Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial
Abstract
1. Introduction
2. Experimental Section
Statistical Analysis
3. Results
3.1. Study Participants
3.2. Primary Outcome
3.3. Secondary Outcomes
3.4. Nasal Endoscopy
3.5. Side Effects
3.6. Results of the Open-Label Phase of the Study
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDonald, J.; Bayrak-Toydemir, P.; Pyeritz, R.E. Hereditary hemorrhagic telangiectasia: An overview of diagnosis, management, and pathogenesis. Genet. Med. Off. J. Am. Coll. Med. Genet. 2011, 13, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Merlo, C.A.; Yin, L.X.; Hoag, J.B.; Mitchell, S.E.; Reh, D.D. The effects of epistaxis on health-related quality of life in patients with hereditary hemorrhagic telangiectasia. Int. Forum Allergy Rhinol. 2014, 4, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Ingrand, I.; Ingrand, P.; Gilbert-Dussardier, B.; Defossez, G.; Jouhet, V.; Migeot, V.; Dufour, X.; Klossek, J.M. Altered quality of life in Rendu-Osler-Weber disease related to recurrent epistaxis. Rhinology 2011, 49, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Loaëc, M.; Morinière, S.; Hitier, M.; Ferrant, O.; Plauchu, H.; Babin, E. Psychosocial quality of life in hereditary haemorrhagic telangiectasia patients. Rhinology 2011, 49, 164–167. [Google Scholar] [CrossRef]
- Sautter, N.B.; Smith, T.L. Treatment of Hereditary Hemorrhagic Telangiectasia-Related Epistaxis. Otolaryngol. Clin. N. Am. 2016, 49, 639–654. [Google Scholar] [CrossRef]
- Ardelean, D.S.; Letarte, M. Anti-angiogenic therapeutic strategies in hereditary hemorrhagic telangiectasia. Front. Genet. 2015, 6, 35. [Google Scholar] [CrossRef]
- Stokes, P.; Rimmer, J. Intranasal bevacizumab in the treatment of HHT -related epistaxis: A systematic review. Rhinology 2018, 56, 3–10. [Google Scholar] [CrossRef]
- Halderman, A.A.; Ryan, M.W.; Marple, B.F.; Sindwani, R.; Reh, D.D.; Poetker, D.M. Bevacizumab for Epistaxis in Hereditary Hemorrhagic Telangiectasia: An Evidence-based Review. Am. J. Rhinol. Allergy 2018, 32, 258–268. [Google Scholar] [CrossRef]
- Rosenberg, T.; Fialla, A.D.; Kjeldsen, J.; Kjeldsen, A.D. Does severe bleeding in HHT patients respond to intravenous bevacizumab? Review of the literature and case series. Rhinology 2019, 57, 242–251. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Dumas de la Roque, E.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Storch, C.H.; Hoeger, P.H. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br. J. Dermatol. 2010, 163, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Mei-Zahav, M.; Blau, H.; Bruckheimer, E.; Zur, E.; Goldschmidt, N. Topical propranolol improves epistaxis in patients with hereditary hemorrhagic telangiectasia—A preliminary report. J. Otolaryngol. Head Neck Surg. J. Oto-Rhino-Laryngol. Chir. Cervico-Faciale 2017, 46, 58. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med. Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Hoag, J.B.; Terry, P.; Mitchell, S.; Reh, D.; Merlo, C.A. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope 2010, 120, 838–843. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE)|Protocol Development|CTEP. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 28 August 2020).
