, 26, 187–192. [Google Scholar] [CrossRef]

| Q1 | Q2 | Q3 | Q4 | Total | Sig. (p) | ||
|---|---|---|---|---|---|---|---|
| n | 54 (25.6) | 51 (24.2) | 54 (25.6) | 52 (24.6) | 211 (100.0) | ||
| Age at diagnosis | 45.8 ± 15.5 | 42.5 ± 17.8 | 43.2 ± 17.0 | 45.9 ± 18.8 | 44.4 ± 17.2 | 0.668 | |
| Age at CMR | 50.6 ± 14.9 | 48.2 ± 17.9 | 47.4 ± 16.5 | 53.1 ± 15.2 | 49.8 ± 16.2 | 0.267 | |
| Proband | 41 (75.9) | 32 (62.7) | 40 (74.1) | 28 (53.8) | 141 (66.8) | 0.059 | |
| Sex | Male | 38 (70.4) | 39 (76.5) | 39 (72.2) | 38 (73.1) | 154 (73) | 0.880 |
| Female | 16 (29.6) | 12 (23.5) | 15 (27.8) | 14 (26.9) | 57 (27) | 0.880 | |
| Reason for diagnosis | Incidental | 18 (33.3) | 18 (29.4) | 16 (29.6) | 12 (23.1) | 61 (28.9) | 0.275 |
| Symptoms | 25 (46.3) | 19 (37.3) | 24 (44.4) | 20 (38.5) | 88 (41.7) | 0.586 | |
| Screening | 9 (16.7) | 15 (29.4) | 13 (24.1) | 14 (26.9) | 51 (24.2) | 0.326 | |
| SD | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0.183 | |
| Genetics | Positive | 20 (37.0) | 18 (35.3) | 26 (48.1) | 21 (40.4) | 85 (40.3) | 0.537 |
| MYBPC3 | 10 (50.0) | 11 (61.1) | 20 (76.9) | 14 (66.7) | 55 (64.7) | 0.136 | |
| MYH7 | 7 (35.0) | 3 (16.7) | 1 (3.8) | 3 (14.3) | 14 (14.5) | 0.135 | |
| Other | 3 (15.0) | 4 (22.2) | 5 (19.2) | 3 (14.3) | 13 (15.3) | 0.866 | |
| HTN | 20 (37.0) | 23 (45.1) | 22 (40.7) | 22 (42.3) | 87 (41.2) | 0.698 | |
| Physical activity | 13 (24.5) | 16 (32.0) | 27 (50.9) | 23 (45.1) | 79 (38.2) | 0.007 | |
| Athlete | 2 (3.8) | 5 (10.0) | 11 (20.8) | 5 (9.8) | 23 (11.1) | 0.131 | |
| NYHA | I | 32 (61.5) | 36 (73.5) | 37 (69.8) | 39 (76.5) | 144 (70.2) | 0.147 |
| II | 16 (30.8) | 13 (26.5) | 13 (24.5) | 9 (17.6) | 51 (24.9) | 0.126 | |
| III | 3 (5.8) | 0 (0.0) | 2 (3.8) | 2 (3.9) | 7 (3.4) | 0.859 | |
| IV | 1 (1.9) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 2 (1.0) | 0.998 | |
| Syncope | 4 (7.7) | 4 (8.2) | 3 (5.7) | 2 (3.8) | 13 (6.3) | 0.356 | |
| Atrial Fibrillation | 11 (20.4) | 10 (19.6) | 12 (22.2) | 9 (17.3) | 42 (19.9) | 0.794 | |
| SD RISK FACTOR | |||||||
| FHSCD | 4 (7.4) | 10 (19.6) | 5 (9.3) | 7 (13.5) | 26 (12.3) | 0.684 | |
| ABPR | 6 (14.3) | 5 (12.8) | 9 (20.9) | 3 (7.1) | 23 (13.9) | 0.580 | |
| NSVT | 16 (30.2) | 9 (18.4) | 9 (18.8) | 5 (10.6) | 39 (19.8) | 0.020 | |
| Sustained VT | 2 (3.7) | 3 (5.9) | 1 (1.9) | 0 (0.0) | 6 (2.8) | 0.144 |
| Q1 | Q2 | Q3 | Q4 | Total | Sig. (p) | ||
|---|---|---|---|---|---|---|---|
| Max LVH (mm) | 21.1 (5.1) | 19.5 (6.3) | 18.1 (4.0) | 16.3 (3.1) | 18.8 (5.1) | <0.001 | |
| Pattern of hypertrophy | ASH | 27 (51.9) | 21 (44.7) | 25 (46.3) | 30 (57.7) | 103 (50.2) | 0.551 |
| Concentric | 10 (19.2) | 11 (23.4) | 17 (31.5) | 13 (25.0) | 51 (24.9) | 0.537 | |
| Apical | 5 (9.6) | 7 (14.9) | 3 (5.6) | 0 (0.0) | 15 (7.3) | 0.032 | |
| Reverse | 14 (28.0) | 7 (14.9) | 6 (11.1) | 3 (5.8) | 30 (14.8) | 0.012 | |
| Neutro | 13 (26.0) | 18 (38.3) | 25 (46.3) | 29 (55.8) | 85 (41.9) | 0.018 | |
| Sigmoid | 3 (6.0) | 3 (6.4) | 4 (7.4) | 4 (7.7) | 14 (6.9) | 0.985 | |
| Obstruction | 25 (47.2) | 13 (25.5) | 16 (29.6) | 10 (19.2) | 64 (30.5) | 0.005 | |
| Severe Obstruction | 12 (22.2) | 6 (12.0) | 5 (9.3) | 4 (8.0) | 27 (13.0) | 0.027 | |
| LVED (ml) | 158.1 (49.0) | 154.5 (40.1) | 165.1 (32.9) | 171.5 (57.4) | 162.5 (46.0) | 0.281 | |
