FLAMSA-RIC for Stem Cell Transplantation in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
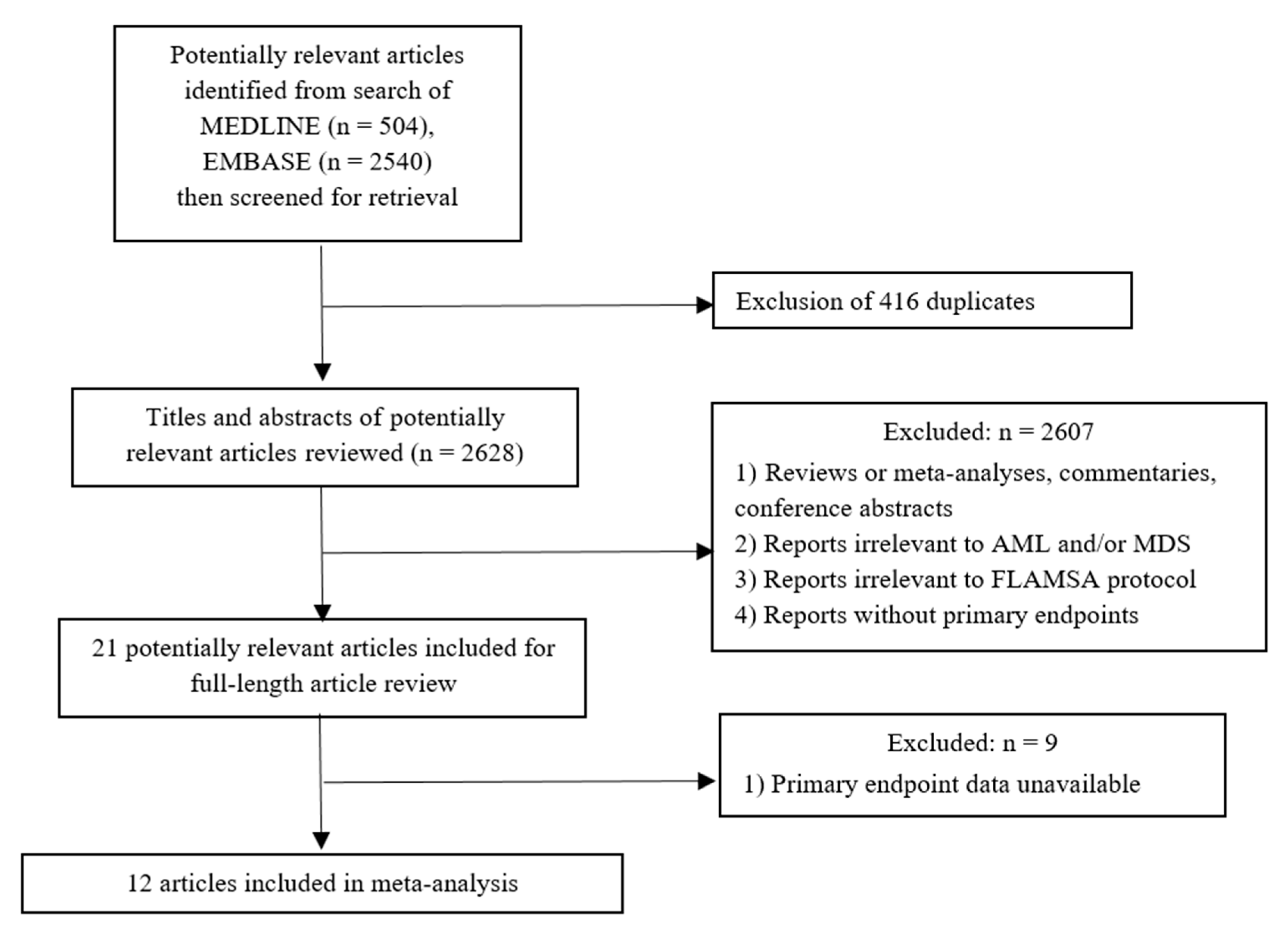
2.2. Selection Criteria and Data Extraction
2.3. Definition of Treatment Response and Outcome
2.4. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. FLAMSA Variations, Stem Sources, and GVHD Prophylaxis
3.3. Survival Outcome
3.4. Complications of HSCT
3.5. Subgroup Analysis
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| Abbreviation | Full Name |
| aGVHD | acute graft-versus-host disease; |
| AML | acute myeloid leukemia |
| Bu | busulfan |
| CI | confidence interval |
| cGVHD | chronic graft-versus-host disease |
| CR | complete remission |
| Cy | cyclophosphamide |
| CyA | cyclosporine A |
| Flu | fludarabine |
| GVHD | graft-versus-host disease |
| HLA | human leukocyte antigen |
| HSCT | hematopoietic stem cell transplantation |
| LFS | leukemia-free survival |
| MAC | myeloablative conditioning |
| MDS | MMUD mismatched unrelated donors |
| MRD | matched related donor |
| MUD | matched unrelated donors |
| NRM | non-relapse mortality |
| OS | overall survival |
| rATG | rabbit anti-thymocyte globulin |
| RIC | reduced-intensity conditioning |
| RR | relapse rate |
| TBI | total body irradiation |
References
- Slavin, S.; Nagler, A.; Naparstek, E.; Kapelushnik, Y.; Aker, M.; Cividalli, G.; Varadi, G.; Kirschbaum, M.; Ackerstein, A.; Samuel, S.; et al. Non-MA stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998, 91, 756–763. [Google Scholar] [PubMed]
- Wais, V.; Kundgen, L.; Bohl, S.R.; von Harsdorf, S.; Schlenk, R.F.; Dohner, K.; Teleanu, V.; Bullinger, L.; Nguyen, T.M.; Drognitz, K.; et al. Reduced-toxicity conditioning for allogeneic hematopoietic cell transplantation in elderly or comorbid patients with AML using fludarabine, BCNU and melphalan: Disease stage at transplant determines outcome. Bone Marrow Transplant. 2018, 53, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Schleuning, M.; Ledderose, G.; Tischer, J.; Kolb, H.-J. Sequential Regimen of Chemotherapy, Reduced-Intensity Conditioning for Allogeneic Stem-Cell Transplantation, and Prophylactic Donor Lymphocyte Transfusion in High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. J. Clin. Oncol. 2005, 23, 5675–5687. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Schleuning, M.; Schwerdtfeger, R.; Hertenstein, B.; Mischak-Weissinger, E.; Bunjes, D.; Harsdorf, S.V.; Scheid, C.; Holtick, U.; Greinix, H.; et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006, 108, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Schleuning, M.; Hentrich, M.; Markl, G.E.; Gerbitz, A.; Tischer, J.; Ledderose, G.; Oruzio, D.; Hiddemann, W.; Kolb, H.