- Yin, L.X.; Reh, D.D.; Hoag, J.B.; Mitchell, S.E.; Mathai, S.C.; Robinson, G.M.; Merlo, C.A. The minimal important difference of the epistaxis severity score in hereditary hemorrhagic telangiectasia. Laryngoscope 2015, 126, 1029–1032. [Google Scholar] [CrossRef]
- Snellings, D.A.; Gallione, C.J.; Clark, D.S.; Vozoris, N.T.; Faughnan, M.E.; Marchuk, D.A. Somatic Mutations in Vascular Malformations of Hereditary Hemorrhagic Telangiectasia Result in Bi-allelic Loss of ENG or ACVRL1. Am. J. Hum. Genet. 2019, 105, 894–906. [Google Scholar] [CrossRef]
- Sadick, H.; Naim, R.; Gössler, U.; Hörmann, K.; Riedel, F. Angiogenesis in hereditary hemorrhagic telangiectasia: VEGF165 plasma concentration in correlation to the VEGF expression and microvessel density. Int. J. Mol. Med. 2005, 15, 15–19. [Google Scholar] [CrossRef]
- Xu, G.; Lv, R.; Zhao, Z.; Huo, R. Topical propranolol for treatment of superficial infantile hemangiomas. J. Am. Acad. Dermatol. 2012, 67, 1210–1213. [Google Scholar] [CrossRef]
- Przewratil, P.; Sitkiewicz, A.; Andrzejewska, E. Local serum levels of vascular endothelial growth factor in infantile hemangioma: Intriguing mechanism of endothelial growth. Cytokine 2010, 49, 141–147. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, X.; Wang, W.; Zhuang, X.; Dong, J.; Qi, Z.; Hu, Q. Circulating level of vascular endothelial growth factor in differentiating hemangioma from vascular malformation patients. Plast. Reconstr. Surg. 2005, 116, 200–204. [Google Scholar] [CrossRef]
- Albiñana, V.; Recio-Poveda, L.; Zarrabeitia, R.; Bernabéu, C.; Botella, L.M. Propranolol as antiangiogenic candidate for the therapy of hereditary haemorrhagic telangiectasia. Thromb. Haemost. 