Benefit of Ambulatory Management of Patients with Chronic Heart Failure by Protocolized Follow-Up Therapeutic Education and Remote Monitoring Solution: An Original Study in 159 Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. Aim
2.2. Method
2.3. Inclusion and Exclusion Criteria
2.4. Study Endpoints and Data Collection
2.4.1. Primary Endpoint
2.4.2. Secondary Endpoints
2.4.3. Data Collection
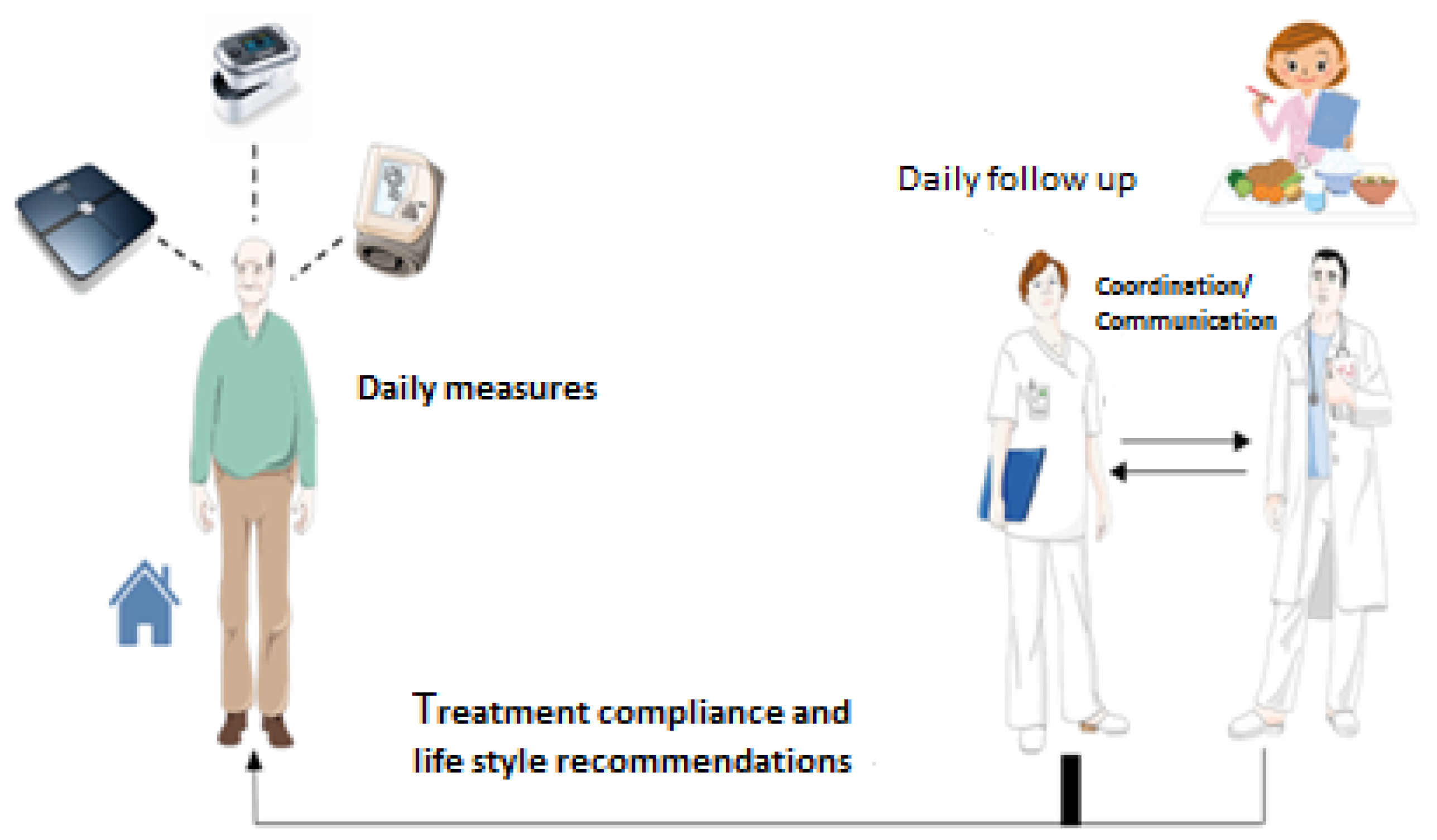
2.5. USICAR HF Unit
2.6. USICAR Program
2.7. Statistical Analysis
2.8. Administrative and Regulatory Aspects
3. Results
3.1. Patient Characteristics
3.2. Results for Primary and Secondary Endpoints
3.2.1. Primary Endpoint
3.2.2. Secondary Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CHF | chronic heart failure |
| USICAR | Unité de Suivi des Patients Insuffisants Cardiaques |
| HF | heart failure |
| ESC | European Society of Cardiology |
| NYHA | New York Heart Association |
| TIM HF 2 | Telemedical Interventional Management in Patients with Heart Failure |
References
- L’état de Santé de la Population en France Rapport 2017. ESP2017_Ouvrage_complet_vdef.pdf. Available online: http://invs.santepubliquefrance.fr/publications/etat_sante_2017/ESP2017_Ouvrage_complet_vdef.pdf (accessed on 8 November 2018).
