Consideration of Migraines Among Risk Factors for Postoperative Nausea and Vomiting
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Migraine, PONV, and Covariates
2.3. Statistical Analysis
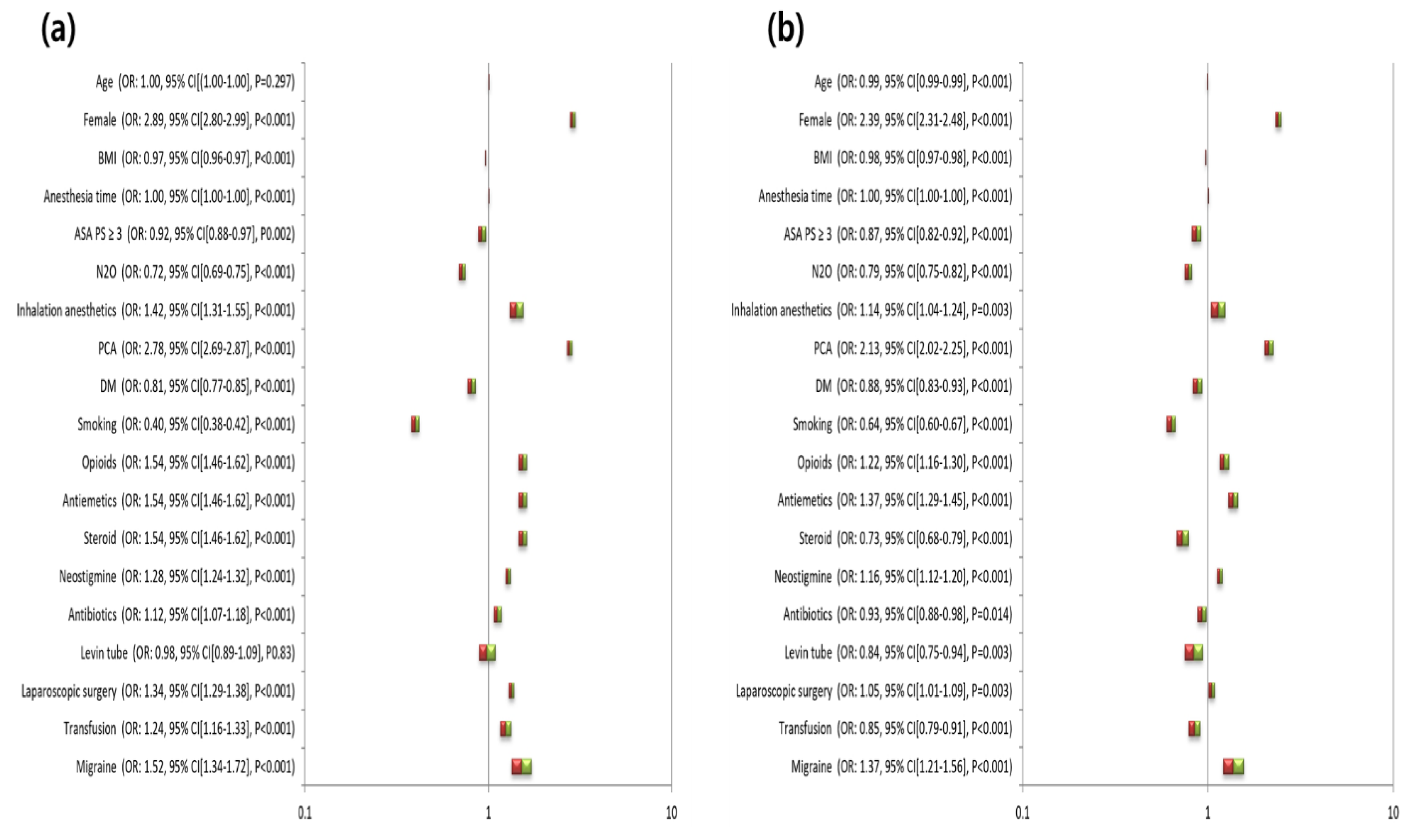
3. Results
3.1. Subject Characteristics
3.2. Odds Ratio for PONV in Migraineurs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eberhart, L.H.; Högel, J.; Seeling, W.; Staack, A.M.; Geldner, G.; Georgieff, M. Evaluation of three risk scores to predict postoperative nausea and vomiting. Acta Anaesthesiol. Scand. 2000, 44, 480–488. [Google Scholar] [CrossRef]
- Apfel, C.C.; Läärä, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef]
- Apfel, C.C.; Kranke, P.; Katz, M.H.; Goepfert, C.; Papenfuss, T.; Rauch, S.; Heineck, R.; Greim, C.A.; Roewer, N. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: A randomized controlled trial of factorial design. Br. J. Anaesth. 2002, 88, 659–668. [Google Scholar] [CrossRef]
- Tramèr, M.R. Treatment of postoperative nausea and vomiting. Br. Med. J. (Clin. Res. Ed.) 2003, 327, 762–763. [Google Scholar] [CrossRef]
- Gan, T.J. Risk factors for postoperative nausea and vomiting. Anesthsia Analg. 2006, 102, 1884–1898. [Google Scholar] [CrossRef]
- Apfel, C.C.; Heidrich, F.M.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.P.; Zhang, K.; Cakmakkaya, O.S. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef]
- Murphy, M.J.; Hooper, V.D.; Sullivan, E.; Clifford, T.; Apfel, C.C. Identification of risk factors for postoperative nausea and vomiting in the perianesthesia adult patient. J. Perianesthesia. Nurs. 2006, 21, 377–384. [Google Scholar] [CrossRef]
- Mythen, M.G. Postoperative gastrointestinal tract dysfunction. Anesthsia Analg. 2005, 100, 196–204. [Google Scholar] [CrossRef]
- Foldes, F.F.; Kepes, E.R.; Ship, A.G. Severe gastrointestinal distension during nitrous oxide and oxygen anesthesia. JAMA 1965, 194, 1146–1148. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Choi, J.W.; Lee, H.S.; Kim, J.H.; Kim, Y.; Lee, J.J. Effect of a preoperative proton pump inhibitor and gastroesophageal reflux disease on postoperative nausea and vomiting. J. Clin. Med. 2020, 9, 825. [Google Scholar] [CrossRef]
- Stefanidis, D.; Hope, W.W.; Kohn, G.P.; Reardon, P.R.; Richardson, W.S.; Fanelli, R.D. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg. Endosc. 2010, 24, 2647–2669. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Papapetropoulos, S.; Kori, S.H.; Kedar, A.; Abell, T.L. Gastric stasis in migraineurs: Etiology, characteristics, and clinical and therapeutic implications. Cephalalgia 2013, 33, 408–415. [Google Scholar] [CrossRef]