2012, 108, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Contis, A.; Gensous, N.; Viallard, J.F.; Goizet, C.; Léauté-Labrèze, C.; Duffau, P. Efficacy and safety of propranolol for epistaxis in hereditary haemorrhagic telangiectasia: Retrospective, then prospective study, in a total of 21 patients. Clin. Otolaryngol. Off. J. ENT-UK Off. J. Neth. Soc. Oto-Rhino-Laryngol. Cervico-Facial Surg. 2017, 42, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Casado, S.; Martín de Rosales Cabrera, A.M.; Usarralde Pérez, A.; Martínez Simón, J.J.; Zhan Zhou, E.; Marcos Salazar, M.S.; Pérez Encinas, M.; Botella Cubells, L. Sclerotherapy and Topical Nasal Propranolol: An Effective and Safe Therapy for HHT-Epistaxis. Laryngoscope 2019, 129, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Brilliant, M.H.; Vidaillet, H. Topical timolol for treatment of epistaxis in hereditary haemorrhagic telangiectasia associated with bradycardia: A look at CYP2D6 metabolising variants. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef]
- Olitsky, S.E. Topical timolol for the treatment of epistaxis in hereditary hemorrhagic telangiectasia. Am. J. Otolaryngol. 2012, 33, 375–376. [Google Scholar] [CrossRef]
- Garg, N.; Khunger, M.; Gupta, A.; Kumar, N. Optimal management of hereditary hemorrhagic telangiectasia. J. Blood Med. 2014, 5, 191–206. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Ginon, I.; Saurin, J.-C.; Marion, D.; Guillot, E.; Decullier, E.; Roux, A.; Carette, M.-F.; Gilbert-Dussardier, B.; Hatron, P.-Y.; et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA 2012, 307, 948–955. [Google Scholar] [CrossRef]
- Riss, D.; Burian, M.; Wolf, A.; Kranebitter, V.; Kaider, A.; Arnoldner, C. Intranasal submucosal bevacizumab for epistaxis in hereditary hemorrhagic telangiectasia: A double-blind, randomized, placebo-controlled trial. Head Neck 2015, 37, 783–787. [Google Scholar] [CrossRef]