- Personnes Prises en Charge Pour Insuffisance Cardiaque (IC) Chronique en 2017. Insuffisance_cardiaque_aigue.pdf. Available online: https://www.ameli.fr/fileadmin/user_upload/documents/Insuffisance_cardiaque_aigue.pdf (accessed on 8 November 2018).
- Gabet, A.; Chin, F.; Olié, V. Mortalité par insuffisance cardiaque en France. Tendances 2000–2010 et inégalités territoriales. Rev. Epidémio. Santé Pub. 2014, 62, S202. [Google Scholar] [CrossRef]
- Dinatolo, E.; Sciatti, E.; Anker, M.; Lombardi, C.; Dasseni, N.; Metra, M. Updates in heart failure: What last year brought to us. ESC Heart Fail. 2018, 5, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef] [PubMed]
- Guide Parcours de Soins Insuffisance Cardiaque. HAS_2014guide_parcours_de_soins_ic_web.pdf. Available online: https://www.has-sante.fr/portail/upload/docs/application/pdf/2012-04/guide_parcours_de_soins_ic_web.pdf (accessed on 7 November 2018).
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Tuppin, P.; Cuerq, A.; de Peretti, C.; Fagot-Campagna, A.; Danchin, N.; Juillière, Y.; Alla, F.; Allemand, H.; Bauters, C.; Drici, M.D.; et al. First hospitalization for heart failure in France in 2009: Patient characteristics and 30-day follow-up. Arch. Cardiovasc. Dis. 2013, 106, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Schiff, G.D.; Fung, S.; Speroff, T.; McNutt, R.A. Decompensated heart failure: Symptoms, patterns of onset, and contributing factors. Am. J. Med. 2003, 114, 625–630. [Google Scholar] [CrossRef]
- Setoguchi, S.; Stevenson, L.W.; Schneeweiss, S. Repeated hospitalizations predict mortality in the community population with heart failure. Am. Heart J. 2007, 154, 260–266. [Google Scholar] [CrossRef] [PubMed]
- PRADO, le Programme de Retour à Domicile. Insuffisance Cardiaque—PDF. Available online: https://docplayer.fr/1613088-Prado-le-programme-de-retour-a-domicile-insuffisance-cardiaque.html (accessed on 9 November 2018).
- ICARLIM_Présentation du Réseau—Santé-Limousin v.2. Available online: http://www.sante-limousin.fr/professionnels/reseaux-de-sante/icarlim/presentation (accessed on 9 November 2018).
- RADIC_CHUR_Poitier. Available online: http://2.chu-poitiers.fr/16e66b26-b174-4dc5-bd80-e5f6e8a8d553.aspx (accessed on 9 November 2018).
- Resic38 > Accueil. Available online: http://www.resic38.org/ (accessed on 9 November 2018).
- ICALOR. Available online: http://www.icalor.org/ (accessed on 9 November 2018).
- RESICARD—Réseau Île-de-France sur L’insuffisance Cardiaque. Available online: http://www.resicard.com/ (accessed on 9 November 2018).
- Agrinier, N.; Altieri, C.; Alla, F.; Jay, N.; Dobre, D.; Thilly, N.; Zannad, F. Effectiveness of a multidimensional home nurse led heart failure disease management program—A French nationwide time-series comparison. Int. J. Cardiol. 2013, 168, 3652–3658. [Google Scholar] [CrossRef] [PubMed]
- Andrès, E.; Talha, S.; Zulfiqar, A.A.; Hajjam, M.; Ervé, S.; Hajjam, J.; Gény, B.; Hajjam El Hassani, A. Current researches and new perspectives of telemedicine in chronic heart failure. J. Clin. Med. 2018, 7, 544. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Saudubray, T.; Saudubray, C.; Viboud, C.; Jondeau, G.; Valleron, A.J.; Flahault, A.; Hanslik, T. Prévalence et prise en charge de l’insuffisance cardiaque en France: Enquête nationale auprès des médecins généralistes du réseau Sentinelles. Rev. Med. Interne 2005, 26, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, A. The crucial role of patient education in heart failure. Eur. J. Heart Fail. 2005, 7, 363–369. [Google Scholar] [CrossRef] [PubMed]
- ÉTAPES: Expérimentations de Télémédecine Pour l’Amélioration des Parcours En Santé. Available online: https://solidarites-sante.gouv.fr/soins-et-maladies/prises-en-charge-specialisees/telemedecine/article/etapes-experimentations-de-telemedecine-pour-l-amelioration-des-parcours-en (accessed on 20 February 2020).
