Dimensional Accuracy and Clinical Value of 3D Printed Models in Congenital Heart Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
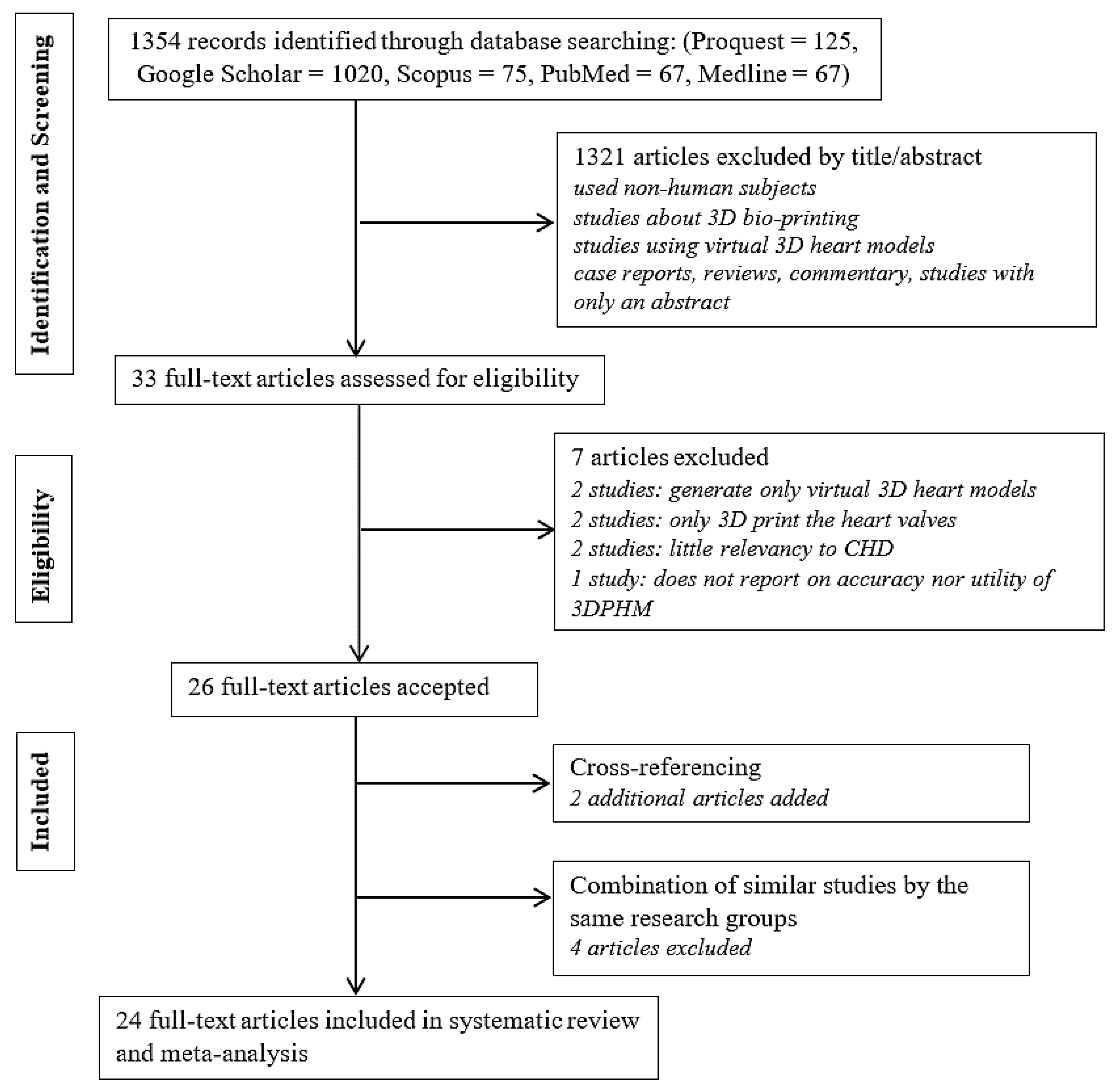
3.1. Literature Search
3.2. Study Characteristics
- In most of the cases, primary CHD is accompanied by secondary CHD. For example, DORV is usually accompanied by ventricular septal defect (VSD). In such cases, only the primary CHD is counted.
