Increased Ratio of Matrix Metalloproteinase-9 (MMP-9)/Tissue Inhibitor Metalloproteinase-1 from Alveolar Macrophages in Chronic Asthma with a Fast Decline in FEV1 at 5-Year Follow-up
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Study Protocol
2.3. Fiberoptic Bronchoscopy
2.4. Preparation of BAL Cells
2.5. Immunocytochemistry
2.6. Hematoxylin and Eosin Staining (H and E)
2.7. MMP-9 and TIMP-1 ELISA
2.8. Statistical Analysis
3. Results
3.1. Demographic Features of Patients
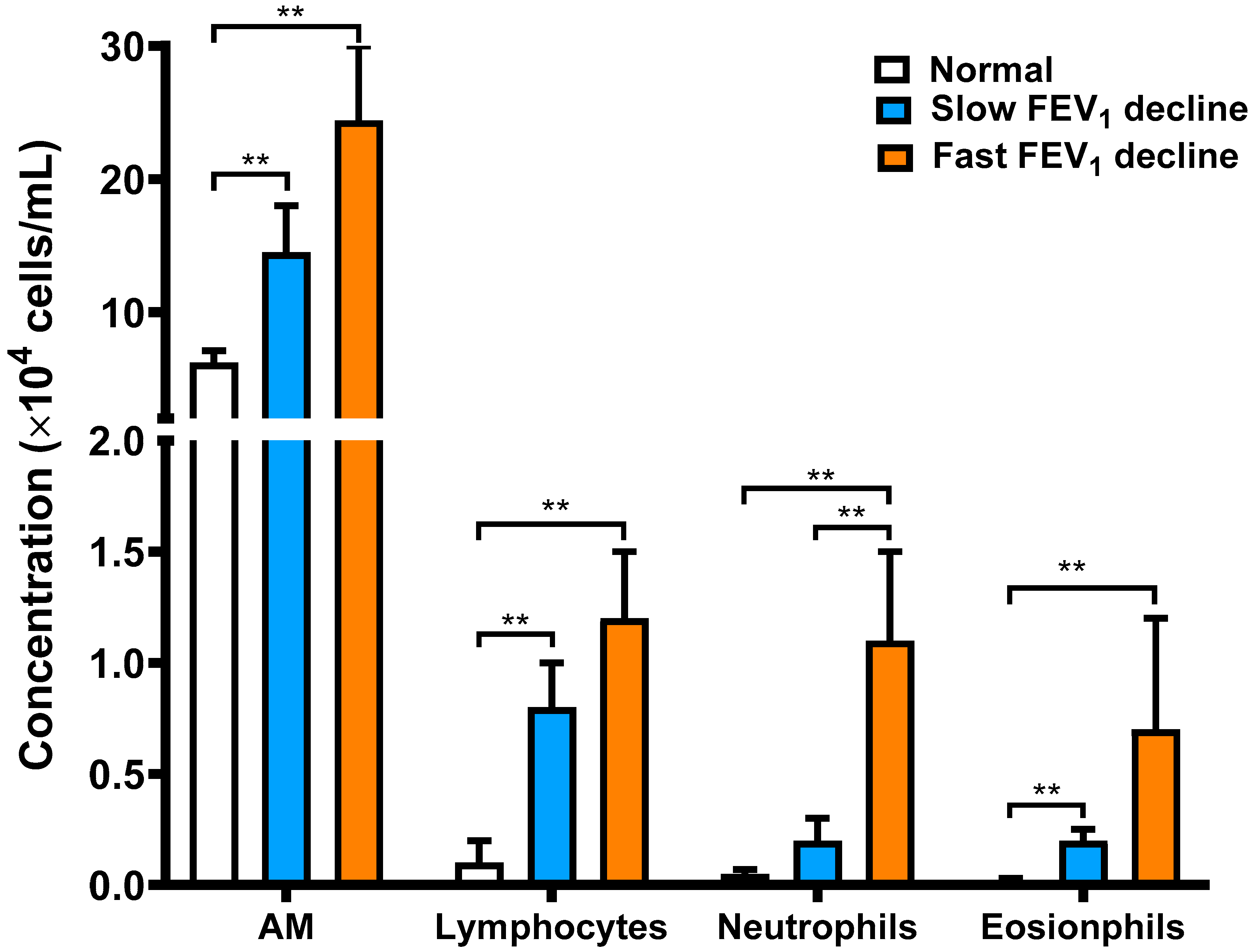
3.2. Cellular Profile Analysis of Bronchoalveolar Lavage
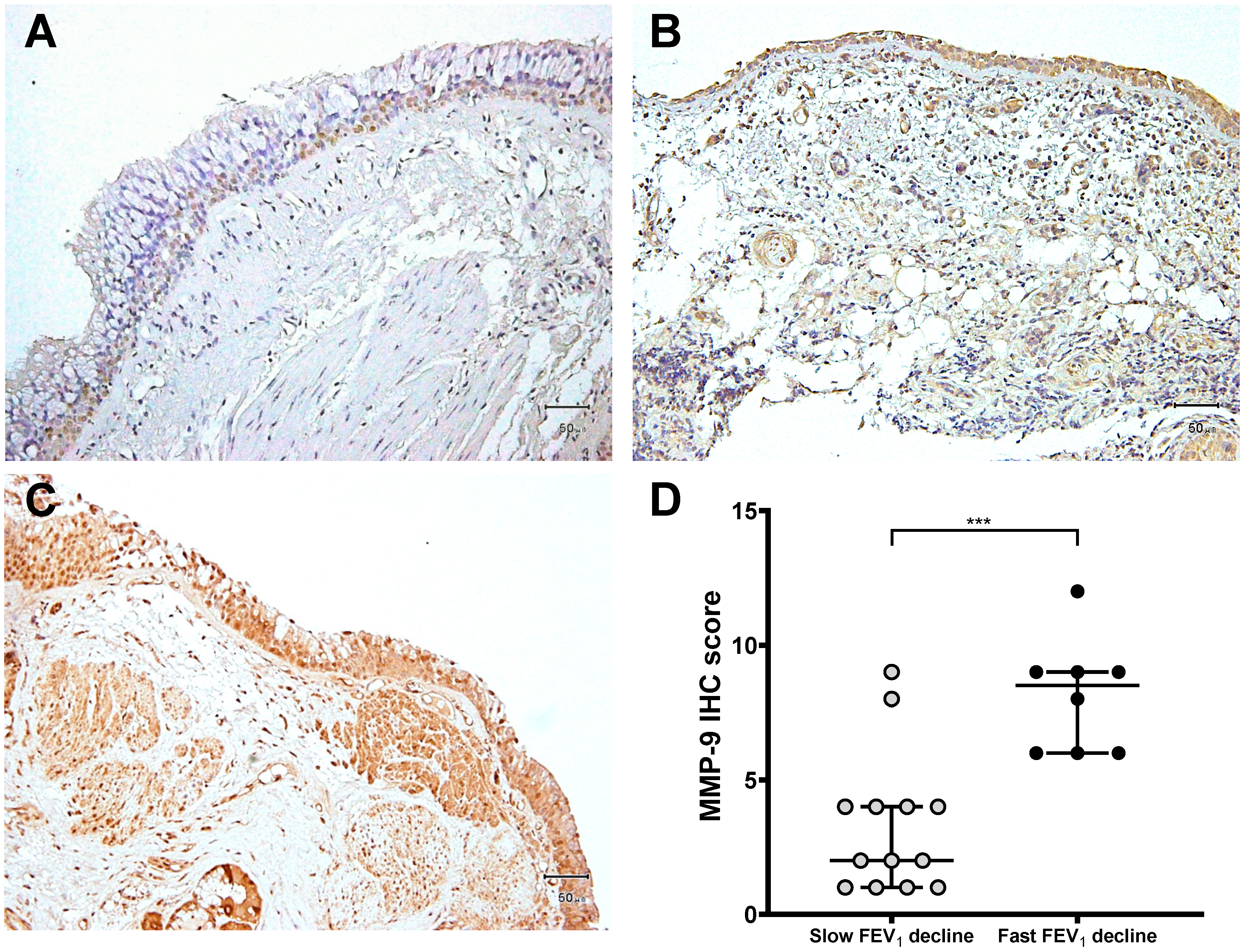
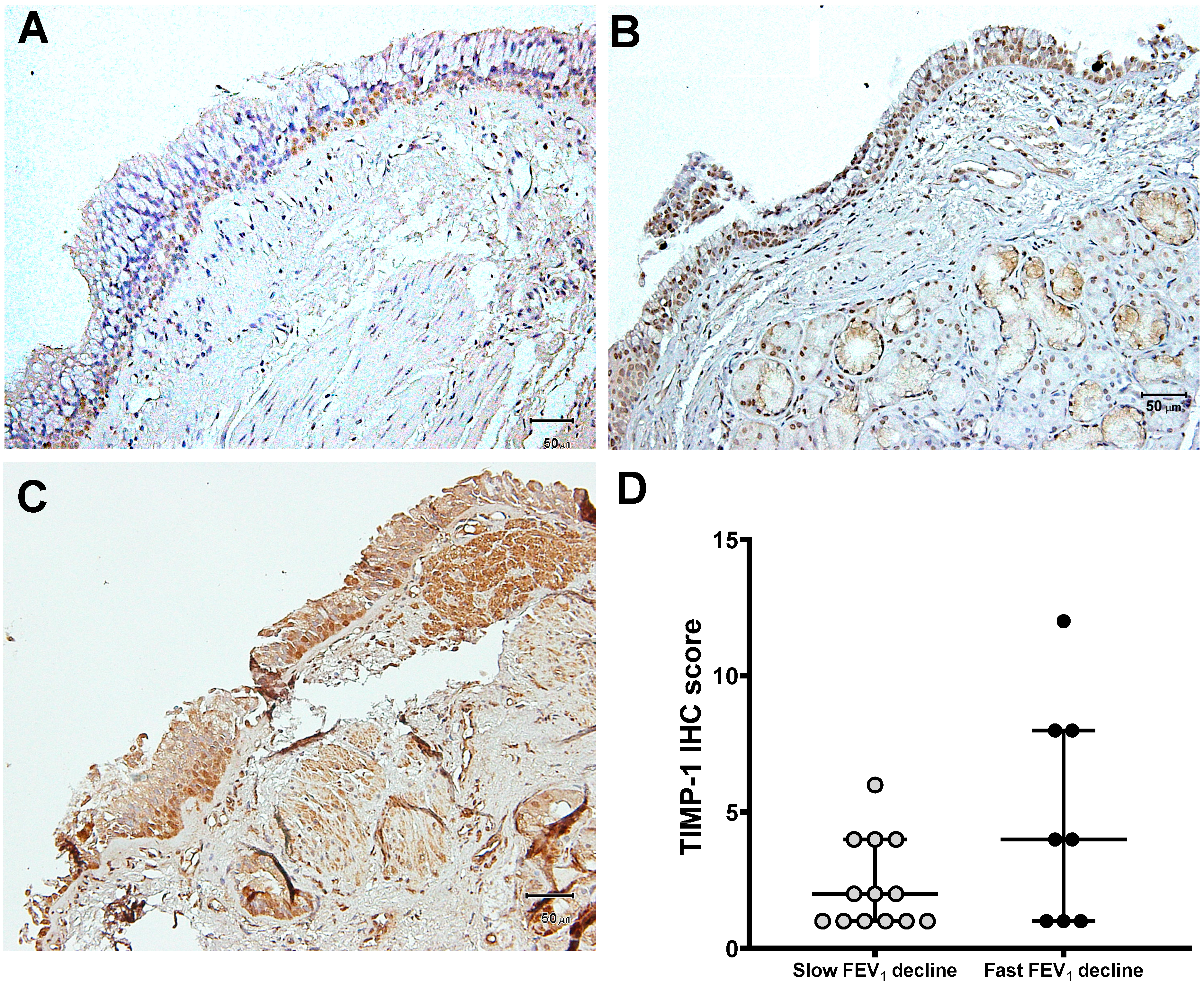
3.3. Expression of MMP-9 and TIMP-1 in Bronchial Biopsies
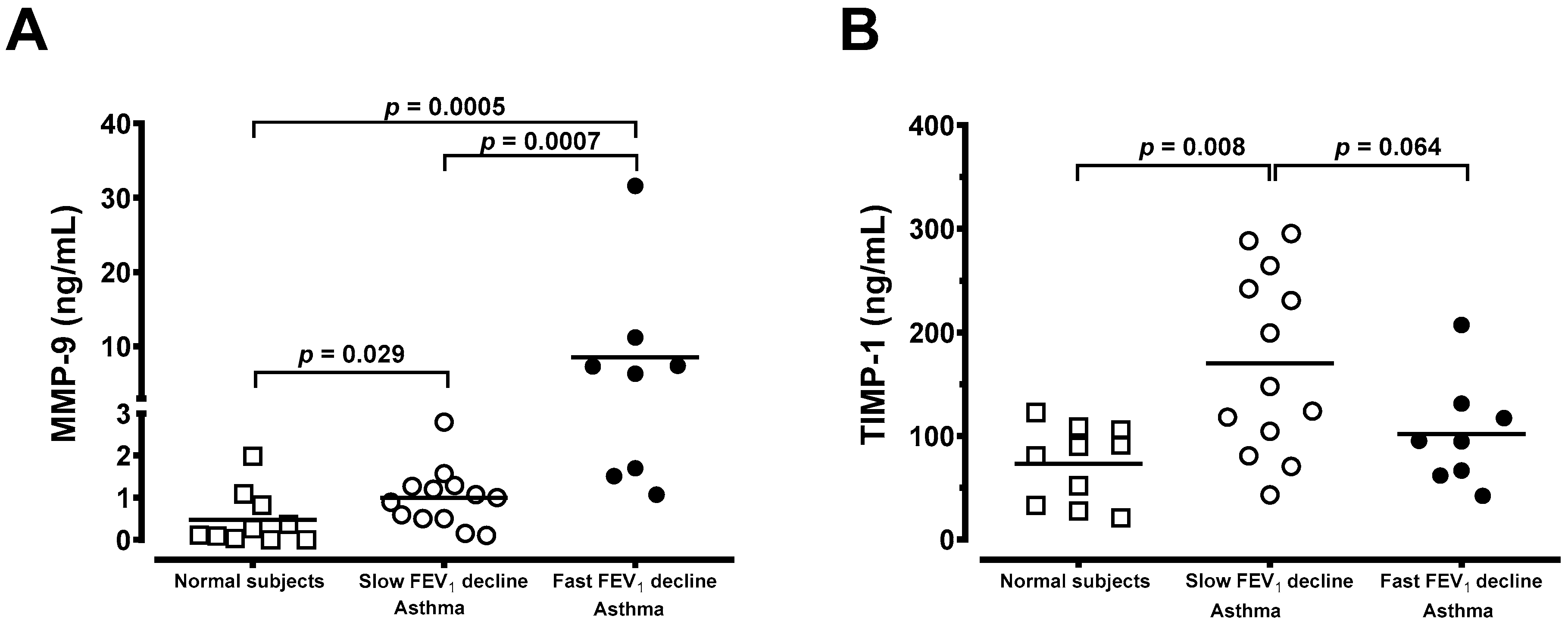
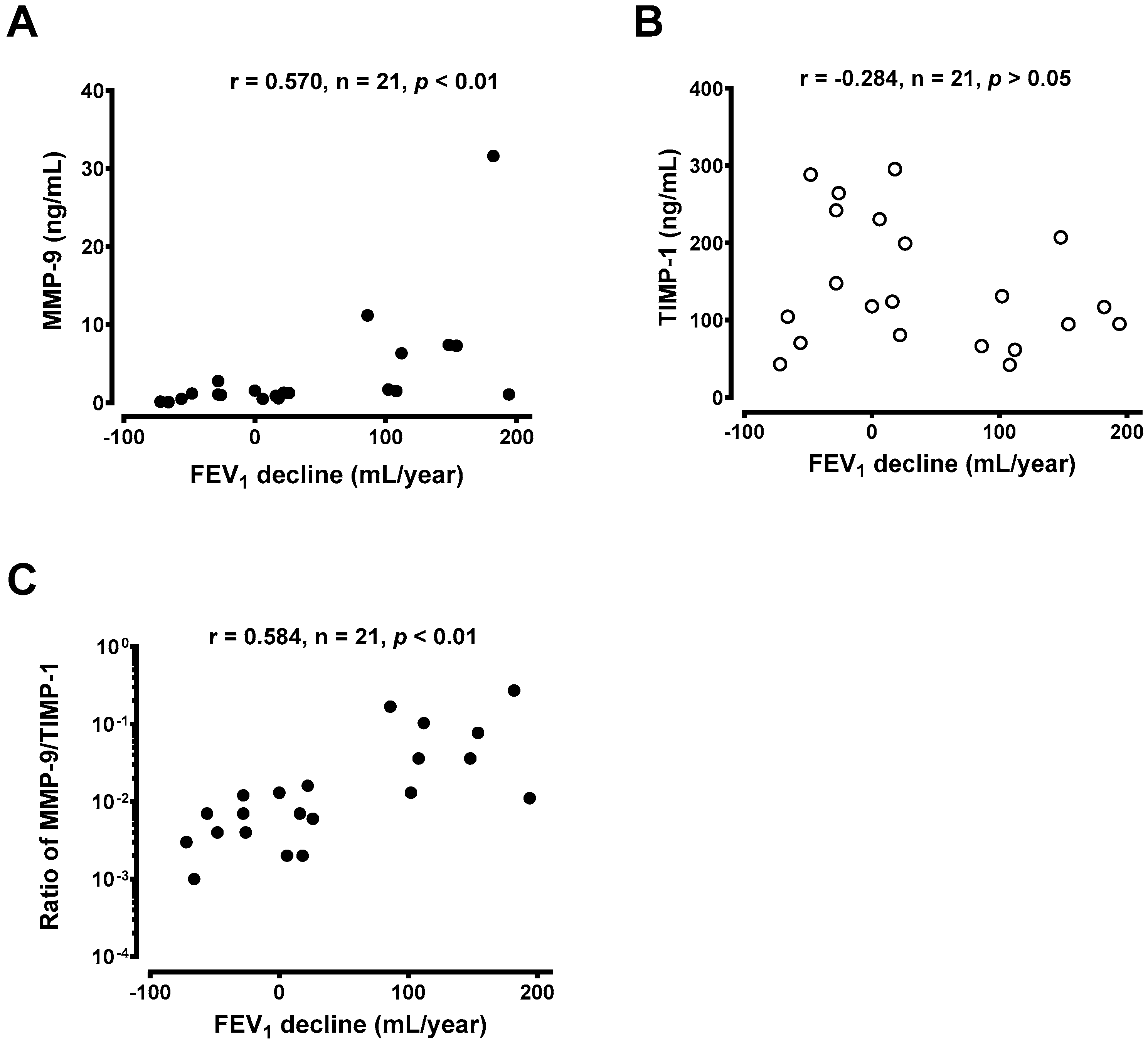
3.4. Generation of MMP-9 and TIMP-1 From Macrophages
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sullivan, P.W.; Campbell, J.D.; Ghushchyan, V.H.; Globe, G. Outcomes before and after treatment escalation to Global Initiative for Asthma steps 4 and 5 in severe asthma. Ann. Allergy Asthma Immunol. 2015, 114, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.; Roche, W.; Holgate, S.T. Inflammatory processes in bronchial asthma. Drugs 1989, 37 (Suppl. 1), 117–122. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Halayko, A.J.; Gosens, R.; Panettieri, R.A.; Camoretti-Mercado, B.; Penn, R.B. ATS Assembly on Respiratory Structure and Function. Am. J. Respir. Crit. Care Med. 2017, 195, e4–e19. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.R.; Cooper, J.; Koelmeyer, T.; Pare, P.D.; Weir, T.D. The effect of age and duration of disease on airway structure in fatal asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Lackie, P.; Wilson, S.; Roche, W.; Davies, D. Bronchial epithelium as a key regulator of airway allergen sensitization and remodeling in asthma. Am. J. Respir. Crit. Care Med. 2000, 162, S113–S117. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Dejonckheer, E.; Libert, C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur. Respir. J. 2011, 38, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.; McSharry, C.; Brady, J.; Donnelly, I.; Grierson, C.; McGuinness, S.; Jolly, L.; Weir, C.J.; Messow, C.M.; Spears, M.; et al. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: Relationship to disease severity. J. Allergy Clin. Immunol. 2012, 129, 655–663. [Google Scholar] [CrossRef]
- Oka, S.; Furukawa, H.; Shimada, K.; Hayakawa, H.; Fukui, N.; Tsuchiya, N.; Tohma, S. Serum biomarker analysis of collagen disease patients with acute-onset diffuse interstitial lung disease. BMC Immunol. 2013, 14, 9. [Google Scholar] [CrossRef]
- Hu, C.; Wang, J.; Xu, Y.; Li, X.; Chen, H.; Bunjhoo, H.; Xiong, W.; Xu, Y.; Zhao, J. Current evidence on the relationship between five polymorphisms in the matrix metalloproteinases (MMP) gene and lung cancer risk: A meta-analysis. Gene 2013, 517, 65–71. [Google Scholar] [CrossRef]
- Craig, V.J.; Quintero, P.A.; Fyfe, S.E.; Patel, A.S.; Knolle, M.D.; Kobzik, L.; Owen, C.A. Profibrotic activities for matrix metalloproteinase-8 during bleomycin-mediated lung injury. J. Immunol. 2013, 190, 4283–4296. [Google Scholar] [CrossRef]
- Ko, F.W.; Diba, C.; Roth, M.; Patel, A.S.; Knolle, M.D.; Kobzik, L.; Owen, C.A. A comparison of airway and serum matrix metalloproteinase-9 activity among normal subjects, asthmatic patients, and patients with asthmatic mucus hypersecretion. Chest 2005, 127, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Castano, R.; Miedinger, D.; Maghni, K.; Ghezzo, H.; Trudeau, C.; Castellanos, L.; Wattiez, M.; Vandenplas, O.; Malo, J.L. Matrix metalloproteinase-9 increases in the sputum from allergic occupational asthma patients after specific inhalation challenge. Int. Arch. Allergy Immunol. 2013, 160, 161–164. [Google Scholar] [CrossRef]
- Hong, Z.; Lin, Y.M.; Qin, X.; Peng, J.L. Serum MMP-9 is elevated in children with asthma. Mol. Med. Rep. 2012, 5, 462–464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bratcher, P.E.; Weathington, N.M.; Nick, H.J.; Jackson, P.L.; Snelgrove, R.J.; Gaggar, A. MMP-9 cleaves SP-D and abrogates its innate immune functions in vitro. PLoS ONE 2012, 7, e41881. [Google Scholar] [CrossRef] [PubMed]