- Cady, R.K.; Farmer, K.; Dexter, J.K.; Hall, J. The bowel and migraine: Update on celiac disease and irritable bowel syndrome. Curr. Pain Headache Rep. 2012, 16, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Katic, B.J.; Golden, W.; Cady, R.K.; Hu, X.H. GERD prevalence in migraine patients and the implication for acute migraine treatment. J. Headache Pain 2009, 10, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kurth, T.; Holtmann, G.; Neufang-Huber, J.; Gerken, G.; Diener, H.C. Prevalence of unexplained upper abdominal symptoms in patients with migraine. Cephalalgia 2006, 26, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, A.H.; Stovner, L.J.; Hagen, K.; Zwart, J.A. Comorbidity of headache and gastrointestinal complaints. The head-hunt study. Cephalalgia 2008, 28, 144–151. [Google Scholar] [CrossRef]
- Koivuranta, M.; Läärä, E.; Snåre, L.; Alahuhta, S. A survey of postoperative nausea and vomiting. Anaesthesia 1997, 52, 443–449. [Google Scholar] [CrossRef]
- Stadler, M.; Bardiau, F.; Seidel, L.; Albert, A.; Boogaerts, J.G. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology 2003, 98, 46–52. [Google Scholar] [CrossRef]
- Grabowska-Gaweł, A.; Porzych, K.; Piskunowicz, G. Risk factors and frequency of postoperative nausea and vomiting in patients operated under general anesthesia. Prz. Lek. 2006, 63, 72–76. [Google Scholar]
- Khoronenko, V.E.; Baskakov, D.S. Role of migraine history in the development of postoperative nausea and vomiting in patients undergoing general and combined general-epidural anesthesia. Anesteziol. Reanimatol. 2014, 2, 41–44. [Google Scholar]
- Brookhart, M.A.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Joffe, M.M.; Rosenbaum, P.R. Invited commentary: Propensity scores. Am. J. Epidemiol. 1999, 150, 327–333. [Google Scholar] [CrossRef]
- Tateosian, V.S.; Champagne, K.; Gan, T.J. What is new in the battle against postoperative nausea and vomiting? Best Pract. Res. Clin. Anaesthesiol. 2018, 32, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Camu, F.; Lauwers, M.H.; Verbessem, D. Incidence and aetiology of postoperative nausea and vomiting. Eur. J. Anaesthesiol. Suppl. 1992, 6, 25–31. [Google Scholar] [PubMed]
- Andrews, P.L. Physiology of nausea and vomiting. Br. J. Anaesth. 1992, 69, 2s–19s. [Google Scholar] [CrossRef]
- Tramèr, M.R. A rational approach to the control of postoperative nausea and vomiting: Evidence from systematic reviews. Part II. Recommendations for prevention and treatment, and research agenda. Acta Anaesthesiol. Scand. 2001, 45, 14–19. [Google Scholar] [CrossRef]
- Palazzo, M.; Evans, R. Logistic regression analysis of fixed patient factors for postoperative sickness: A model for risk assessment. Br. J. Anaesth. 1993, 70, 135–140. [Google Scholar] [CrossRef]