- Each type of CHD is counted once per study, which means the number of cases per study does not contribute to the count.
- CHD that have been repaired are also included in the count. For example, the study that produced 3DPHM of repaired TGA is counted.
3.3. Risk of Bias of the Included Studies
3.4. Meta-Analyses
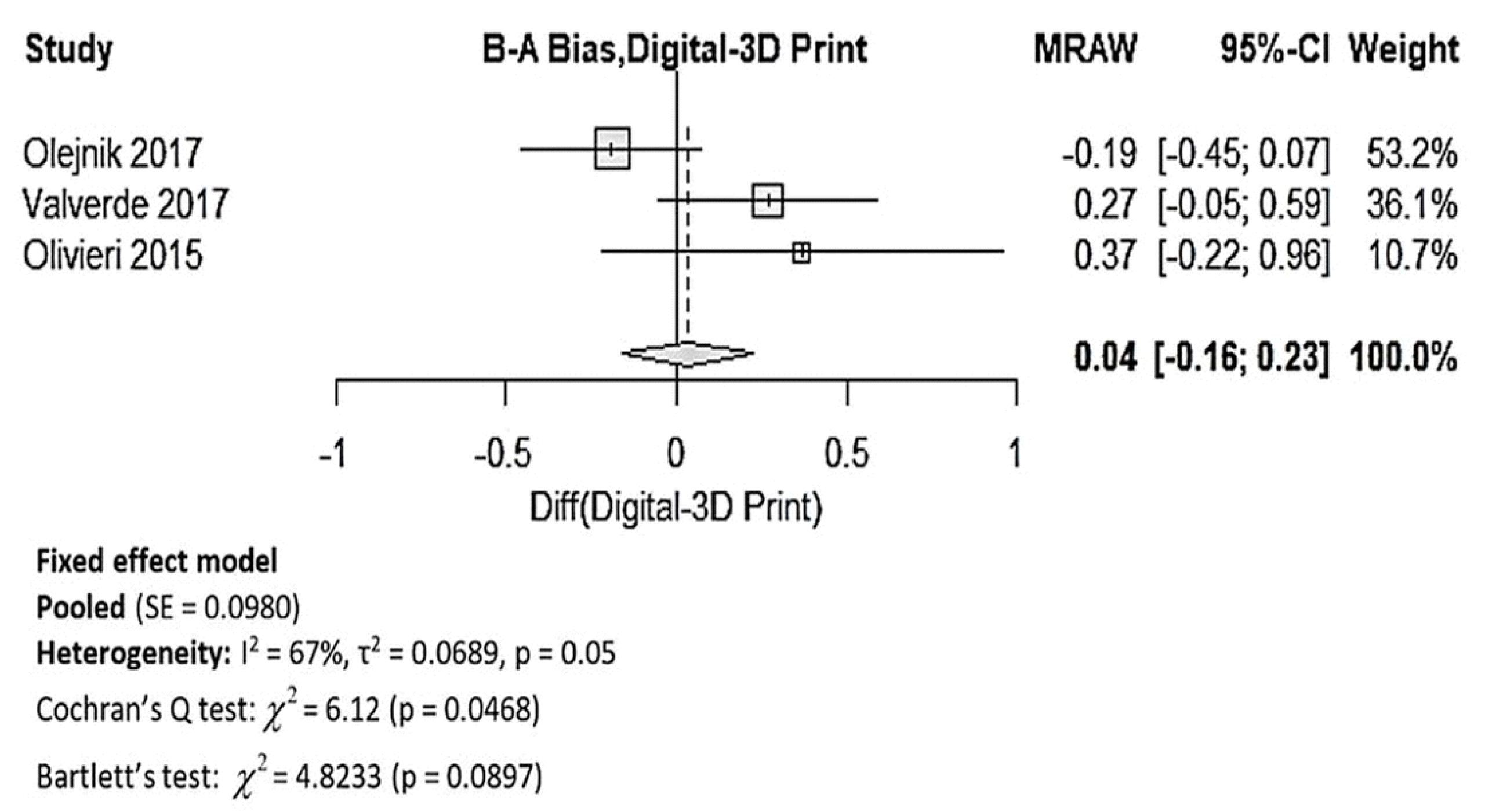
3.4.1. Dimensional Accuracy of 3DPHM
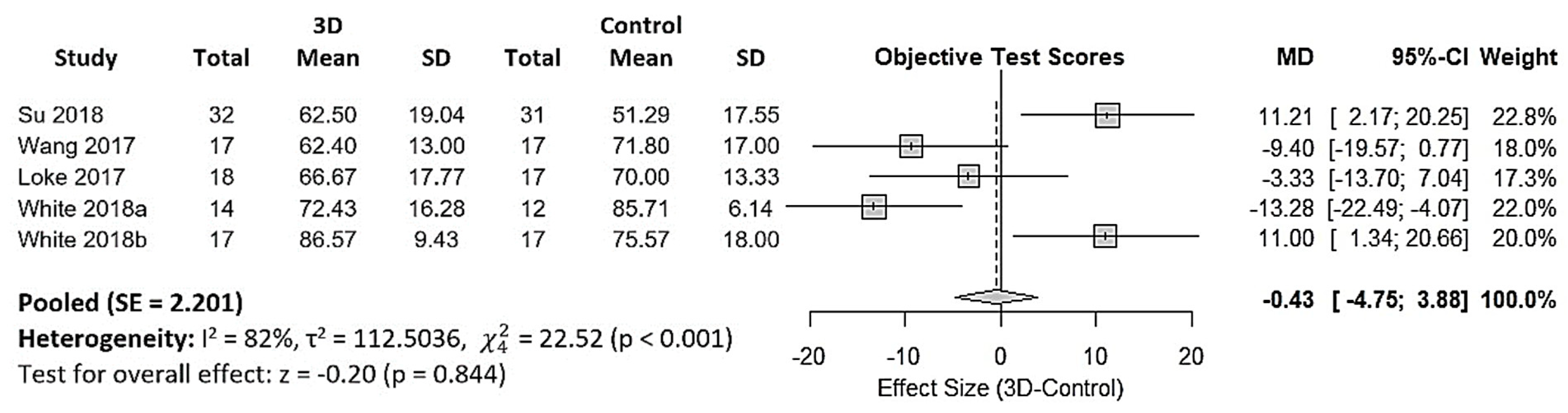
3.4.2. 3DPHM in Medical Education
4. Discussion
4.1. Dimensional Accuracy of 3DPHM
4.2. 3DPHM in Medical Education
4.3. 3DPHM in Pre-Operative Planning
4.4. 3DPHM in Communication within Medical Practice
4.5. Limitations
5. Conclusions and Implications of Future Work
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valverde, I.; Gomez, G.; Gonzalez, A.; Suarez-Mejias, C.; Adsuar, A.; Coserria, J.F.; Uribe, S.; Gomez-Cia, T.; Hosseinpour, A.R. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol. Young 2015, 25, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.; Liu, D.; Xu, L.; Fan, Z.; Sun, Z. Clinical value of patient-specific three-dimensional printing of congenital heart disease: Quantitative and qualitative assessments. PLoS ONE 2018, 13, e0194333. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lau, I.; Wong, Y.; Yeong, C. Personalized Three-Dimensional Printed Models in Congenital Heart Disease. J. Clin. Med. 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Biglino, G.; Koniordou, D.; Gasparini, M.; Capelli, C.; Leaver, L.-K.; Khambadkone, S.; Schievano, S.; Taylor, A.; Wray, J. Piloting the Use of Patient-Specific Cardiac Models as a Novel Tool to Facilitate Communication During Clinical Consultations. Pediatr. Cardiol. 2017, 38, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Tao, L.; Chen, X.; Li, W.; Peng, Z.Y.; Chen, Y.; Jin, J.; Zhang, X.L.; Xiong, Q.F.; Zhong, Z.L.; et al. Clinical application of three-dimensional reconstruction and rapid prototyping technology of multislice spiral computed tomography angiography for the repair of ventricular septal defect of tetralogy of Fallot. Genet. Mol. Res. 2015, 14, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Riesenkampff, E.; Rietdorf, U.; Wolf, I.; Schnackenburg, B.; Ewert, P.; Huebler, M.; Alexi-Meskishvili, V.; Anderson, R.H.; Engel, N.; Meinzer, H.-P.; et al. The practical clinical value of three-dimensional models of complex congenitally malformed hearts. J. Thorac. Cardiovasc. Surg. 2009, 138, 571–580. [Google Scholar] [CrossRef]
