Confocal Laser Endomicroscopy Assessment of Pituitary Tumor Microstructure: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Feasibility of CLE Positioning for Endonasal Procedures
2.2. Biopsy Specimens
2.3. Imaging Protocol
2.4. Tissue Sampling, Histology, and Processing
3. Results
3.1. Feasibility of CLE Positioning for Transsphenoidal Surgery
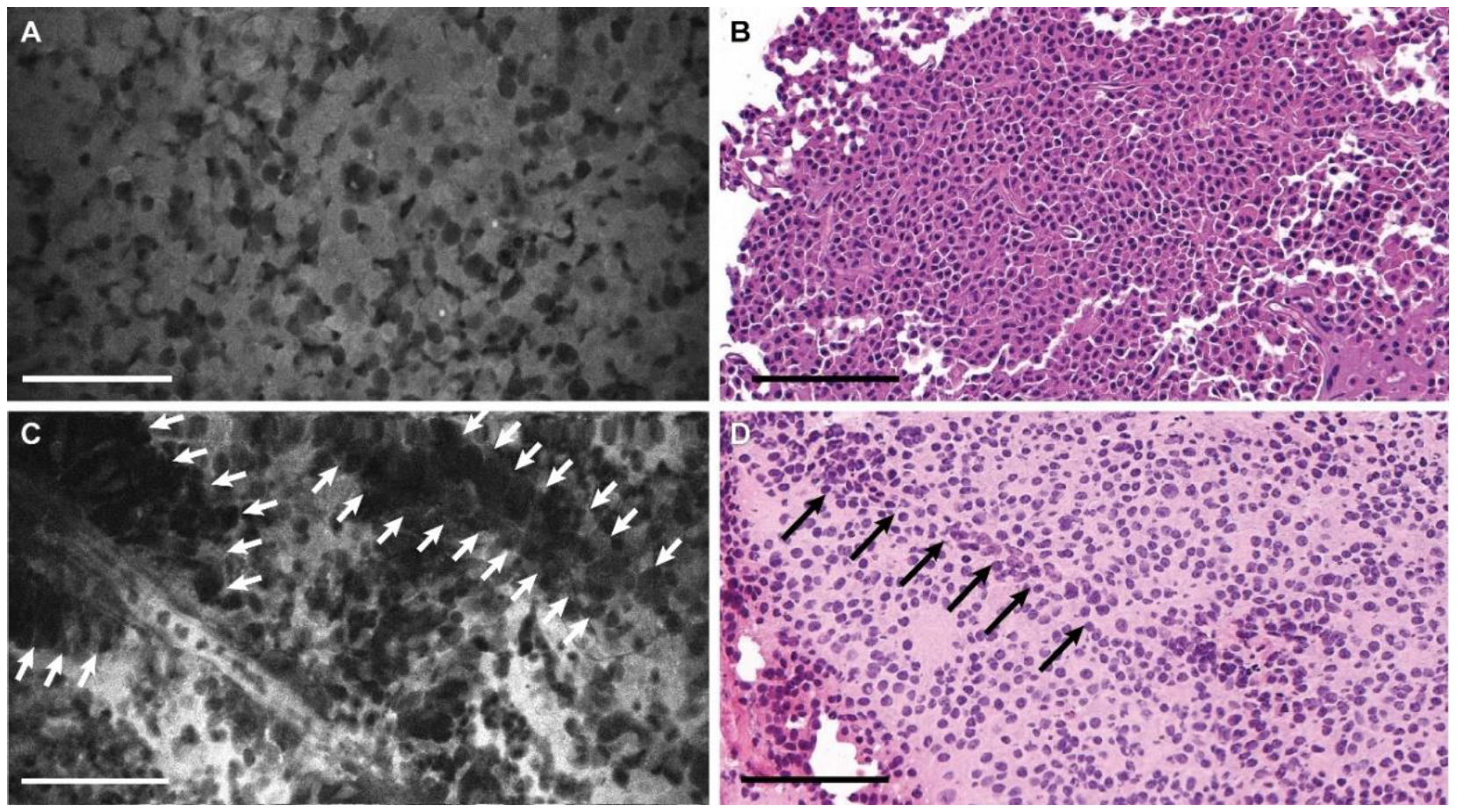
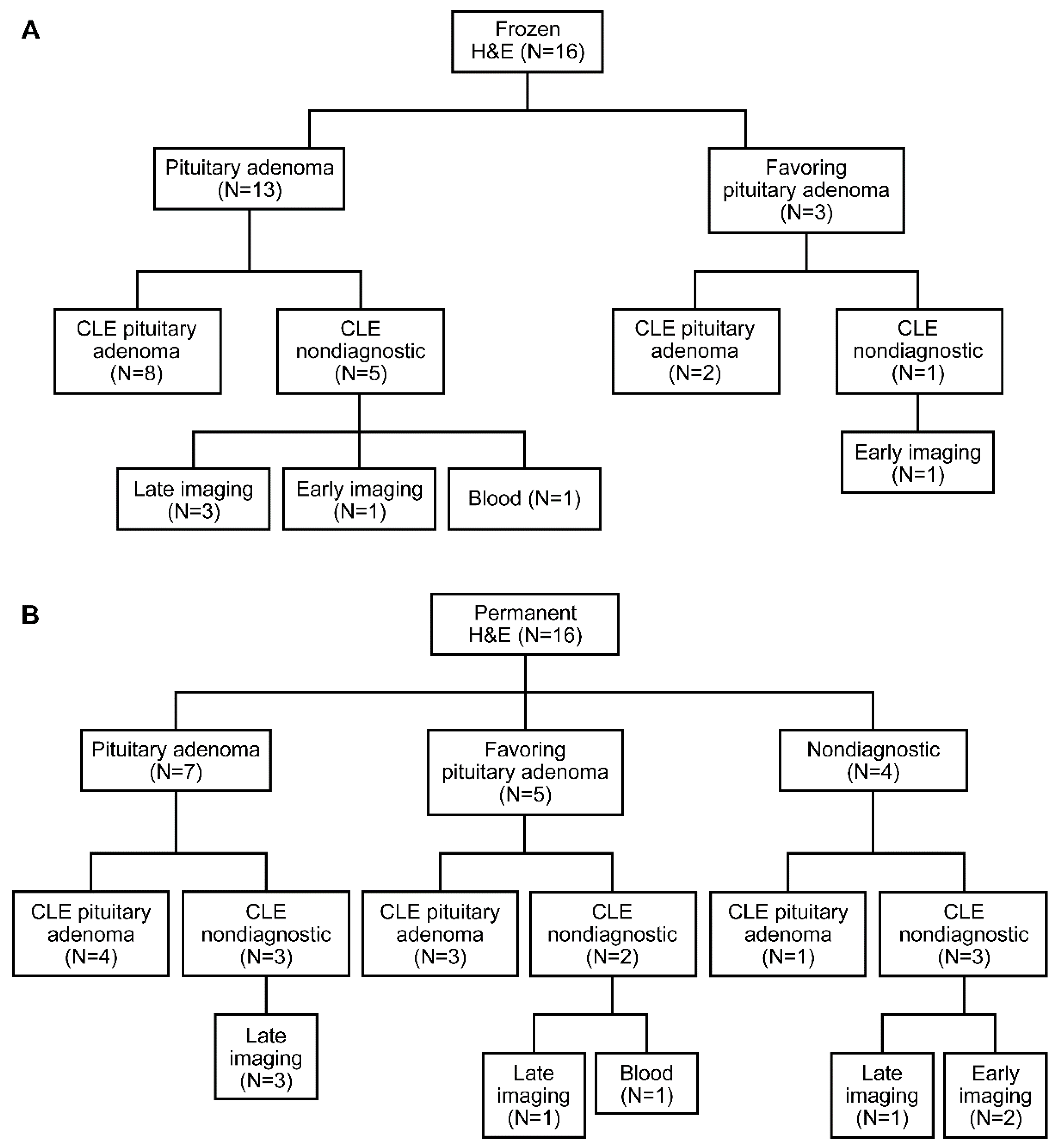
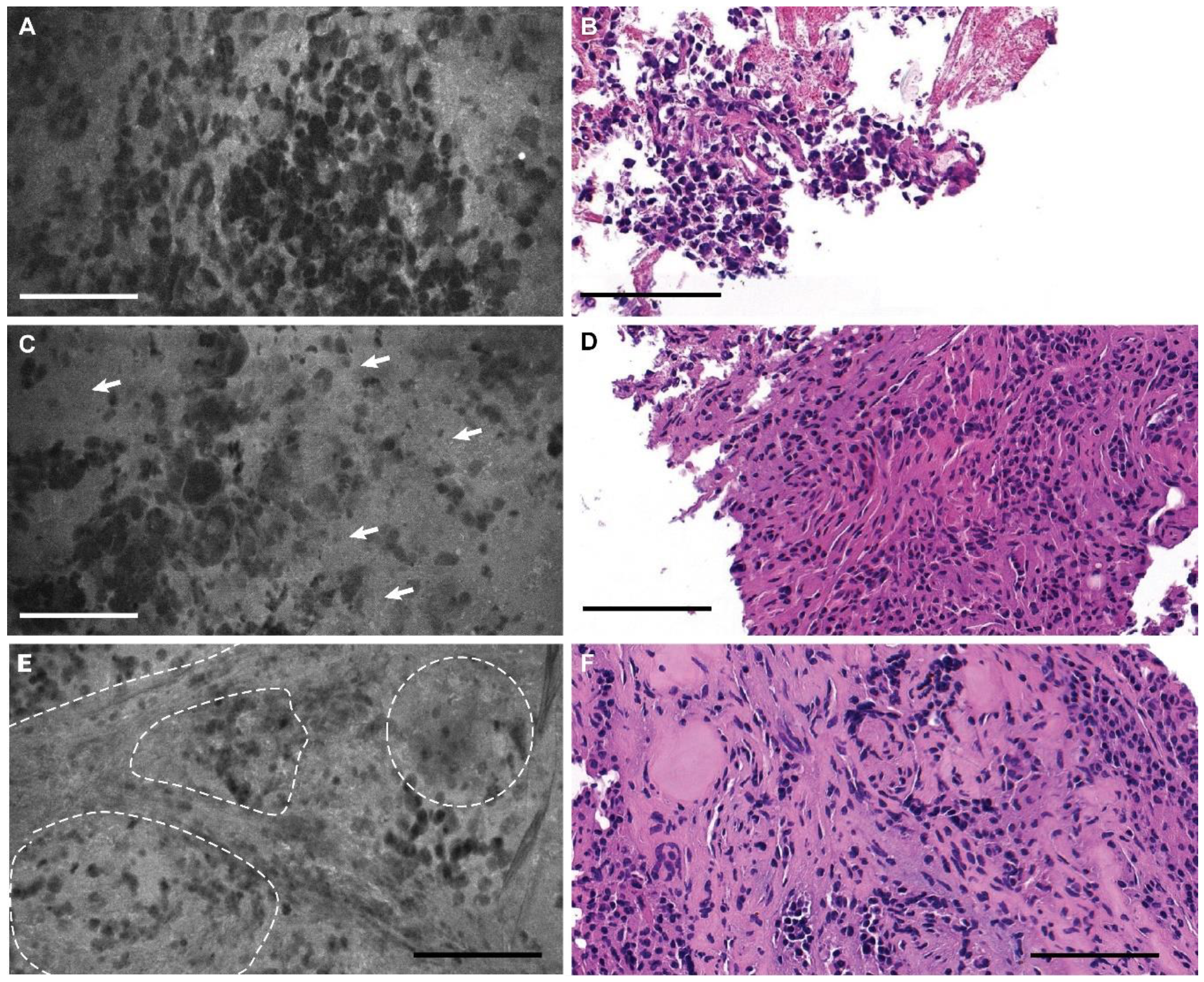
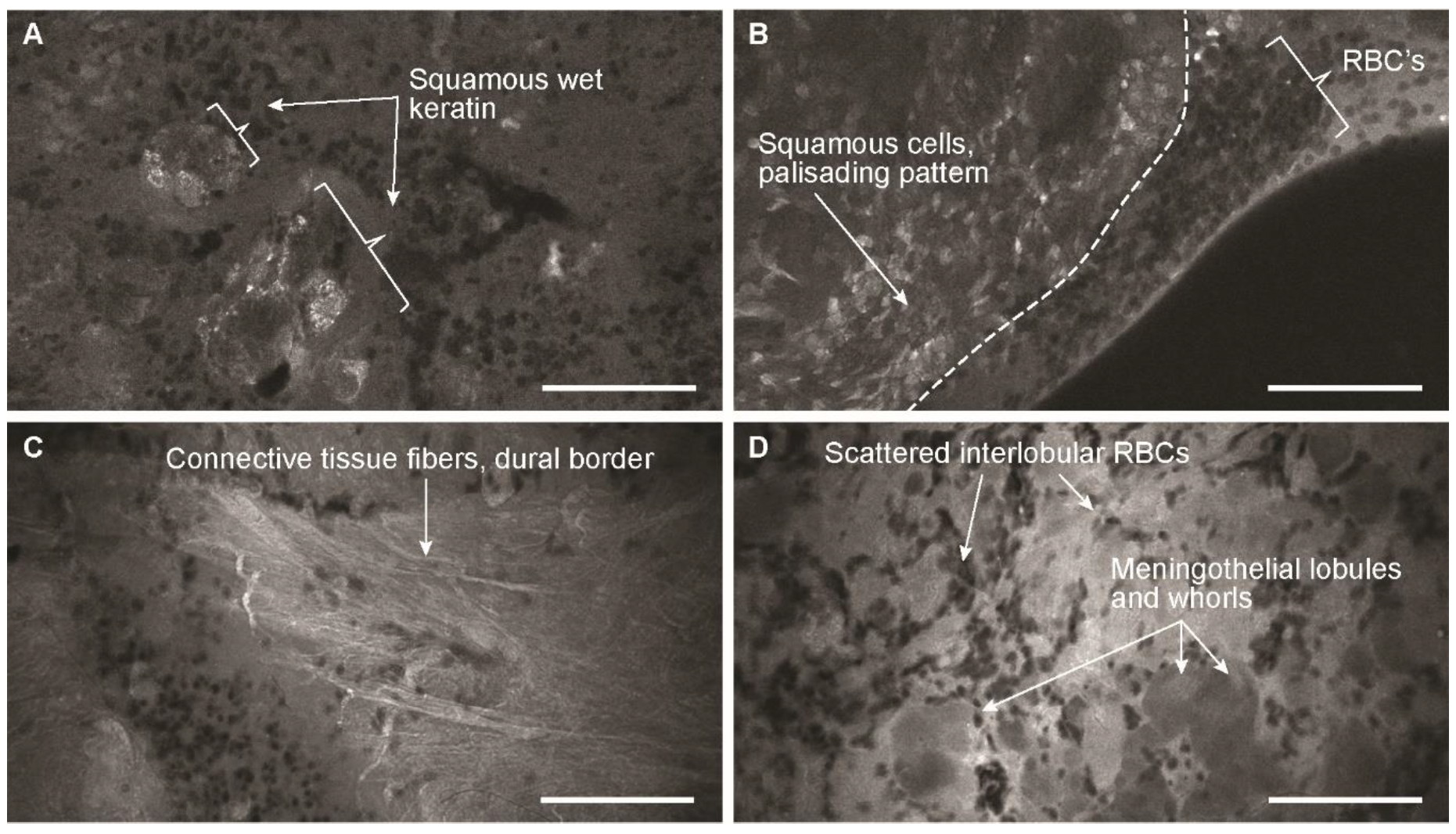
3.2. Descriptive Analysis
3.3. Blinded Review
4. Discussion
4.1. Confocal Endomicroscopy Technology
4.2. Biopsy Acquisition
4.3. Optimal Dosing and Timing of Fluorescein Injection
4.4. Feasibility for Endonasal Procedures
4.5. Other Fluorophores
4.6. Other Imaging Technologies
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tilgner, J.; Herr, M.; Ostertag, C.; Volk, B. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: Intraoperative versus final diagnosis—influence of clinical factors. Neurosurgery 2005, 56, 257–265, discussion 257–265. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, Y.; Owai, Y.; Okita, R.; Tanaka, Y.; Itakura, T. The usefulness and problem of intraoperative rapid diagnosis in surgical neuropathology. Brain Tumor Pathol. 2007, 24, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Plesec, T.P.; Prayson, R.A. Frozen section discrepancy in the evaluation of central nervous system tumors. Arch. Pathol. Lab. Med. 2007, 131, 1532–1540. [Google Scholar] [PubMed]
- Behbahaninia, M.; Martirosyan, N.L.; Georges, J.; Udovich, J.A.; Kalani, M.Y.S.; Feuerstein, B.G.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Intraoperative fluorescent imaging of intracranial tumors: A review. Clin. Neurol. Neurosurg. 