- Apfel, C.C.; Greim, C.A.; Haubitz, I.; Goepfert, C.; Usadel, J.; Sefrin, P.; Roewer, N. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiol. Scand. 1998, 42, 495–501. [Google Scholar] [CrossRef]
- Sinclair, D.R.; Chung, F.; Mezei, G. Can postoperative nausea and vomiting be predicted? Anesthesiology 1999, 91, 109–118. [Google Scholar] [CrossRef]
- Eberhart, L.H.; Geldner, G.; Kranke, P.; Morin, A.M.; Schäuffelen, A.; Treiber, H.; Wulf, H. The development and validation of a risk score to predict the probability of postoperative vomiting in pediatric patients. Anesth. Analg. 2004, 99, 1630–1637. [Google Scholar] [CrossRef]
- Van den Bosch, J.E.; Moons, K.G.; Bonsel, G.J.; Kalkman, C.J. Does measurement of preoperative anxiety have added value for predicting postoperative nausea and vomiting? Anesth. Analg. 2005, 100, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Rudra, A.; Sengupta, S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol. Res. Pract. 2011, 748031. [Google Scholar] [CrossRef]
- Shaikh, S.I.; Nagarekha, D.; Hegade, G.; Marutheesh, M. Postoperative nausea and vomiting: A simple yet complex problem. Anesth. Essays Res. 2016, 10, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Kreis, M.E. Postoperative nausea and vomiting. Auton. Neurosci. 2006, 129, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Holte, K.; Kehlet, H. Postoperative ileus: Progress towards effective management. Drugs 2002, 62, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Sehhati, G.; Frey, R.; Star, E.G. The action of inhalation anesthetics upon the lower oesophageal sphincter. Acta Anaesthesiol. Belg. 1980, 31, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Cotton, B.R.; Smith, G. The lower oesophageal sphincter and anaesthesia. Br. J. Anaesth. 1984, 56, 37–46. [Google Scholar] [CrossRef]
- Lind, J.F.; Crispin, J.S.; McIver, D.K. The effect of atropine on the gastroesophageal sphincter. Can. J. Physiol. Pharmacol. 1968, 46, 233–238. [Google Scholar] [CrossRef]
- Powell, P.W.W.; Vasudevan, M.; Rall, M.; Carrithers, J.A. Gastroesophageal reflux disease as a predictor for post operative nausea and vomiting. In Proceedings of the 2010 Annual Meeting of the American Society Anesthesiologists, San Diego, CA, USA, 16 October 2010; p. A213. [Google Scholar]
- Lee, S.H.; Lee, J.J.; Kwon, Y.; Kim, J.H.; Sohn, J.H. Clinical implications of associations between headache and gastrointestinal disorders: A study using the hallym smart clinical data warehouse. Front. Neurol. 2017, 8, 526. [Google Scholar] [CrossRef]
- Peskersoy, C.; Peker, S.; Kaya, A.; Unalp, A.; Gokay, N. Evaluation of the relationship between migraine disorder andoral comorbidities: Multicenter randomized clinical trial. Turk. Med. Sci. 2016, 46, 712–718. [Google Scholar] [CrossRef]