| NYHA class: | |
| n = 59 (37.1%) n = 82 (51.6%) n = 18 (11.3%) |
| Type of heart failure: | |
| n = 80 (50.3%) n = 30 (18.9%) n = 49 (30.8%) |
| Mean BNP (range) | 519 pg/mL (6–3277) |
| Type of heart disease: | |
| n = 81 (50.9%) n = 43 (27.1%) n = 16 (10.1%) n = 10 (6.3%) n = 8 (5%) n = 1 (0.6%) |
| Time Period | TOTAL in the Year Preceding Enrollment | TOTAL at 1 Year of Follow-Up | TOTAL at 2 Years of Follow-Up |
|---|---|---|---|
| Number of patients | 159 | 159 | 99 |
| Number of days hospitalized for HF | 1343 | 368 | 219 |
| Number of patients hospitalized for HF | 112 | 23 | 15 |
| Number of days hospitalized for a heart condition other than HF | 274 | 259 | 112 |
| Number of deaths | - | 11 | 10 |
| Number of lost to follow-up patients | - | 28 | 14 |
| Number of patients included for < 2 years and being followed up | - | 21 | - |
| Ratios of Means [95% CI] | Adjusted p-Value | |
|---|---|---|
| 1 year/before | 0.31 [0.18–0.54] | <0.001 |
| 2 years/before | 0.34 [0.16–0.73] | <0.001 |
| 2 years/1 year | 1.08 [0.47–2.48] | 0.818 |
| Time Period | Already Acquired at the Time of Inclusion | At 1 Year of Follow-Up | At 2 Years of Follow-Up |
|---|---|---|---|
| Weighs himself/herself regularly | 45% | 85% | 89% |
| Describes the clinical signs of HF | 23% | 79% | 86% |
| Knows the warning signs | 16% | 78% | 83% |
| Good treatment compliance | 82% | 94% | 96% |
| Good knowledge of his/her treatment | 39% | 79% | 83% |
| Knows which foods are salty | 27% | 82% | 86% |
| Good compliance with low-salt diet | 16% | 62% | 70% |
| Is able to quantify his/her water intake | 31% | 80% | 85% |
| Compliance with water restriction | 36% | 77% | 82% |
| Engages in physical activity | 27% | 55% | 60% |
| For All the Patients | For the Subgroup of Patients with the Remote Monitoring System | |
|---|---|---|
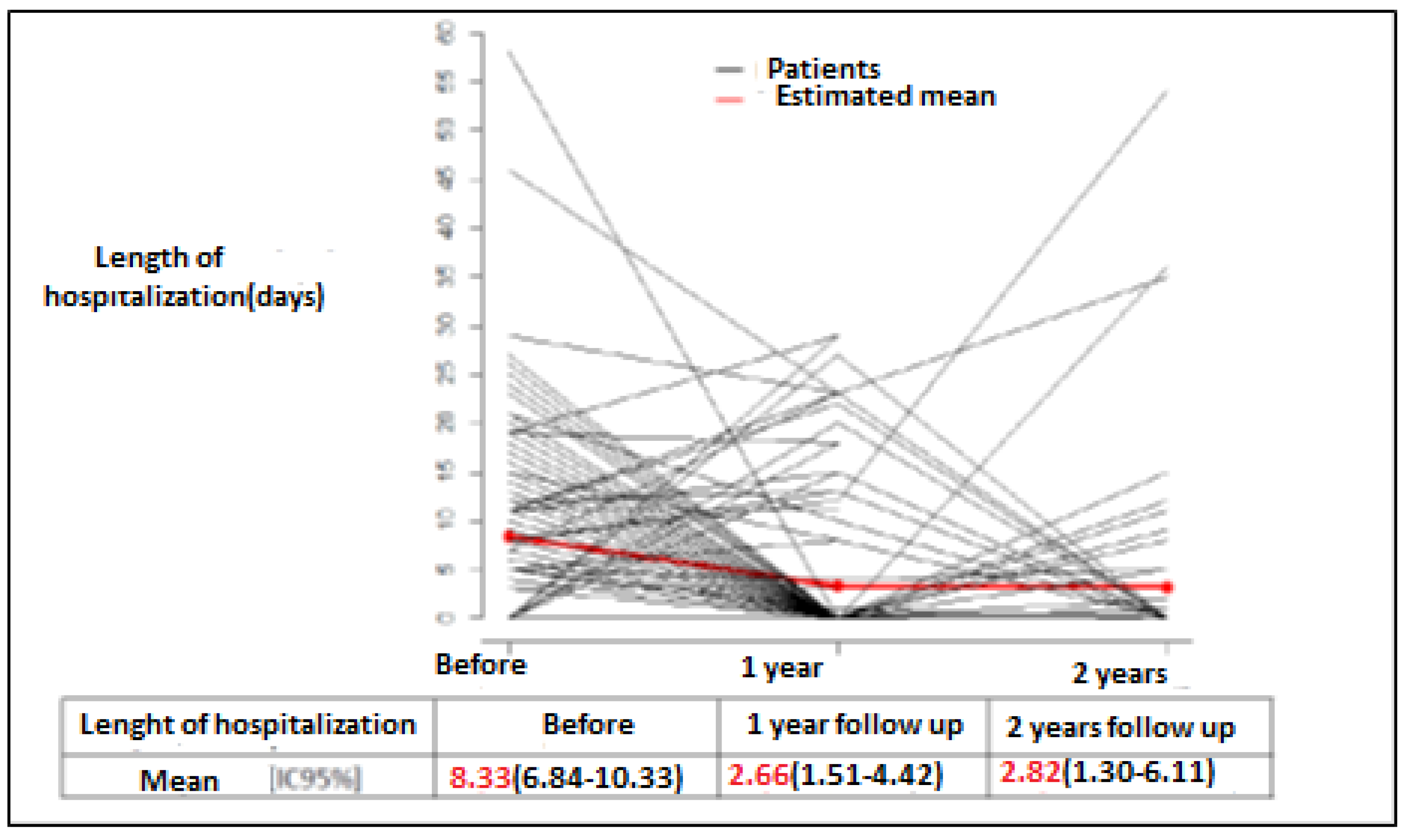