- Page, K.; Ledford, J.R.; Zhou, P.; Wills-Karp, M. A TLR2 agonist in German cockroach frass activates MMP-9 release and is protective against allergic inflammation in mice. J. Immunol. 2009, 183, 3400–3408. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.P.; Spanevello, A.; Palladino, G.P.; Salerno, F.G.; Lacedonia, D.; Carpagnano, G.E. Exhaled matrix metalloproteinase-9 (MMP-9) in different biological phenotypes of asthma. Eur. J. Intern. Med. 2014, 25, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta. 2010, 1803, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Roche, W.R.; Beasley, R.; Williams, J.H.; Holgate, S.T. Subepithelial fibrosis in the bronchi of asthmatics. Lancet 1989, 1, 520–524. [Google Scholar] [CrossRef]
- Lo, C.Y.; Huang, H.Y.; He, J.R.; Huang, T.T.; Heh, C.C.; Sheng, T.F.; Chung, K.F.; Kuo, H.P.; Wang, C.H. Increased matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio in smokers with airway hyperresponsiveness and accelerated lung function decline. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1135–1144. [Google Scholar] [CrossRef]
- Vignola, A.M.; Riccobono, L.; Mirabella, A.; Profita, M.; Chanez, P.; Bellia, V.; Mautino, G.; D’accardi, P.; Bousquet, J.; Bonsignore, G. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1998, 158, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Miyazaki, N.; Oashi, K.; Tanaka, S.; Ohmichi, M.; Abe, S. Sputum matrix metalloproteinase-9: Tissue inhibitor of metalloproteinase-1 ratio in acute asthma. J. Allergy Clin. Immunol. 2000, 105, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Bosse, M.; Chakir, J.; Rouabhia, M.; Boulet, L.P.; Audette, M.; Laviolette, M. Serum matrix metalloproteinase-9: Tissue inhibitor of metalloproteinase-1 ratio correlates with steroid responsiveness in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 1999, 159, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Kapsner, R.K.; Osberg, I. Induced sputum matrix metalloproteinase-9 correlates with lung function and airway inflammation in children with cystic fibrosis. Pediatric Pulmonol. 