2013, 115, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, P.; Zehri, A.H.; Ramey, W.; Georges, J.F.; Mooney, M.A.; Martirosyan, N.L.; Preul, M.C. Neurosurgical confocal endomicroscopy: A review of contrast agents, confocal systems, and future imaging modalities. Surg. Neurol. Int. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Quinn, M.; Pyman, J.; Delaney, P.; McLaren, W.; Quinn, M.A. Detection of cervical intraepithelial neoplasia in vivo using confocal endomicroscopy. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 1663–1670. [Google Scholar] [CrossRef]
- Broek, F.V.D.; Van Es, J.; Van Eeden, S.; Stokkers, P.; Ponsioen, C.; Reitsma, J.; Fockens, P.; Dekker, E. Pilot study of probe-based confocal laser endomicroscopy during colonoscopic surveillance of patients with longstanding ulcerative colitis. Endoscopy 2010, 43, 116–122. [Google Scholar] [CrossRef]
- Eschbacher, J.; Martirosyan, N.L.; Nakaji, P.; Sanai, N.; Preul, M.C.; Smith, K.A.; Coons, S.W.; Spetzler, R.F. In vivo intraoperative confocal microscopy for real-time histopathological imaging of brain tumors. J. Neurosurg. 2012, 116, 854–860. [Google Scholar] [CrossRef]
- Fenton, K.E.; Martirosyan, N.L.; Abdelwahab, M.G.; Coons, S.W.; Zabramski, J.M.; Scheck, A.C. In vivo visualization of GL261-luc2 mouse glioma cells by use of Alexa Fluor–labeled TRP-2 antibodies. Neurosurg. Focus 2014, 36, E12. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Cavalcanti, D.; Eschbacher, J.M.; Delaney, P.M.; Scheck, A.C.; Abdelwahab, M.G.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Use of in vivo near-infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumor. J. Neurosurg. 2011, 115, 1131–1138. [Google Scholar] [CrossRef]