- Schmauss, D.; Haeberle, S.; Hagl, C.; Sodian, R. Three-dimensional printing in cardiac surgery and interventional cardiology: A single-centre experience. Eur. J. Cardiothorac. Surg. 2015, 47, 1044–1052. [Google Scholar] [CrossRef]
- Shiraishi, I.; Yamagishi, M.; Hamaoka, K.; Fukuzawa, M.; Yagihara, T. Simulative operation on congenital heart disease using rubber-like urethane stereolithographic biomodels based on 3D datasets of multislice computed tomography. Eur. J. Cardiothorac. Surg. 2010, 37, 302–306. [Google Scholar] [CrossRef]
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suarez-Mejias, C.; Velasco-Forte, M.N.; Byrne, N.; Ordoñez, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G.; et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: An international multicentre study. Eur. J. Cardiothorac. Surg. 2017, 52, 1139–1148. [Google Scholar] [CrossRef]
- Bhatla, P.; Tretter, J.; Ludomirsky, A.; Argilla, M.; Latson, L.; Chakravarti, S.; Barker, P.; Yoo, S.-J.; McElhinney, D.; Wake, N.; et al. Utility and Scope of Rapid Prototyping in Patients with Complex Muscular Ventricular Septal Defects or Double-Outlet Right Ventricle: Does it Alter Management Decisions? Pediatr. Cardiol. 2017, 38, 103–114. [Google Scholar] [CrossRef]
- Biglino, G.; Capelli, C.; Wray, J.; Schievano, S.; Leaver, L.-K.; Khambadkone, S.; Giardini, A.; Derrick, G.; Jones, A.; Taylor, A.M. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: Feasibility and acceptability. BMJ Open 2015, 5, e007165. [Google Scholar] [CrossRef] [PubMed]
- Biglino, G.; Capelli, C.; Leaver, L.-K.; Schievano, S.; Taylor, A.; Wray, J. Involving patients, families and medical staff in the evaluation of 3D printing models of congenital heart disease. Commun. Med. 2015, 12, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Biglino, G.; Capelli, C.; Koniordou, D.; Robertshaw, D.; Leaver, L.K.; Schievano, S.; Taylor, A.M.; Wray, J. Use of 3D models of congenital heart disease as an education tool for cardiac nurses. Congenit. Heart. Dis. 2017, 12, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.P.; Olivieri, L.J.; Krieger, A.; Thabit, O.; Marshall, M.B.; Yoo, S.-J.; Kim, P.C.; Jonas, R.A.; Nath, D.S. Utilizing Three-Dimensional Printing Technology to Assess the Feasibility of High-Fidelity Synthetic Ventricular Septal Defect Models for Simulation in Medical Education. World J. Pediatr. Congenit. Heart. Surg. 2014, 5, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.P.; Olivieri, L.J.; Su, L.; Krieger, A.; Alfares, F.; Thabit, O.; Marshall, M.B.; Yoo, S.J.; Kim, P.C.; Jonas, R.A.; et al. Incorporating Three-dimensional Printing into a Simulation-based Congenital Heart Disease and Critical Care Training Curriculum for Resident Physicians. Congenit. Heart Dis. 2015, 10, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, F.; Ryan, J.; Henriksen, M.; Stomski, L.; Feith, M.; Osborn, M.; Pophal, S.; Richardson, R.; Frakes, D. Color-coded patient-specific physical models of congenital heart disease. Rapid Prototyp. J. 2014, 20, 336–343. [Google Scholar] [CrossRef]