2005, 39, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Niimi, A.; Takemura, M.; Ueda, T.; Minakuchi, M.; Tabuena, R.; Chin, K.; Mio, T.; Ito, Y.; Muro, S.; et al. Relationship of airway wall thickening to an imbalance between matrix metalloproteinase-9 and its inhibitor in asthma. Thorax 2005, 60, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Mercer, P.F.; Shute, J.K.; Bhowmik, A.; Donaldson, G.C.; Wedzicha, J.A.; Warner, J.A. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir. Res. 2005, 6, 151. [Google Scholar] [CrossRef]
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am. Rev. Respir. Dis. 1987, 136, 225–244. [Google Scholar]
- Broekema, M.; Volbeda, F.; Timens, W.; Dijkstra, A.; Lee, N.A.; Lee, J.J.; Lodewijk, M.E.; Postma, D.S.; Hylkema, M.N.; Ten Hacken, N.H. Airway eosinophilia in remission and progression of asthma: Accumulation with a fast decline of FEV(1). Respir. Med. 2010, 104, 1254–1262. [Google Scholar] [CrossRef]
- Wang, C.H.; Liu, C.Y.; Lin, H.C.; Yu, C.T.; Chung, K.F.; Kuo, H.P. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur. Respir. J. 1998, 11, 809–815. [Google Scholar] [CrossRef]
- Hoshino, M.; Takahashi, M.; Takai, Y.; Sim, J. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in asthma. J. Allergy Clin. Immunol. 1999, 104, 356–363. [Google Scholar] [CrossRef]
- Kelly, E.A.; Busse, W.W.; Jarjour, N.N. Increased matrix metalloproteinase-9 in the airway after allergen challenge. Am. J. Respir. Crit. Care Med. 2000, 162, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Kolls, J.K. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Nakamura, Y.; Sim, J.; Shimojo, J.; Isogai, S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J. Allergy Clin. Immunol. 1998, 102, 783–788. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, X.; Shapiro, S.D.; Shipley, J.M.; Twining, S.S.; Diaz, L.A.; Senior, R.M.; Werb, Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell 2000, 102, 647–655. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Liu, X.D.; Skold, C.M.; Umino, T.; Wang, H.J.; Spurzem, J.R.; Kohyama, T.; Ertl, R.F.; Rennard, S.I. Synergistic neutrophil elastase-cytokine interaction degrades collagen in three-dimensional culture. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L868–L878. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Pure, E. CD44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrixremodeling and tensile strength during cutaneous wound healing. Matrix Biol. 2019, 75–76, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Min, K.H.; Kim, S.R.; Park, S.J.; Park, H.S.; Jin, G.Y.; Lee, Y.C. Vascular endothelial growth factor modulates matrix metalloproteinase-9 expression in asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Fredrich, K.; Weihrauch, D.; Hattan, N.; Chilian, W.M.; Patterson, B.C.; Sang, Q.A. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am. J. Physiol. Ren. Physiol. 2004, 286, F893–F902. [Google Scholar] [CrossRef]
- Cataldo, D.D.; Gueders, M.; Munaut, C.; Rocks, N.; Bartsch, P.; Foidart, J.M.; Noël, A.; Louis, R. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases mRNA transcripts in the bronchial secretions of asthmatics. Lab. Investig. 2004, 84, 418–424. [Google Scholar] [CrossRef]
- Russell, R.E.; Culpitt, S.V.; DeMatos, C.; Donnelly, L.; Smith, M.; Wiggins, J.; Barnes, P.J. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2002, 26, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Corticosteroid resistance in airway disease. Proc. Am. Thorac. Soc. 2004, 1, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur. Respir. J. 2003, 22, 470–477. [Google Scholar]
- Bergeron, C.; Tulic, M.K.; Hamid, Q. Airway remodelling in asthma: From bench side to clinical practice. Can. Respir. J. 2010, 17, e85–e93. [Google Scholar] [CrossRef]
- Grzela, K.; Litwiniuk, M.; Zagorska, W.; Grzela, T. Airway remodeling in chronic obstructive pulmonary disease and asthma: The role of matrix metalloproteinase-9. Arch. Immunol. Ther. Exp. 2016, 64, 47–55. [Google Scholar] [CrossRef]