-J. High antileukemic efficacy of an intermediate intensity conditioning regimen for allogeneic stem cell transplantation in patients with high-risk acute myeloid leukemia in first complete remission. Bone Marrow Transplant. 2008, 41, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Holtick, U.; Shimabukuro-Vornhagen, A.; Chakupurakal, G.; Theurich, S.; Leitzke, S.; Burst, A.; Hallek, M.; von Bergwelt-Baildon, M.; Scheid, C.; Chemnitz, J.M. FLAMSA reduced-intensity conditioning is equally effective in AML patients with primary induction failure as well as in first or second complete remission. Eur. J. Haematol. 2016, 96, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Krejci, M.; Doubek, M.; Dušek, J.; Brychtova, Y.; Racil, Z.; Navrátil, M.; Tomiska, M.; Horky, O.; Pospisilova, S.; Mayer, J. Combination of fludarabine, amsacrine, and cytarabine followed by reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation in patients with high-risk acute myeloid leukemia. Ann. Hematol. 2013, 92, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Bohl, S.; Von Harsdorf, S.; Mulaw, M.; Hofmann, S.; Babiak, A.; Maier, C.P.; Schnell, J.; Hütter-Krönke, L.-M.; Scholl, K.; Wais, V.; et al. Strong impact of extramedullary involvement in high-risk AML patients with active disease receiving the FLAMSA conditioning regimen for HSCT. Bone Marrow Transplant. 2016, 51, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Saure, C.; Schroeder, T.; Zohren, F.; Groten, A.; Bruns, I.; Czibere, A.; Galonska, L.; Kondakci, M.; Weigelt, C.; Fenk, R.; et al. Upfront Allogeneic Blood Stem Cell Transplantation for Patients with High-Risk Myelodysplastic Syndrome or Secondary Acute Myeloid Leukemia Using a FLAMSA-Based High-Dose Sequential Conditioning Regimen. Biol. Blood Marrow Transplant. 2012, 18, 466–472. [Google Scholar] [CrossRef][Green Version]
- Schneidawind, D.; Federmann, B.; Faul, C.; Vogel, W.; Kanz, L.; Bethge, W.A. Allogeneic hematopoietic cell transplantation with reduced-intensity conditioning following FLAMSA for primary refractory or relapsed acute myeloid leukemia. Ann. Hematol. 2013, 92, 1389–1395. [Google Scholar] [CrossRef]
- Pfrepper, C.; Klink, A.; Behre, G.; Schenk, T.; Franke, G.N.; Jentzsch, M.; Schwind, S.; Al-Ali, H.K.; Hochhaus, A.; Niederwieser, D.; et al. Risk factors for outcome in refractory acute myeloid leukemia patients treated with a combination of fludarabine, cytarabine, and amsacrine followed by a reduced-intensity conditioning and allogeneic stem cell transplantation. J. Cancer Res. Clin. Oncol. 2016, 142, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ringdén, O.; Labopin, M.; Schmid, C.; Sadeghi, B.; Polge, E.; Tischer, J.; Ganser, A.; Michallet, M.; Kanz, L.; Schwerdtfeger, R.; et al. Sequential chemotherapy followed by reduced-intensity conditioning and allogeneic haematopoietic stem cell transplantation in adult patients with relapse or refractory acute myeloid leukaemia: A survey from the Acute Leukaemia Working Party of EBMT. Br. J. Haematol. 