- Garekar, S.; Bharati, A.; Chokhandre, M.; Mali, S.; Trivedi, B.; Changela, V.P.; Solanki, N.; Gaikwad, S.; Agarwal, V. Clinical Application and Multidisciplinary Assessment of Three Dimensional Printing in Double Outlet Right Ventricle With Remote Ventricular Septal Defect. World. J. Pediatr. Congenit. Heart. Surg. 2016, 7, 344–350. [Google Scholar] [CrossRef]
- Hoashi, T.; Ichikawa, H.; Nakata, T.; Shimada, M.; Ozawa, H.; Higashida, A.; Kurosaki, K.; Kanzaki, S.; Shiraishi, I. Utility of a super-flexible three-dimensional printed heart model in congenital heart surgery. Interact. CardioVasc. Thorac. Surg. 2018, 27, 749–755. [Google Scholar] [CrossRef]
- Loke, Y.-H.; Harahsheh, A.S.; Krieger, A.; Olivieri, L.J. Usage of 3D models of tetralogy of Fallot for medical education: Impact on learning congenital heart disease. BMC. Med. Educ. 2017, 17, 54–61. [Google Scholar] [CrossRef]
- McGovern, E.; Kelleher, E.; Snow, A.; Walsh, K.; Gadallah, B.; Kutty, S.; Redmond, J.M.; McMahon, C.J. Clinical application of three-dimensional printing to the management of complex univentricular hearts with abnormal systemic or pulmonary venous drainage. Cardiol. Young 2017, 27, 1248–1256. [Google Scholar] [CrossRef]
- Ngan, E.M.; Rebeyka, I.M.; Ross, D.B.; Hirji, M.; Wolfaardt, J.F.; Seelaus, R.; Grosvenor, A.; Noga, M.L. The rapid prototyping of anatomic models in pulmonary atresia. J. Thorac. Cardiovasc. Surg. 2006, 132, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Olejník, P.; Nosal, M.; Havran, T.; Furdova, A.; Cizmar, M.; Slabej, M.; Thurzo, A.; Vitovic, P.; Klvac, M.; Acel, T.; et al. Utilisation of three-dimensional printed heart models for operative planning of complex congenital heart defects. Kardiol. Pol. 2017, 75, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, L.J.; Krieger, A.; Loke, Y.H.; Nath, D.S.; Kim, P.C.W.; Sable, C.A. Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: Feasibility and relative accuracy. J. Am. Soc. Echocardiogr. 