- Mautino, G.; Oliver, N.; Chanez, P.; Bousquet, J.; Capony, F. Increased release of matrix metalloproteinase-9 in bronchoalveolar lavage fluid and by alveolar macrophages of asthmatics. Am. J. Respir. Cell Mol. Biol. 1997, 17, 583–591. [Google Scholar] [CrossRef]
- Han, Z.; Zhong, N. Expression of matrix metalloproteinases MMP-9 within the airways in asthma. Respir. Med. 2003, 97, 563–567. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Balzar, S.; Cundall, M.; Chu, H.W. Subepithelial basement membrane immunoreactivity for matrix metalloproteinase 9: Association with asthma severity, neutrophilic inflammation, and wound repair. J. Allergy Clin. Immunol. 2003, 111, 1345–1352. [Google Scholar] [CrossRef]
- Ventura, I.; Vega, A.; Chacón, P.; Chamorro, C.; Aroca, R.; Gómez, E.; Bellido, V.; Puente, Y.; Blanca, M.; Monteseirín, J. Neutrophils from allergic asthmatic patients produce and release metalloproteinase-9 upon direct exposure to allergens. Allergy 2014, 69, 898–905. [Google Scholar] [CrossRef]
- Kostamo, K.; Tervahartiala, T.; Sorsa, T.; Richardson, M.; Toskala, E. Metalloproteinase function in chronic rhinosinusitis with nasal polyposis. Laryngoscope 2007, 117, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Vignola, A.M.; Kips, J.; Bousquet, J. Tissue remodeling as a feature of persistent asthma. J. Allergy Clin. Immunol. 2000, 105, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Pais, M.; Bish, R.; Reid, D.; Feltis, B.; Johns, D.; Walters, E.H. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax 2002, 57, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Naveed, S.U.; Clements, D.; Jackson, D.J.; Philp, C.; Billington, C.K.; Soomro, I.; Reynolds, C.; Harrison, T.W.; Johnston, S.L.; Shaw, D.E.; et al. Matrix metalloproteinase-1 activation contributes to airway smooth muscle growth and asthma severity. Am. J. Respir. Crit. Care Med. 2017, 195, 1000–1009. [Google Scholar] [CrossRef]