2017, 176, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Labopin, M.; Stuhler, G.; Bittenbring, J.; Ganser, A.; Tischer, J.; Michallet, M.; Kröger, N.; Schmid, C.; Huynh, A.; et al. Sequential Intensified Conditioning Regimen Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients with Intermediate- or High-Risk Acute Myeloid Leukemia in Complete Remission: A Study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 278–284. [Google Scholar] [PubMed]
- Heinicke, T.; Labopin, M.; Schmid, C.; Polge, E.; Socié, G.; Blaise, D.; Mufti, G.J.; Huynh, A.; Brecht, A.; LeDoux, M.-P.; et al. Reduced Relapse Incidence with FLAMSA–RIC Compared with Busulfan/Fludarabine for Acute Myelogenous Leukemia Patients in First or Second Complete Remission: A Study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 2224–2232. [Google Scholar] [PubMed]
- Sheth, V.; Labopin, M.; Canaani, J.; Volin, L.; Brecht, A.; Ganser, A.; Mayer, J.; Labussière-Wallet, H.; Bittenbring, J.; Shouval, R.; et al. Comparison of FLAMSA-based reduced intensity conditioning with treosulfan/fludarabine conditioning for patients with acute myeloid leukemia: An ALWP/EBMT analysis. Bone Marrow Transplant. 2019, 54, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Saraceni, F.; Labopin, M.; Brecht, A.; Kröger, N.; Eder, M.; Tischer, J.; Labussière-Wallet, H.; Einsele, H.; Beelen, D.; Bunjes, D.; et al. Fludarabine-treosulfan compared to thiotepa-busulfan-fludarabine or FLAMSA as conditioning regimen for patients with primary refractory or relapsed acute myeloid leukemia: A study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). J. Hematol. Oncol. 2019, 12, 44. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Mathes, T.; Kuss, O. A comparison of methods for meta-analysis of a small number of studies with binary outcomes. Res. Synth. Methods 2018, 9, 366–381. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Bhatt, V.R.; Chen, B.; Gyawali, B.; Lee, S.J. Socioeconomic and health system factors associated with lower utilization of hematopoietic cell transplantation in older patients with acute myeloid leukemia. Bone Marrow Transplant. 2018, 53, 1288–1294. [Google Scholar] [CrossRef]
- De Lima, M.; Anagnostopoulos, A.; Munsell, M.; Shahjahan, M.; Ueno, N.; Ippoliti, C.; Andersson, B.S.; Gajewski, J.; Couriel, D.; Cortes, J.; et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: Dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood 2004, 104, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.L.; Pasquini, M.C.; Logan, B.R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.D.; Fernandez, H.F.; Alyea, E.P.; et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2017, 35, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Bornhäuser, M.; Kienast, J.; Trenschel, R.; Burchert, A.; Hegenbart, U.; Stadler, M.; Baurmann, H.; Schäfer-Eckart, K.; Holler, E.; Kröger, N.; et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: A prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012, 13, 1035–1044. [Google Scholar] [CrossRef]