2015, 28, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, L.J.; Su, L.; Hynes, C.F.; Krieger, A.; Alfares, F.A.; Ramakrishnan, K.; Zurakowski, D.; Marshall, M.B.; Kim, P.C.W.; Jonas, R.A.; et al. “Just-In-Time” Simulation Training Using 3-D Printed Cardiac Models After Congenital Cardiac Surgery. World J. Pediatr. Congenit. Heart Surg. 2016, 7, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Parimi, M.; Buelter, J.; Thanugundla, V.; Condoor, S.; Parkar, N.; Danon, S.; King, W. Feasibility and Validity of Printing 3D Heart Models from Rotational Angiography. Pediatr. Cardiol. 2018, 39, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Plasencia, J.; Richardson, R.; Velez, D.; Nigro, J.; Pophal, S.; Frakes, D. 3D printing for congenital heart disease: A single site’s initial three-yearexperience. 3D Print. Med. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Su, W.; Xiao, Y.; He, S.; Huang, P.; Deng, X. Three-dimensional printing models in congenital heart disease education for medical students: A controlled comparative study. BMC Med. Educ. 2018, 18, 178. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Luo, H.; Gao, C.; Zhang, J.; Dai, Y. Is a Three-Dimensional Printing Model Better Than a Traditional Cardiac Model for Medical Education? A Pilot Randomized Controlled Study. Acta Cardiol. Sin. 2017, 33, 664. [Google Scholar]
- White, S.C.; Sedler, J.; Jones, T.W.; Seckeler, M. Utility of three-dimensional models in resident education on simple and complex intracardiac congenital heart defects. Congenit. Heart Dis. 2018, 13, 1045–1049. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, S.; Fan, T.; Li, B.; Liang, W.; Dong, H. Three-dimensional printing enhances preparation for repair of double outlet right ventricular surgery. J. Card. Surg. 2018, 33, 24–27. [Google Scholar] [CrossRef]
- Zuniga, J.; Katsavelis, D.; Peck, J.; Stollberg, J.; Petrykowski, M.; Carson, A.; Fernandez, C. Cyborg beast: A low-cost 3d-printed prosthetic hand for children with upper-limb differences. BMC Res. Notes 2015, 8, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ebert, J.; Ozkol, E.; Zeichner, A.; Uibel, K.; Weiss, O.; Koops, U.; Telle, R.; Fischer, H. Direct Inkjet Printing of dental Prostheses Made of Zirconia. J. Dent. Res. 2009, 88, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Ploch, C.C.; Mansi, C.S.S.A.; Jayamohan, J.; Kuhl, E. Using 3D Printing to Create Personalized Brain Models for Neurosurgical Training and Preoperative Planning. World Neurosurg. 2016, 90, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.; Sun, Z. Three-dimensional printing in congenital heart disease: A systematic review. J. Med. Radiat. Sci. 2018, 65, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data: The PRISMA-IPD Statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 12 July 2019).