- Iyer, V.N.; Apala, D.R.; Pannu, B.S.; Kotecha, A.; Brinjikji, W.; Leise, M.D.; Kamath, P.S.; Misra, S.; Begna, K.H.; Cartin-Ceba, R.; et al. Intravenous Bevacizumab for Refractory Hereditary Hemorrhagic Telangiectasia-Related Epistaxis and Gastrointestinal Bleeding. Mayo Clin. Proc. 2018, 93, 155–166. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Kasthuri, R.S.; Parambil, J.G.; Albitar, H.A.; Almodallal, Y.A.; Vázquez, C.; Serra, M.M.; Dupuis-Girod, S.; Wilsen, C.B.; McWilliams, J.P.; et al. An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: The InHIBIT-Bleed study. Haematologica 2020. [Google Scholar] [CrossRef]
- Kabbinavar, F.; Hurwitz, H.I.; Fehrenbacher, L.; Meropol, N.J.; Novotny, W.F.; Lieberman, G.; Griffing, S.; Bergsland, E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, A.; Figueras, A.; Cerdà, P.; Mora, J.M.; Jucglà, A.; Penín, R.; Viñals, F.; Riera-Mestre, A. PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1. Cells 2019, 8, 971. [Google Scholar] [CrossRef]
- Ruiz, S.; Zhao, H.; Chandakkar, P.; Papoin, J.; Choi, H.; Nomura-Kitabayashi, A.; Patel, R.; Gillen, M.; Diao, L.; Chatterjee, P.K.; et al. Correcting Smad1/5/8, mTOR, and VEGFR2 treats pathology in hereditary hemorrhagic telangiectasia models. J. Clin. Investig. 2020, 130, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Lesca, G.; Olivieri, C.; Burnichon, N.; Pagella, F.; Carette, M.-F.; Gilbert-Dussardier, B.; Goizet, C.; Roume, J.; Rabilloud, M.; Saurin, J.-C.; et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: Data from the French-Italian HHT network. Genet. Med. Off. J. Am. Coll. Med. Genet. 2007, 9, 14–22. [Google Scholar] [CrossRef]
- Mora-Luján, J.M.; Iriarte, A.; Alba, E.; Sánchez-Corral, M.A.; Cerdà, P.; Cruellas, F.; Ordi, Q.; Corbella, X.; Ribas, J.; Castellote, J.; et al. Gender differences in hereditary hemorrhagic telangiectasia severity. Orphanet J. Rare Dis. 2020, 15, 63. [Google Scholar] [CrossRef] [PubMed]