- Eapen, M.; Brazauskas, R.; Hemmer, M.; Perez, W.S.; Steinert, P.; Horowitz, M.M.; Deeg, H.J. Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic syndrome: Conditioning regimen intensity. Blood Adv. 2018, 2, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Wattad, M.; Amlsg, F.T.G.-A.; Weber, D.; Döhner, K.; Krauter, J.; Gaidzik, V.I.; Paschka, P.; Heuser, M.; Thol, F.; Kindler, T.; et al. Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia 2017, 31, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.D.; Zhang, M.J.; Wang, H.L.; Akpek, G.; Copelan, E.A.; Freytes, C.; Gale, R.P.; Hamadani, M.; Inamoto, Y.; Kamble, R.T.; et al. Allogeneic hematopoietic cell transplant for AML: No impact of pre-transplant extramedullary disease on outcome. Bone Marrow Transplant. 2015, 50, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Lian, X.-Y.; Yao, D.-M.; He, P.-F.; Ma, J.-C.; Xu, Z.-J.; Guo, H.; Zhang, W.; Lin, J.; Qian, J. Reduced intensity conditioning of allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia in patients older than 50 years of age: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1853–1864. [Google Scholar] [CrossRef] [PubMed]





| References | No. | Sex (M/F) | Median Age (Years, Range) | Diseases | Disease Status | Stem Cell Source | Donor Source | CD34+ (×106 cells/kg) | Study Period | Median Follow Up (Months, Range) | Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Schmid et al. (2005) [4] | 75 | 42/33 | 52.3 (18.5–65.8) | 50 dAML, 15 sAML, 10 MDS | 8 CR1, 8 CR2, 49 R/R, 10 MDS | 61 PBSC, 14 BM | 31 MRD, 30 MUD 6 MMRD, 8 MMUD | 9.6 | 1999–2002 | 31.5 (13.6–47.6) | P |
| Saure et al. (2012) [9] | 30 | 20/10 | 49 (36–66) | 10 sAML, 20 MDS | 10 untreated AML, 20 MDS | 30 PBSC | 13 MRD, 13 MUD 4 MMUD | 7.7 | 2003–2010 | 28 (7–81) | P |
| Krejci et al. (2013) [7] | 60 | 28/32 | 52 (20–63) | 50 dAML, 10 sAML | 34 CR1, 26 R/R | 56 PBSC, 4 BM | 15 MRD, 29 MUD, 16 MMUD | 6.3 | 2006–2011 | 37 (10–69) | P |
| Schneidawind et al. (2013) [10] | 62 | 34/28 | 55 (20–72) | 35 dAML, 27 sAML | 62 R/R | 62 PBSC | 11 MRD, 22 MUD 4 MMRD, 25 MMUD | 5.4 | 2005–2012 | 17.5 (2.2–77.6) | R |
| Bohl et al. (2016) [8] | 84 | 46/38 | 48.7 | 67 dAML, 17 sAML | 13 CR1, 12 CR2, 59 R/R | NR | NR | NR | 2000–2012 | NR | R |
| Holtick et al. (2016) [6] | 130 | 59/71 | 50.9 (19–73) | NR | 47 CR1, 26 CR2, 57 R/R | 127 PBSC, 3 BM | 42 MRD, 64 MUD 1 MMRD, 23MMUD | 7.07 | 2004–2015 | 37 (10–125) | R |