| Number of Days Hospitalized for HF Per patient per year: | ||
| 8.33 2.6 2.82 | 8.33 2.3 1.7 |
| Number of days hospitalized for of days hospitalized for a heart condition other than HF: | ||
| 274 259 112 | 274 181 36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenneve, A.; Lorenzo-Villalba, N.; Courdier, G.; Talha, S.; Séverac, F.; Zulfiqar, A.-A.; Arnold, P.; Lang, P.; Roul, G.; Andrès, E. Benefit of Ambulatory Management of Patients with Chronic Heart Failure by Protocolized Follow-Up Therapeutic Education and Remote Monitoring Solution: An Original Study in 159 Patients. J. Clin. Med. 2020, 9, 3106. https://doi.org/10.3390/jcm9103106
Jenneve A, Lorenzo-Villalba N, Courdier G, Talha S, Séverac F, Zulfiqar A-A, Arnold P, Lang P, Roul G, Andrès E. Benefit of Ambulatory Management of Patients with Chronic Heart Failure by Protocolized Follow-Up Therapeutic Education and Remote Monitoring Solution: An Original Study in 159 Patients. Journal of Clinical Medicine. 2020; 9(10):3106. https://doi.org/10.3390/jcm9103106
Chicago/Turabian StyleJenneve, Anne, Noel Lorenzo-Villalba, Guy Courdier, Samy Talha, François Séverac, Abrar-Ahmad Zulfiqar, Patrick Arnold, Philippe Lang, Gérald Roul, and Emmanuel Andrès. 2020. "Benefit of Ambulatory Management of Patients with Chronic Heart Failure by Protocolized Follow-Up Therapeutic Education and Remote Monitoring Solution: An Original Study in 159 Patients" Journal of Clinical Medicine 9, no. 10: 3106. https://doi.org/10.3390/jcm9103106
APA StyleJenneve, A., Lorenzo-Villalba, N., Courdier, G., Talha, S., Séverac, F., Zulfiqar, A.-A., Arnold, P., Lang, P., Roul, G., & Andrès, E. (2020). Benefit of Ambulatory Management of Patients with Chronic Heart Failure by Protocolized Follow-Up Therapeutic Education and Remote Monitoring Solution: An Original Study in 159 Patients. Journal of Clinical Medicine, 9(10), 3106. https://doi.org/10.3390/jcm9103106