- Todorova, L.; Bjermer, L.; Miller-Larsson, A.; Westergren-Thorsson, G. Relationship between matrix production by bronchial fibroblasts and lung function and AHR in asthma. Respir. Med. 2010, 104, 1799–1808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corry, D.B.; Kiss, A.; Song, L.Z.; Song, L.; Xu, J.; Lee, S.H.; Werb, Z.; Kheradmand, F. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 2004, 18, 995–997. [Google Scholar] [CrossRef]
- Churg, A.; Zhou, S.; Wright, J.L. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur. Respir. J. 2012, 39, 197–209. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Melgert, B.N.; ten Hacken, N.H.; Rutgers, B.; Timens, W.; Postma, D.S.; Hylkema, M.N. More alternative activation of macrophages in lungs of asthmatic patients. J. Allergy Clin. Immunol. 2011, 127, 831–833. [Google Scholar] [CrossRef]
- Girodet, P.O.; Nguyen, D.; Mancini, J.D.; Hundal, M.; Zhou, X.; Israel, E.; Cernadas, M. Alternative macrophage activation is increased in asthma. Am. J. Respir. Cell Mol. Biol. 2016, 55, 467–475. [Google Scholar] [CrossRef]
- Huang, W.C.; Sala-Newby, G.B.; Susana, A.; Johnson, J.L.; Newby, A.C. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS ONE 2012, 7, e42507. [Google Scholar] [CrossRef]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef]


| Clinical Features | Normal Subjects (n = 10) | Asthma (n = 13) Slow FEV1 Decline | Asthma (n = 8) Fast FEV1 Decline |
|---|---|---|---|
| Age, year | 47.6 ± 2.8 | 49.2 ± 3.4 | 49.4 ± 3.7 |
| Gender, F/M | 5/5 | 5/8 | 4/4 |
| FVC, L | 3.4 ± 0.3 | 3.0 ± 0.3 | 3.3 ± 0.2 |
| FVC, % pred. | 95.2 ± 4.7 | 88.8 ± 5.7 | 94.0 ± 5.0 |
| FEV1, L | 2.6 ± 0.3 | 2.1 ± 0.3 | 2.4 ± 0.2 |
| FEV1, % pred. | 82.4 ± 4.5 | 72.2 ± 6.3 | 77.6 ± 4.8 |
| FEV1/FVC | 83.3 ± 2.7 | 68.7 ± 3.6 | 73.8 ± 4.0 |
| Annual FEV1 decline, mL/year | −18.2 ± 9.7 | 135.8 ± 14.0 *** | |
| PC20, mg/dL | > 25 | 1.9 ± 0.5 | 3.5 ± 1.0 |
| Cell Profile | Normal (n = 10) | Asthma (n = 13) Slow FEV1 Decline | Asthma (n = 8) Fast FEV1 Decline |
|---|---|---|---|
| Total cell count, ×106 cells | 11.9 ± 1.7 | 21.2 ± 6.6 ** | 34.2 ± 10.1 ** |
| Recovered volume, % | 62.9 ± 3.7 | 44.6 ± 5.0 | 47.5 ± 5.8 |
| Cell viability, % | 94.4 ± 1.4 | 94.1 ± 1.3 | 91.6 ± 2.5 |