| Pfrepper et al. (2016) [11] | 44 | 25/19 | 52 (21–65) | NR | 44 R/R | 44 PBSC | 3 MRD, 27 MUD, 14 MMUD | NR | 2006–2013 | 34 (6–71) | R |
| Ringden et al. (2016) [12] | 267 | 131/136 | 51.7 (19.4–72.5) | NR | 267 R/R | 256 PBSC, 11 BM | 77 MRD, 190 MUD | NR | NR | 68.2 (2–157) | R |
| Malard et al. (2017) [13] | 265 | 143/122 | 55 (19–76) | 156 dAML, 109 sAML | 216 CR1, 49 CR2 | 251 PBSC, 14 BM | 74 MRD, 191 MUD | NR | 2002–2014 | 46 (1–145) | R |
| Heinicke et al. (2018) [14] | 399 | 206/193 | (18–74.4) | NR | 305 CR1, 94 CR2 | 379 PBSC, 20 BM | 139 MRD, 198 MUD, 62 MMUD | NR | 2005–2016 | (0.7–121.5) | R |
| Sheth et al. (2019) [15] | 348 | 179/169 | (40.1–65) | 294 dAML, 54 sAML | 264 CR1, 84 CR2 | 330 PBSC, 18 BM | 113 MRD, 182 MUD, 53 MMUD | NR | 2007–2016 | NR | R |
| Saraceni et al. (2019) [16] | 631 | 336/295 | 51.5 (18.1–76) | NR | 631 RR | 616 PBSC, 15 BM | 252 MRD, 268 MUD, 111 MMUD | NR | 2005–2016 | 53 (4–35) | R |
| Number of Patients (N = 2395) | Percent (%) or Range | ||
|---|---|---|---|
| Sex | Male | 1249 | 52.2 |
| Female | 1146 | 47.8 | |
| Age range in years | - | 18.1–6.0 | |
| Diseases (n = 924) | dAML | 652 | 70.6 |
| sAML | 242 | 26.2 | |
| MDS | 30 | 3.2 | |
| Disease status (n = 2395) | CR1 | 887 | 37.0 |
| CR2 | 273 | 11.4 | |
| R/R | 1195 | 49.9 | |
| Untreated AML | 10 | 0.4 | |
| High-risk MDS | 30 | 1.3 | |
| Stem cell source (n = 2311) | PBSC | 2212 | 95.7 |
| BM | 99 | 4.3 | |
| Donor source (n = 2311) | MRD | 770 | 33.3 |
| MUD | 1214 | 52.5 | |
| MMRD | 11 | 0.5 | |
| MMUD | 316 | 13.7 | |
| CD 34+ in 106 cells/kg (n = 357) | - | 1.2–23.1 | |
| Follow up duration in months (n = 933) | - | 0.7–145 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owattanapanich, W.; Ungprasert, P.; Wais, V.; Kungwankiattichai, S.; Bunjes, D.; Kuchenbauer, F. FLAMSA-RIC for Stem Cell Transplantation in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1437. https://doi.org/10.3390/jcm8091437
Owattanapanich W, Ungprasert P, Wais V, Kungwankiattichai S, Bunjes D, Kuchenbauer F. FLAMSA-RIC for Stem Cell Transplantation in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(9):1437. https://doi.org/10.3390/jcm8091437
Chicago/Turabian StyleOwattanapanich, Weerapat, Patompong Ungprasert, Verena Wais, Smith Kungwankiattichai, Donald Bunjes, and Florian Kuchenbauer. 2019. "FLAMSA-RIC for Stem Cell Transplantation in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 8, no. 9: 1437. https://doi.org/10.3390/jcm8091437
APA StyleOwattanapanich, W., Ungprasert, P., Wais, V., Kungwankiattichai, S., Bunjes, D., & Kuchenbauer, F. (2019). FLAMSA-RIC for Stem Cell Transplantation in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 8(9), 1437. https://doi.org/10.3390/jcm8091437