| LVES (ml) | 53.7 (37.6) | 51.4 (20.7) | 58.5 (25.3) | 71.9 (47.3) | 59.1 (35.2) | 0.019 | |
| LVEF (%) | 67.9 (10.7) | 67.2 (7.5) | 64.5 (11.4) | 60.1 (14.4) | 64.8 (11.7) | 0.004 | |
| LVEF < 50% | 2 (4.2) | 3 (6.3) | 6 (11.8) | 8 (16.3) | 19 (9.7%) | 0.023 | |
| LGE | 31 (63.3) | 31 (63.3) | 27 (55.1) | 17 (34.7) | 106 (54.1) | 0.003 |
| Female | Male | Total | Sig. (p) | |
|---|---|---|---|---|
| CM | 124.8 ± 55.5 | 173.1 ± 59.3 | 160.0 ± 62.0 | <0.0001 |
| TM | 49.8 ± 18.5 | 57.6 ± 18.5 | 55.5 ± 18.7 | 0.007 |
| TM% | 29.7 ± 7.2 | 25.6 ± 5.8 | 26.7 ± 6.4 | <0.0001 |
| CM (indexed) | 72.2 ± 32.0 | 87.9 ± 30.1 | 83.6 ± 31.3 | 0.001 |
| TM (indexed) | 29.1 ± 11.6 | 29.1 ± 9.3 | 29.1 ± 10.0 | 0.977 |
| Q1 | Q2 | Q3 | Q4 | Total | Sig. (p) | |
|---|---|---|---|---|---|---|
| Pacemaker | 7 (13.0) | 1 (2.0) | 0 (0.0) | 1 (1.9) | 9 (4.3) | 0.004 |
| ICD | 9 (16.7) | 6 (11.8) | 8 (14.8) | 3 (5.8) | 26 (12.3) | 0.145 |
| Sustained VT | 2 (3.7) | 3 (5.9) | 1 (1.9) | 0 (0.0) | 6 (2.8) | 0.144 |
| Stroke | 7 (13.0) | 1 (2.0) | 2 (3.7) | 1 (1.9) | 11 (5.3) | 0.020 |
| Transplant | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 1 (0.5) | 0.178 |
| SD | 2 (3.7) | 3 (5.9) | 1 (1.9) | 2 (3.8) | 8 (3.8) | 0.762 |
| HF Death | 1 (1.9) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 2 (0.9) | 0.993 |
| CV death | 3 (5.6) | 3 (5.9) | 2 (3.7) | 4 (7.7) | 12 (5.7) | 0.774 |
| Beta | 95% CI | Sig. (p) | |
|---|---|---|---|
| Female | 4.294 | (2.466, 6.122) | <0.0001 |
| LVEF (%) | −0.124 | (−0.195, −0.053) | 0.0007 |
| Obstruction (absence) | 1.819 | (0.043, 3.595) | 0.0448 |
| MWT (mm) | −0.423 | (−0.605, −0.242) | <0.0001 |
| Pattern (neutre) | 1.725 | (0.021, 3.429) | 0.0473 |
| Exp (B) | 95% CI | Sig. (p) | |
|---|---|---|---|
| Compacted myocardium (g) | 1.011 | (1.002, 1.021) | 0.0197 |
| Atrial Fibrillation | 8.914 | (2.267, 35.050) | 0.0017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, J.D.; Carrillo, J.G.; Jiménez, J.M.; Muñoz, J.C.; Esparza, C.M.; Alvárez, M.S.; Escribá, R.; Milla, E.B.; de la Pompa, J.L.; Raya, Á.; et al. Trabeculated Myocardium in Hypertrophic Cardiomyopathy: Clinical Consequences. J. Clin. Med. 2020, 9, 3171. https://doi.org/10.3390/jcm9103171
Casanova JD, Carrillo JG, Jiménez JM, Muñoz JC, Esparza CM, Alvárez MS, Escribá R, Milla EB, de la Pompa JL, Raya Á, et al. Trabeculated Myocardium in Hypertrophic Cardiomyopathy: Clinical Consequences. Journal of Clinical Medicine. 2020; 9(10):3171. https://doi.org/10.3390/jcm9103171
Chicago/Turabian StyleCasanova, José David, Josefa González Carrillo, Jesús Martín Jiménez, Javier Cuenca Muñoz, Carmen Muñoz Esparza, Marcos Siguero Alvárez, Rubén Escribá, Esther Burillo Milla, José Luis de la Pompa, Ángel Raya, and et al. 2020. "Trabeculated Myocardium in Hypertrophic Cardiomyopathy: Clinical Consequences" Journal of Clinical Medicine 9, no. 10: 3171. https://doi.org/10.3390/jcm9103171
APA StyleCasanova, J. D., Carrillo, J. G., Jiménez, J. M., Muñoz, J. C., Esparza, C. M., Alvárez, M. S., Escribá, R., Milla, E. B., de la Pompa, J. L., Raya, Á., Gimeno, J. R., Molina, M. S., & García, G. B. (2020). Trabeculated Myocardium in Hypertrophic Cardiomyopathy: Clinical Consequences. Journal of Clinical Medicine, 9(10), 3171. https://doi.org/10.3390/jcm9103171