- Clément, B.; Moussa, A.H.; Damien, B. 3D-Printed Models for Surgical Planning in Complex Congenital Heart Diseases: A Systematic Review. Front. Pediatr. 2019, 7, 23. [Google Scholar]
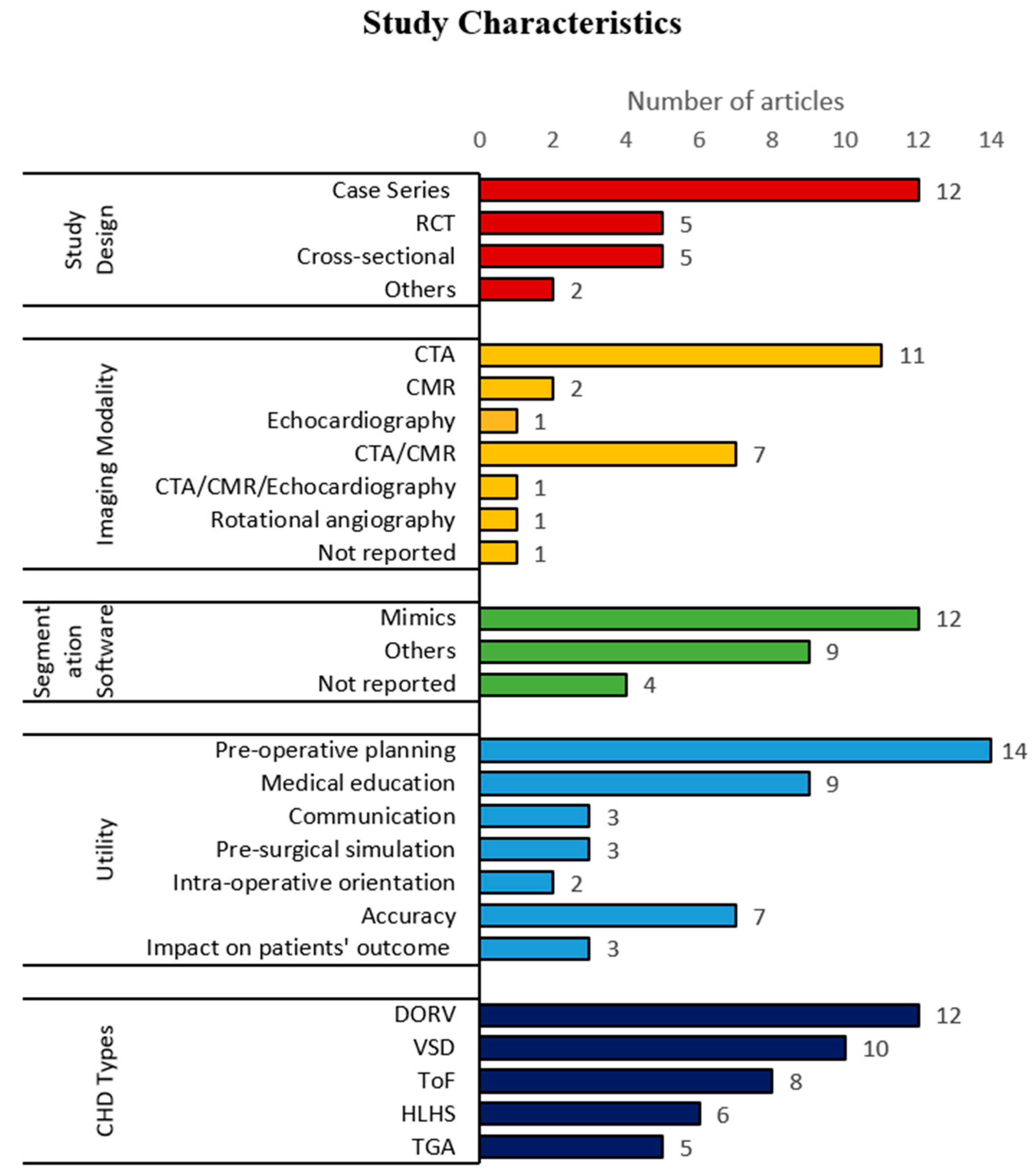
| First Author/Year | Study Design | CHD Types | Imaging Modality | Segmentation Software | Utility |
|---|---|---|---|---|---|
| * Lau et al. 2018 [2] | Cross-sectional | DORV with sub-aortic VSD | CTA | Mimics | Accuracy, pre-operative planning, communication, medical education |
| * Ma et al. 2015 [5] | Case series | ToF, ToF with ASD, ToF with PDA | CTA | Philips EBW Comp-Cardiac post-processing software | Accuracy, intraoperative orientation, impact on patients’ outcomes a |
| * Riesenkampff et al. 2009 [6] | Case series | DORV, VSD, LVOTO, CoA, RVOTO, AVSD, pulmonary atresia, pulmonary stenosis, TGA, congenitally corrected TGA | CTA/CMR | Medical Imaging and Interaction Toolkit | Pre-operative planning |
| * Schmauss et al. 2015 [7] | Case series | subpulmonary VSD, HLHS, pulmonary atresia and hypoplastic right ventricle, aortic stenosis | CTA/CMR | Amira, MeVisLab-Environment | Pre-operative planning, intraoperative orientation, pre-surgical simulation |
| * Shiraishi et al. 2009 [8] | Case series | CoA, DORV, VSD, HLHS | CTA | NR | Pre-operative planning, pre-surgical simulation |
| * Valverde et al. 2017 [9] | Prospective case-crossover | DORV, Complex TGA, univentricle, VSD, criss-cross heart, LVOTO, discordant AV and VA connections | CTA/CMR | ITK Snap | Accuracy, pre-operative planning, communication, medical education |
| * Bhatla et al. 2017 [10] | Case series | Complex muscular VSD, DORV | CTA/CMR | Mimics | Pre-operative planning |
| * Ejaz et al. 2013 [16] | RCT | NR | CTA | Advance Workstation (GE Health Systems), Mimics | Medical education, pre-operative planning |
| * Garekar et al. 2016 [17] | Case series | DORV with remote VSD | CTA/CMR | NR | Pre-operative planning |
| * Hoashi et al. 2018 [18] | Case series | DORV, TGA, congenitally corrected TGA, interrupted aortic arch Type B, ToF and MAPCA, HLHS, functional single ventricle, mitral stenosis, AVSD | CTA | NR | Pre-operative planning, pre-surgical simulation |
| * Loke et al. 2017 [19] | RCT | Unrepaired ToF, repaired ToF | CTA/CMR/echocardiography | Mimics | Medical education |