- Masaoka, T.; Tack, J. Gastroparesis: Current concepts and management. Gut Liver 2009, 3, 166–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parkman, H.P. Migraine and gastroparesis from a gastroenterologist’s perspective. Headache 2013, 53, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Kori, S.H.; Barrodale, P.; McDonald, S.A.; Haseley, D. Gastric stasis in migraine: More than just a paroxysmal abnormality during a migraine attack. Headache 2006, 46, 57–63. [Google Scholar] [CrossRef]
- Aurora, S.; Kori, S.; Barrodale, P.; Nelsen, A.; McDonald, S. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache 2007, 47, 1443–1446. [Google Scholar] [CrossRef]
- Deen, M.; Correnti, E.; Kamin, K.; Keiderman, T.; Papeti, L.; Rubio Beltran, E.; Vigneri, S.; Edvisson, L.; Van Den Brink, A.M. On the behalf of the European headache federation school of advanced studies (EHF-SAS) blocking CGRP in migraine patients-a review of pros and cons. J. Headache Pain 2017, 18, 96. [Google Scholar] [CrossRef]
- Tiseo, C.; Ornello, R.; Pistoia, F.; Sacco, S. How to integrate monoclonal antibodies targeting the calcitonin gene-related peptide or its receptor in daily clinical practice. J. Headache Pain 2019, 20, 49. [Google Scholar] [CrossRef]
- Jurgen, L.H. Calcitonin and CGRP inhibit gastrointestinal transit via distinct neuronal pathways. Am. J. Phys. 1988, 254, G920–G924. [Google Scholar] [CrossRef]
- L’Heureux, M.C.; St-Pierre, S.; Trudel, L.; Plourde, V.; Lepage, R.; Poitraus, P. Digestive motor effects and vascular actions of CGRP in dog are expressed by different receptor subtypes. Peptides 2000, 21, 425–430. [Google Scholar] [CrossRef]
- Frattale, L.; Ornello, R.; Pistoia, F.; Caponnetto, V.; Colangeli, E.; Sacco, S. Paralytic ileus after planned abdominal surgery in a patient on treatment with erenmab. Intern. Emerg. Med. 2020, 17. [Google Scholar] [CrossRef]
- Haanes, K.A.; Edvinsson, L.; Sams, A. Understanding side-effects of anti-CGRP and anti-CGRP receptor antibodies. J. Headache Pain 2020, 21, 26. [Google Scholar] [CrossRef]
- Haug, T.T.; Svebak, S.; Hausken, T.; Wilhelmsen, I.; Berstad, A.; Ursin, H. Low vagal activity as mediating mechanism for the relationship between personality factors and gastric symptoms in functional dyspepsia. Psychosom. Med. 1994, 56, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hausken, T.; Svebak, S.; Wilhelmsen, I.; Haug, T.T.; Olafsen, K.; Pettersson, E.; Hveem, K.; Berstad, A. Low vagal tone and antral dysmotility in patients with functional dyspepsia. Psychosom. Med. 1993, 55, 12–22. [Google Scholar] [CrossRef]