| AMs, % | 97.5 ± 0.6 | 87.9 ± 2.3 ** | 84.3 ± 5.3 ** |
| Lymphocytes, % | 1.5 ± 0.5 | 8.2 ± 1.8 ** | 8.0 ± 3.6 ** |
| Neutrophils, % | 0.7 ± 0.2 | 1.4 ± 0.3 | 5.0 ± 1.5 **# |
| Eosinophils, % | 0.2 ± 0.1 | 2.4 ± 1.1 * | 2.6 ± 1.1 * |
| Airway Structure | Asthma (n = 13) Slow FEV1 Decline | Asthma (n = 8) Fast FEV1 Decline | p Value |
|---|---|---|---|
| Epithelium, μm | 27.2 ± 6.3 | 23.8 ± 7.4 | 0.733 |
| Basement membrane, μm | 7.0 ± 0.6 | 15.5 ± 2.2 | 0.0002 |
| Subepithelium, μm | 37.8 ± 4.2 | 138.3 ± 12.2 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, F.-T.; Huang, H.-Y.; Lo, C.-Y.; Huang, Y.-C.; Lin, C.-W.; He, C.-C.; He, J.-R.; Sheng, T.-F.; Wang, C.-H. Increased Ratio of Matrix Metalloproteinase-9 (MMP-9)/Tissue Inhibitor Metalloproteinase-1 from Alveolar Macrophages in Chronic Asthma with a Fast Decline in FEV1 at 5-Year Follow-up. J. Clin. Med. 2019, 8, 1451. https://doi.org/10.3390/jcm8091451
Chung F-T, Huang H-Y, Lo C-Y, Huang Y-C, Lin C-W, He C-C, He J-R, Sheng T-F, Wang C-H. Increased Ratio of Matrix Metalloproteinase-9 (MMP-9)/Tissue Inhibitor Metalloproteinase-1 from Alveolar Macrophages in Chronic Asthma with a Fast Decline in FEV1 at 5-Year Follow-up. Journal of Clinical Medicine. 2019; 8(9):1451. https://doi.org/10.3390/jcm8091451
Chicago/Turabian StyleChung, Fu-Tsai, Hung-Yu Huang, Chun-Yu Lo, Yu-Chen Huang, Chang-Wei Lin, Chih-Chen He, Jung-Ru He, Te-Fang Sheng, and Chun-Hua Wang. 2019. "Increased Ratio of Matrix Metalloproteinase-9 (MMP-9)/Tissue Inhibitor Metalloproteinase-1 from Alveolar Macrophages in Chronic Asthma with a Fast Decline in FEV1 at 5-Year Follow-up" Journal of Clinical Medicine 8, no. 9: 1451. https://doi.org/10.3390/jcm8091451
APA StyleChung, F.-T., Huang, H.-Y., Lo, C.-Y., Huang, Y.-C., Lin, C.-W., He, C.-C., He, J.-R., Sheng, T.-F., & Wang, C.-H. (2019). Increased Ratio of Matrix Metalloproteinase-9 (MMP-9)/Tissue Inhibitor Metalloproteinase-1 from Alveolar Macrophages in Chronic Asthma with a Fast Decline in FEV1 at 5-Year Follow-up. Journal of Clinical Medicine, 8(9), 1451. https://doi.org/10.3390/jcm8091451