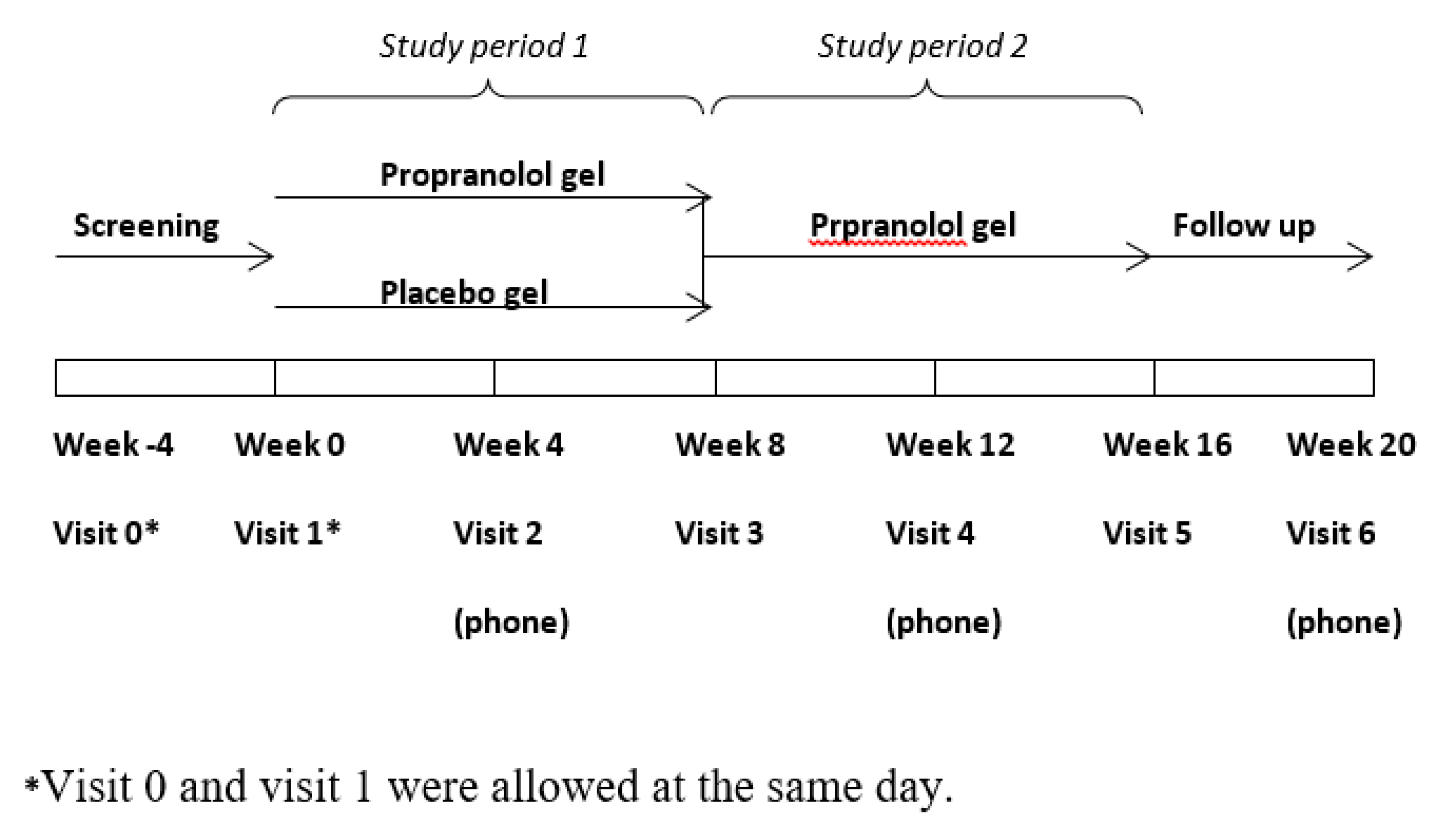
| Inclusion criteria: |
Exclusion criteria:
|
| Placebo (n = 10) | Propranolol (n = 10) | p | |
|---|---|---|---|
| Age—years (mean ± SD) | 51 ± 9 | 57 ± 11 | 0.262 |
| Gender (F,M) | 9:1 | 6:4 | 0.152 |
| Gene mutation (number of participants) | ACVRL1-8 Endoglin-1 ND-1 | ACVRL1-5 Endoglin-3 ND-2 | 0.223 |
| ESS | 5.68 ± 1.8 | 6.50 ± 1.84 | 0.323 |
| QOL | 34.75 ± 10.9 | 35.1 ± 6.7 | 0.932 |
| Hemoglobin (g/dL) | 10.7 ± 2.52 | 10.57 ± 2.6 | 0.918 |
| IV iron */IV PC * | 16/3 | 9/7 | 0.739/0.481 |
| Ferritin | 65.4 ± 86.19 | 89.65 ± 212.04 | 0.715 |
| Rhinology grading (number of patients) | 0.601 | ||
| Grade I | 4 | 3 | |
| Grade II | 5 | 5 | |
| Grade III | 1 | 2 | |
| Systolic BP mmHg (mean ± SD) | 110.2 ± 9.6 | 119.3 ± 12.3 | 0.09 |
| Diastolic BP mmHg (mean ± SD) | 63.3 ± 9.2 | 69.0 ± 9.2 | 0.20 |
| HR per minute (mean ± SD) | 76.0 ± 12.9 | 73.9 ± 111.7 | 0.71 |
| Outcome Measure | Placebo (n = 10) | Propranolol (n = 10) | ||||
|---|---|---|---|---|---|---|
| Baseline | End of DB Phase | p | Baseline | End of DB Phase | p | |
| Primary outcome | ||||||
| ESS | 5.68 ± 1.8 | 5.33 ± 2.1 | 0.133 | 6.50 ± 1.84 | 4.47 ± 1.75 | 0.004 |
| Secondary outcomes | ||||||
| Hb g/dL | 10.7 ± 2.5 | 10.7 ± 2.3 | 0.91 | 10.5 ± 2.6 | 11.4 ± 2.02 | <0.001 |
| Total PC units required * | 3 | 5 | 0.346 | 7 | 3 | <0.001 |
| Total IV iron ** | 16 | 14 | 0.233 | 9 | 4 | 0.15 |
| doses required * | ||||||
| QOL | 34.75 ± 10.9 | 40.6 ± 9.11 | 0.03 | 35.1 ± 6.7 | 41 ± 7.39 | 0.048 |
| Outcome Measure | Placebo | Propranolol | p |
|---|---|---|---|
| Primary outcome | |||
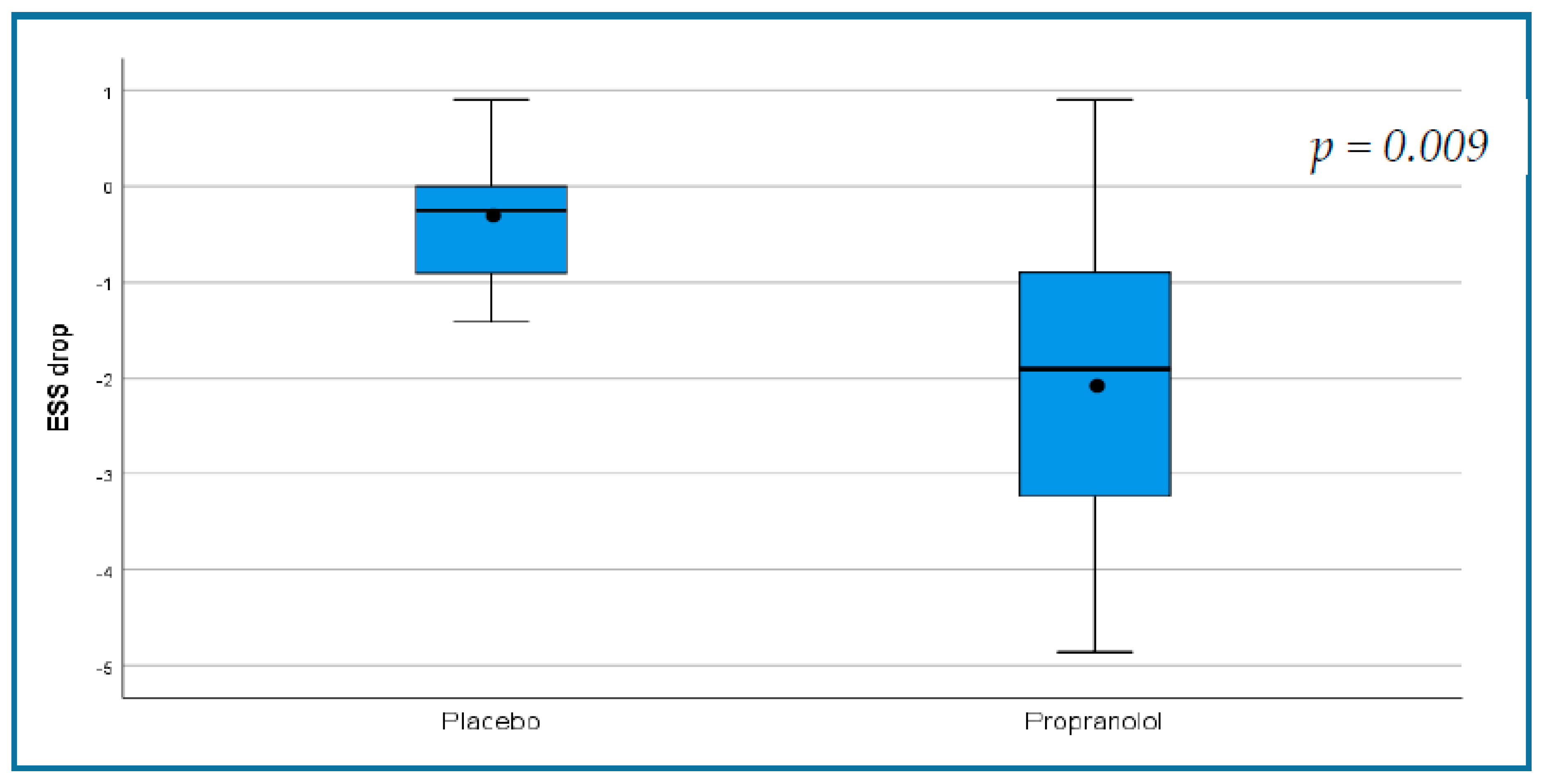
| Change in ESS | −0.35 ± 0.68 | −2.03 ± 1.7 | 0.009 |
| Secondary outcomes | |||