| * McGovern et al. 2017 [20] | Case series | univentricular heart, abnormal systemic or pulmonary venous drainage, dextrocardia, TGA, HLHS | CTA | Mimics | Pre-operative planning |
| * Ngan et al. 2006 [21] | Case series | VSD, pulmonary atresia, MAPCA | CTA | Mimics | Pre-operative planning |
| * Olejnik et al. 2017 [22] | Case series | interrupted aortic arch type A with aortopulmonary window type 2, dextroversion, DORV with subaortic VSD, CoA, ToF | CTA | 3D Slicer | Accuracy, pre-operative planning |
| * Olivieri et al. 2015 [23] | Case series | VSD | echocardiography | Mimics | Accuracy |
| * Olivieri et al. 2016 [24] | Cross-sectional | HLHS with total anomalous pulmonary venous connection, supravalvar aortic stenosis, DORV with hypoplastic and stenotic aortic valve and hypoplastic aortic arch, aortic regurgitation, right partial anomalous pulmonary venous connection, left pulmonary artery sling, RVOTO, truncal valve regurgitation, double aortic arch, TGA with VSD and pulmonary atresia | CTA/CMR | Mimics | Medical education |
| * Parimi et al. 2018 [25] | Case series | HLHS post Glenn shunt, CoA, ToF with MAPCAs, pulmonary atresia | Rotational angiography | Osirix | Accuracy |
| * Ryan et al. 2018 [26] | Case control study | pulmonary atresia, ToF, DORV, truncus arteriosus, single ventricle | CTA/CMR | Mimics | Pre-operative planning, impact on patients’ outcomes |
| * Su et al. 2018 [27] | RCT | 3 different subtypes of VSD | CTA | NR | Medical education |
| * Wang et al. 2017 [28] | RCT | VSD, pulmonary atresia, MAPCA | CTA | Mimics | Medical education |
| * White et al. 2018 [29] | RCT | 3 different subtypes of VSD, ToF | NR | Philips IntelliSpace Portal | Medical education |
| * Zhao et al. 2018 [30] | Cross-sectional | DORV | CTA | Mimics | Accuracy, pre-operative planning, impact on patients’ outcomes |
| * Biglino et al. 2017a [4] | Pre-post study | ToF, TGA, CoA, pulmonary atresia, aortic stenosis with dilated ascending aorta, DORV, Ebstein’s anomaly | CMR | Simpleware | Communication |
| Biglino et al. 2015a [11] | RCT | CoA, pulmonary atresia, ToF, TGA, aortic stenosis, bicuspid aortic valve, total anomalous pulmonary venous drainage, double-inlet left ventricle | CMR | Mimics | Communication |