- Sankar, T.; Delaney, P.M.; Ryan, R.W.; Eschbacher, J.; Abdelwahab, M.; Nakaji, P.; Coons, S.W.; Scheck, A.C.; Smith, K.A.; Spetzler, R.F.; et al. Miniaturized handheld confocal microscopy for neurosurgery. Neurosurgery 2010, 66, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.S.; Turner, J.D.; Belykh, E.G.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef] [PubMed]
- Fugazza, A.; Gaiani, F.; Carra, M.C.; Brunetti, F.; Lévy, M.; Sobhani, I.; Azoulay, D.; Catena, F.; De’Angelis, G.L.; De’Angelis, N. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases: A systematic review and meta-analysis. BioMed Res. Int. 2016, 2016, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Goorsenberg, A.; Kalverda, K.A.; Annema, J.; Bonta, P.I. Advances in optical coherence tomography and confocal laser endomicroscopy in pulmonary diseases. Respiration 2019, 99, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Georges, J.; Eschbacher, J.M.; Cavalcanti, D.; Elhadi, A.M.; Abdelwahab, M.G.; Scheck, A.C.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg. Focus 2014, 36, E16. [Google Scholar] [CrossRef] [PubMed]
- Brommeland, T.; Lindal, S.; Straume, B.; Dahl, I.L.; Hennig, R. Does imprint cytology of brain tumours improve intraoperative diagnoses? Acta Neurol. Scand. 2003, 108, 153–156. [Google Scholar] [CrossRef]
- 17Mooney, M.A.; Georges, J.; Yazdanabadi, M.I.; Goehring, K.Y.; White, W.L.; Little, A.S.; Zabramski, J.M.; Coons, S.W.; Nakaji, P.; Eschbacher, J.M. Immediate ex-vivo diagnosis of pituitary adenomas using confocal reflectance microscopy: A proof-of-principle study. J. Neurosurg. 2018, 128, 1072–1075. [Google Scholar]
- Eschbacher, J.M.; Georges, J.F.; Belykh, E.; Yazdanabadi, M.I.; Martirosyan, N.L.; Szeto, E.; Seiler, C.; Mooney, M.A.; Daniels, J.K.; Goehring, K.Y.; et al. Immediate label-free ex vivo evaluation of human brain tumor biopsies with confocal reflectance microscopy. J. Neuropathol. Exp. Neurol. 2017, 76, 1008–1022. [Google Scholar] [CrossRef]