- Shechter, A.; Stewart, W.F.; Silberstein, S.D.; Lipton, R.B. Migraine and autonomic nervous system function: A population-based, case-control study. Neurology 2002, 58, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lorena, S.L.; Figueiredo, M.J.; Almeida, J.R.; Mesquita, M.A. Autonomic function in patients with functional dyspepsia assessed by 24-hour heart rate variability. Dig. Dis. Sci. 2002, 47, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Heitkemper, M.; Burr, R.L.; Jarrett, M.; Hertig, V.; Lustyk, M.K.; Bond, E.F. Evidence for autonomic nervous system imbalance in women with irritable bowel syndrome. Dig. Dis. Sci. 1998, 43, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Peroutka, S.J. Migraine: A chronic sympathetic nervous system disorder. Headache 2004, 44, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.T.; De Dhaem, O.B.; Van Diest, A.K.; Powers, S.; Schwedt, T.J.; Lipton, R.; Silbersweig, D. Migraine and its psychiatric comorbidities. J. Neurol. Neurosurg. Psychiatry 2016, 87, 741–749. [Google Scholar] [CrossRef]
- Lampl, C.; Rapoport, A.; Levin, M.; Bräutigam, E. Migraine and episodic Vertigo: A cohort survey study of their relationship. J. Headache Pain 2019, 20, 33. [Google Scholar] [CrossRef]
- Neuhauser, H.K.; Radtke, A.; von Brevern, M.; Feldmann, M.; Lezius, F.; Ziese, T.; Lempert, T. Migrainous vertigo: Prevalence and impact on quality of life. Neurology 2006, 67, 1028–1033. [Google Scholar] [CrossRef]
- Bisdorff, A.; Andree, C.; Vaillant, M.; Sandor, P.S. Headache-associated dizziness in a headache population: Prevalence and impact. Cephalalgia 2010, 30, 815–820. [Google Scholar] [CrossRef]
| Non-PONV n = 167,920 | PONV n = 19,786 | p-Value | |
|---|---|---|---|
| Age (Years, mean ± SD) | 49.3 ± 16.9 | 49.5 ± 16.2 | 0.282 |
| Female (n (%)) | 83,803(49.9) | 14,696 (74.3) | <0.0001 |
| BMI (Mean ± SD) | 24.2 ± 3.8 | 23.8 ± 3.7 | <0.0001 |
| Anesthesia Time (Minutes, Mean ± SD) | 135.7 ± 91.2 | 153.4 ± 89.8 | <0.0001 |
| ASA PS ≥ 3 (n, (%)) | 20,199 (12.0) | 2227 (11.3) | 0.002 |
| N2O (n (%)) | 38,080 (22.7) | 3473 (17.6) | <0.0001 |
| Inhalation Anesthetics (n (%)) | 160,757 (95.7) | 19,187 (97.0) | <0.0001 |
| PCA (n (%)) | 73,583 (43.8) | 13,548 (68.5) | <0.0001 |
| DM (n (%)) | 19,589 (11.7) | 1920 (9.7) | <0.0001 |
| Smoking (n (%)) | 32,408 (19.3) | 1731 (8.7) | <0.0001 |
| Opioids (n (%)) | 146,743 (87.4) | 18,091 (91.4) | <0.0001 |
| Antiemetics (n (%)) | 90,457 (53.9) | 14,573 (73.7) | <0.0001 |
| Steroid (n (%)) | 10,828 (6.4) | 796 (4.0) | <0.0001 |
| Neostigmine (n (%)) | 47,437 (28.2) | 6551 (33.6) | <0.0001 |
| Antibiotics (n (%)) | 151,231 (90.1) | 18,023 (91.1) | <0.0001 |
| Levin tube (n (%)) | 3416 (2.0) | 398 (2.0) | 0.028 |