| Biglino et al. 2015b [12] | Cross-sectional | TGA, ToF, pulmonary atresia, CoA, HLHS, TCPC | CMR | Mimics | Pre-operative planning, medical education, communication |
| Biglino et al. 2017b [13] | Cross-sectional | repaired TGA, CoA, ToF, pulmonary atresia with intact ventricular septum, palliated HLHS | CMR | NR | Medical education |
| * Costello et al.2015 [15] | Pre-post study | 5 different subtypes of VSD | CMR | Mimics | Medical education |
| Costello et al. 2014 [14] | Pre-post study | 5 different subtypes of VSD | CMR | Mimics | Medical education |
| Studies | Quality Rating |
|---|---|
| Lau et al. 2018 [2] | Fair |
| Biglino et al. 2017a [4] | Fair |
| Ma et al. 2015 [5] | Good |
| Riesenkampff et al. 2009 [6] | Fair |
| Schmauss et al. 2015 [7] | Good |
| Shiraishi et al. 2009 [8] | Fair |
| Valverde et al. 2017 [9] | Good |
| Bhatla et al. 2017 [10] | Good |
| Costello et al. 2015 [15] | Fair |
| Ejaz et al. 2013 [16] | Fair |
| Garekar et al. 2016 [17] | Good |
| Hoashi et al. 2018 [18] | Good |
| Loke et al. 2017 [19] | Fair |
| McGovern et al. 2017 [20] | Good |
| Ngan et al. 2006 [21] | Good |
| Olejnik et al. 2017 [22] | Good |
| Olivieri et al. 2015 [23] | Good |
| Olivieri et al. 2016 [24] | Fair |
| Parimi et al. 2018 [25] | Good |
| Ryan et al. 2018 [26] | Good |
| Su et al. 2018 [27] | Good |
| Wang et al. 2017 [28] | Fair |
| White et al. 2018 [29] | Good |
| Zhao et al. 2018 [30] | Fair |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, I.W.W.; Sun, Z. Dimensional Accuracy and Clinical Value of 3D Printed Models in Congenital Heart Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1483. https://doi.org/10.3390/jcm8091483
Lau IWW, Sun Z. Dimensional Accuracy and Clinical Value of 3D Printed Models in Congenital Heart Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(9):1483. https://doi.org/10.3390/jcm8091483
Chicago/Turabian StyleLau, Ivan Wen Wen, and Zhonghua Sun. 2019. "Dimensional Accuracy and Clinical Value of 3D Printed Models in Congenital Heart Disease: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 8, no. 9: 1483. https://doi.org/10.3390/jcm8091483
APA StyleLau, I. W. W., & Sun, Z. (2019). Dimensional Accuracy and Clinical Value of 3D Printed Models in Congenital Heart Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 8(9), 1483. https://doi.org/10.3390/jcm8091483