- Georges, J.; Zehri, A.; Carlson, E.; Nichols, J.; Mooney, M.A.; Martirosyan, N.L.; Ghaffari, L.; Kalani, M.Y.S.; Eschbacher, J.; Feuerstein, B.; et al. Label-free microscopic assessment of glioblastoma biopsy specimens prior to biobanking [corrected]. Neurosurg. Focus 2014, 36, E8. [Google Scholar] [CrossRef]
- Forest, F.; Cinotti, E.; Yvorel, V.; Habougit, C.; Vassal, F.; Nuti, C.; Perrot, J.-L.; Labeille, B.; Péoc’H, M. Ex vivo confocal microscopy imaging to identify tumor tissue on freshly removed brain sample. J. Neuro-Oncol. 2015, 124, 157–164. [Google Scholar] [CrossRef]
- Orringer, D.A.; Pandian, B.; Niknafs, Y.S.; Hollon, T.C.; Boyle, J.; Lewis, S.; Garrard, M.; Hervey-Jumper, S.L.; Garton, H.J.L.; Maher, C.O.; et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 2017, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Charalampaki, P.; Nakamura, M.; Athanasopoulos, D.; Heimann, A. Confocal-assisted multispectral fluorescent microscopy for brain tumor surgery. Front. Oncol. 2019, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Charalampaki, P.; Javed, M.; Daali, S.; Heiroth, H.-J.; Igressa, A.; Weber, F. Confocal laser endomicroscopy for real-time histomorphological diagnosis. Neurosurgery 2015, 62, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Patel, A.A.; Miller, E.J.; Bozkurt, B.; Yağmurlu, K.; Woolf, E.C.; Scheck, A.C.; Eschbacher, J.M.; Nakaji, P.; Preul, M.C. Probe-based three-dimensional confocal laser endomicroscopy of brain tumors: Technical note. Cancer Manag. Res. 2018, 10, 3109–3123. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Georges, J.; Eschbacher, J.M.; Belykh, E.; Carotenuto, A.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Confocal scanning microscopy provides rapid, detailed intraoperative histological assessment of brain neoplasms: Experience with 106 cases. Clin. Neurol. Neurosurg. 2018, 169, 21–28. [Google Scholar] [CrossRef]