| Change in Hb level (g/dL) | 0.68 ± 0.01 | 1.96 ± 0.85 | 0.216 |
| Change in number of PC required | −0.20 ± 0.63 | −0.40 ± 0.69 | 0.029 |
| Change in number of IV iron doses required * | −0.20 ± 0.63 | −0.50 ± 0.97 | 0.304 |
| QOL | 6.11 ± 5.85 | 6.06 ± 5.90 | 0.986 |
| Placebo (10) | Propranolol (10) | p | |
|---|---|---|---|
| Any burning sensation | 2 | 9 | 0.005 |
| Sustained burning sensation | 0 | 4 | 0.086 |
| Rhinorrhea | 0 | 3 | 0.06 |
| Nasal dryness | 1 | 0 | 1 |
| Otitis media | 0 | 1 | 1 |
| Outcome Measure (mean ± SD) n = 15 | Double Blind Period | Open-Label Period | p |
|---|---|---|---|
| Epistaxis frequency, bleeds/day | 1.71 ± 1.34 | 1.24 ± 1.24 | <0.001 |
| Epistaxis severity | 1.42 ± 0.64 | 1.03 ± 0.55 | <0.001 |
| Epistaxis duration, minutes/day | 10.68 ± 9.01 | 6.13 ± 4.67 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei-Zahav, M.; Gendler, Y.; Bruckheimer, E.; Prais, D.; Birk, E.; Watad, M.; Goldschmidt, N.; Soudry, E. Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 3130. https://doi.org/10.3390/jcm9103130
Mei-Zahav M, Gendler Y, Bruckheimer E, Prais D, Birk E, Watad M, Goldschmidt N, Soudry E. Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial. Journal of Clinical Medicine. 2020; 9(10):3130. https://doi.org/10.3390/jcm9103130
Chicago/Turabian StyleMei-Zahav, Meir, Yulia Gendler, Elchanan Bruckheimer, Dario Prais, Einat Birk, Muhamad Watad, Neta Goldschmidt, and Ethan Soudry. 2020. "Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial" Journal of Clinical Medicine 9, no. 10: 3130. https://doi.org/10.3390/jcm9103130
APA StyleMei-Zahav, M., Gendler, Y., Bruckheimer, E., Prais, D., Birk, E., Watad, M., Goldschmidt, N., & Soudry, E. (2020). Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial. Journal of Clinical Medicine, 9(10), 3130. https://doi.org/10.3390/jcm9103130