| Laparoscopic surgery (n (%)) | 36,211 (21.6) | 5333 (27.0) | <0.0001 |
| Transfusion (n, (%)) | 6914 (4.1) | 1005 (5.1) | <0.0001 |
| Migraine (n (%)) | 1682 (1.0) | 300 (1.5) | <0.0001 |
| Migraine n = 1982 | Normal n = 1982 | D | p-Value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 47.82 ± 15.21 | 47.37 ± 16.80 | 0.03 | 0.373 |
| Female (n (%)) | 1459 (73.6%) | 1454 (73.4%) | 0.01 | 0.857 |
| BMI (mean ± SD) | 24.57±4.39 | 24.48±4.11 | 0.01 | 0.510 |
| Anesthesia time (Minutes, Mean ± SD) | 121.30 ± 78.60 | 119.12 ± 73.95 | 0.03 | 0.369 |
| ASA PS ≥ 3 (n (%)) | 309 (15.6%) | 299 (15.1%) | 0.02 | 0.659 |
| N2O (n, (%)) | 321 (16.2%) | 333 (16.8%) | 0.02 | 0.608 |
| Inhalation Anesthetics (n (%)) | 1892 (95.5%) | 1901 (95.9%) | 0.06 | 0.482 |
| PCA (n (%)) | 778 (39.3%) | 775 (39.1%) | <0.01 | 0.922 |
| DM (n (%)) | 170 (8.6%) | 159 (8.0%) | 0.04 | 0.527 |
| Smoking (n (%)) | 258 (13.0%) | 292 (14.7%) | 0.08 | 0.118 |
| Opioids (n (%)) | 1787 (90.2%) | 1795 (1795%) | 0.03 | 0.667 |
| Antiemetics (n (%)) | 1000 (50.5%) | 1007 (50.8%) | 0.01 | 0.824 |
| Steroid (n (%)) | 134 (6.8%) | 152 (7.7) | 0.07 | 0.269 |
| Neostigmine (n (%)) | 691 (34.9%) | 663 (33.5%) | 0.03 | 0.348 |
| Antibiotics (n (%)) | 1776 (89.6%) | 1778 (89.7%) | 0.01 | 0.917 |
| Levin tube (n (%)) | 16 (0.8%) | 13 (0.7%) | 0.10 | 0.576 |
| Laparoscopic surgery (n (%)) | 405 (20.4%) | 385 (19.4%) | 0.03 | 0.426 |
| Transfusion (n (%)) | 50 (2.5%) | 57 (2.9%) | 0.07 | 0.493 |
| Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| Unadjusted | 1.36 (1.13–1.63) | 0.001 |
| Fully Adjusted | 1.37 (1.13–1.66) | 0.001 |
| All Covariates + Propensity Score-Adjusted | 1.37 (1.13–1.66) | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lim, M.-s.; Lee, S.-H.; Kwon, Y.-S.; Lee, J.J.; Sohn, J.-H. Consideration of Migraines Among Risk Factors for Postoperative Nausea and Vomiting. J. Clin. Med. 2020, 9, 3154. https://doi.org/10.3390/jcm9103154
Kim J-H, Lim M-s, Lee S-H, Kwon Y-S, Lee JJ, Sohn J-H. Consideration of Migraines Among Risk Factors for Postoperative Nausea and Vomiting. Journal of Clinical Medicine. 2020; 9(10):3154. https://doi.org/10.3390/jcm9103154
Chicago/Turabian StyleKim, Jong-Ho, Man-sup Lim, Sang-Hwa Lee, Young-Suk Kwon, Jae Jun Lee, and Jong-Hee Sohn. 2020. "Consideration of Migraines Among Risk Factors for Postoperative Nausea and Vomiting" Journal of Clinical Medicine 9, no. 10: 3154. https://doi.org/10.3390/jcm9103154
APA StyleKim, J.-H., Lim, M.-s., Lee, S.-H., Kwon, Y.-S., Lee, J. J., & Sohn, J.-H. (2020). Consideration of Migraines Among Risk Factors for Postoperative Nausea and Vomiting. Journal of Clinical Medicine, 9(10), 3154. https://doi.org/10.3390/jcm9103154