- Cho, S.S.; Jeon, J.; Buch, L.; Nag, S.; Nasrallah, M.; Low, P.S.; Grady, M.S.; Singhal, S.; Lee, J.Y.K. Intraoperative near-infrared imaging with receptor-specific versus passive delivery of fluorescent agents in pituitary adenomas. J. Neurosurg. 2019, 131, 1974–1984. [Google Scholar] [CrossRef]
- Cho, S.S.; Zeh, R.; Pierce, J.T.; Jeon, J.; Nasrallah, M.; Adappa, N.D.; Palmer, J.N.; Newman, J.G.; White, C.; Kharlip, J.; et al. Folate receptor near-infrared optical imaging provides sensitive and specific intraoperative visualization of nonfunctional pituitary adenomas. Oper. Neurosurg. 2018, 16, 59–70. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, S.S.; Zeh, R.; Pierce, J.T.; Martinez-Lage, M.; Adappa, N.D.; Palmer, J.N.; Newman, J.G.; Learned, K.O.; White, C.; et al. Folate receptor overexpression can be visualized in real time during pituitary adenoma endoscopic transsphenoidal surgery with near-infrared imaging. J. Neurosurg. 2018, 129, 390–403. [Google Scholar] [CrossRef]
- Evans, C.-O.; Reddy, P.; Brat, D.J.; O’Neill, E.B.; Craige, B.; Stevens, V.L.; Oyesiku, N.M. Differential expression of folate receptor in pituitary adenomas. Cancer Res. 2003, 63, 4218–4224. [Google Scholar]
- Evans, C.O.; Yao, C.; Laborde, D.; Oyesiku, N.M. Folate receptor expression in pituitary adenomas cellular and molecular analysis. Vitam Horm. 2008, 79, 235–266. [Google Scholar]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Boil. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Padalkar, M.V.; Pleshko, N. Wavelength-dependent penetration depth of near infrared radiation into cartilage. Analyst 2015, 140, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Lee, J.Y. Intraoperative fluorescent visualization of pituitary adenomas. Neurosurg. Clin. N. Am. 2019, 30, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Meza, D.; Wang, D.; Wang, Y.; Ms, S.B.; Sanai, N.; Liu, J.T. Comparing high-resolution microscopy techniques for potential intraoperative use in guiding low-grade glioma resections. Lasers Surg. Med. 2015, 47, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Miller, E.J.; Carotenuto, A.; Patel, A.A.; Cavallo, C.; Martirosyan, N.L.; Healey, D.R.; Byvaltsev, V.A.; Scheck, A.C.; Lawton, M.T.; et al. Progress in confocal laser endomicroscopy for neurosurgery and technical nuances for brain tumor imaging with fluorescein. Front. Oncol. 2019, 9, 554. [Google Scholar] [CrossRef]
- Poulon, F.; Pallud, J.; Varlet, P.; Zanello, M.; Chretien, F.; Dezamis, E.; Abi-Lahoud, G.; Nataf, F.; Turak, B.; Devaux, B.; et al. Real-time brain tumor imaging with endogenous fluorophores: A diagnosis proof-of-concept study on fresh human samples. Sci. Rep. 2018, 8, 14888. [Google Scholar] [CrossRef]
- Uckermann, O.; Galli, R.; Mark, G.; Meinhardt, M.; Koch, E.; Schackert, G.; Steiner, G.; Kirsch, M. Label-free multiphoton imaging allows brain tumor recognition based on texture analysis—A study of 382 tumor patients. Neuro-Oncol. Adv. 2020, 2, vdaa035. [Google Scholar] [CrossRef]
- Hollon, T.; Lewis, S.; Freudiger, C.W.; Xie, X.S.; Orringer, D.A. Improving the accuracy of brain tumor surgery via Raman-based technology. Neurosurg. Focus 2016, 40, E9. [Google Scholar] [CrossRef]
- Belykh, E.; Cavallo, C.; Gandhi, S.; Zhao, X.; Veljanoski, D.; Yazdanabadi, M.I.; Martirosyan, N.L.; Byvaltsev, V.A.; Eschbacher, J.; Preul, M.C.; et al. Utilization of intraoperative confocal laser endomicroscopy in brain tumor surgery. J. Neurosurg. Sci. 2018, 62. [Google Scholar] [CrossRef]
- Meira, J.; Marques, M.L.; Falcão-Reis, F.; Rebelo Gomes, E.; Carneiro, Â. Immediate reactions to fluorescein and indocyanine green in retinal angiography: Review of literature and proposal for patient’s evaluation. Clin. Ophthalmol. 2020, 14, 171–178. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belykh, E.; Ngo, B.; Farhadi, D.S.; Zhao, X.; Mooney, M.A.; White, W.L.; Daniels, J.K.; Little, A.S.; Eschbacher, J.M.; Preul, M.C. Confocal Laser Endomicroscopy Assessment of Pituitary Tumor Microstructure: A Feasibility Study. J. Clin. Med. 2020, 9, 3146. https://doi.org/10.3390/jcm9103146
Belykh E, Ngo B, Farhadi DS, Zhao X, Mooney MA, White WL, Daniels JK, Little AS, Eschbacher JM, Preul MC. Confocal Laser Endomicroscopy Assessment of Pituitary Tumor Microstructure: A Feasibility Study. Journal of Clinical Medicine. 2020; 9(10):3146. https://doi.org/10.3390/jcm9103146
Chicago/Turabian StyleBelykh, Evgenii, Brandon Ngo, Dara S. Farhadi, Xiaochun Zhao, Michael A. Mooney, William L. White, Jessica K. Daniels, Andrew S. Little, Jennifer M. Eschbacher, and Mark C. Preul. 2020. "Confocal Laser Endomicroscopy Assessment of Pituitary Tumor Microstructure: A Feasibility Study" Journal of Clinical Medicine 9, no. 10: 3146. https://doi.org/10.3390/jcm9103146
APA StyleBelykh, E., Ngo, B., Farhadi, D. S., Zhao, X., Mooney, M. A., White, W. L., Daniels, J. K., Little, A. S., Eschbacher, J. M., & Preul, M. C. (2020). Confocal Laser Endomicroscopy Assessment of Pituitary Tumor Microstructure: A Feasibility Study. Journal of Clinical Medicine, 9(10), 3146. https://doi.org/10.3390/jcm9103146



