Vascular Patterns in Retinitis Pigmentosa on Swept-Source Optical Coherence Tomography Angiography
Abstract
1. Introduction
2. Methods
2.1. Ophthalmologic Assessment
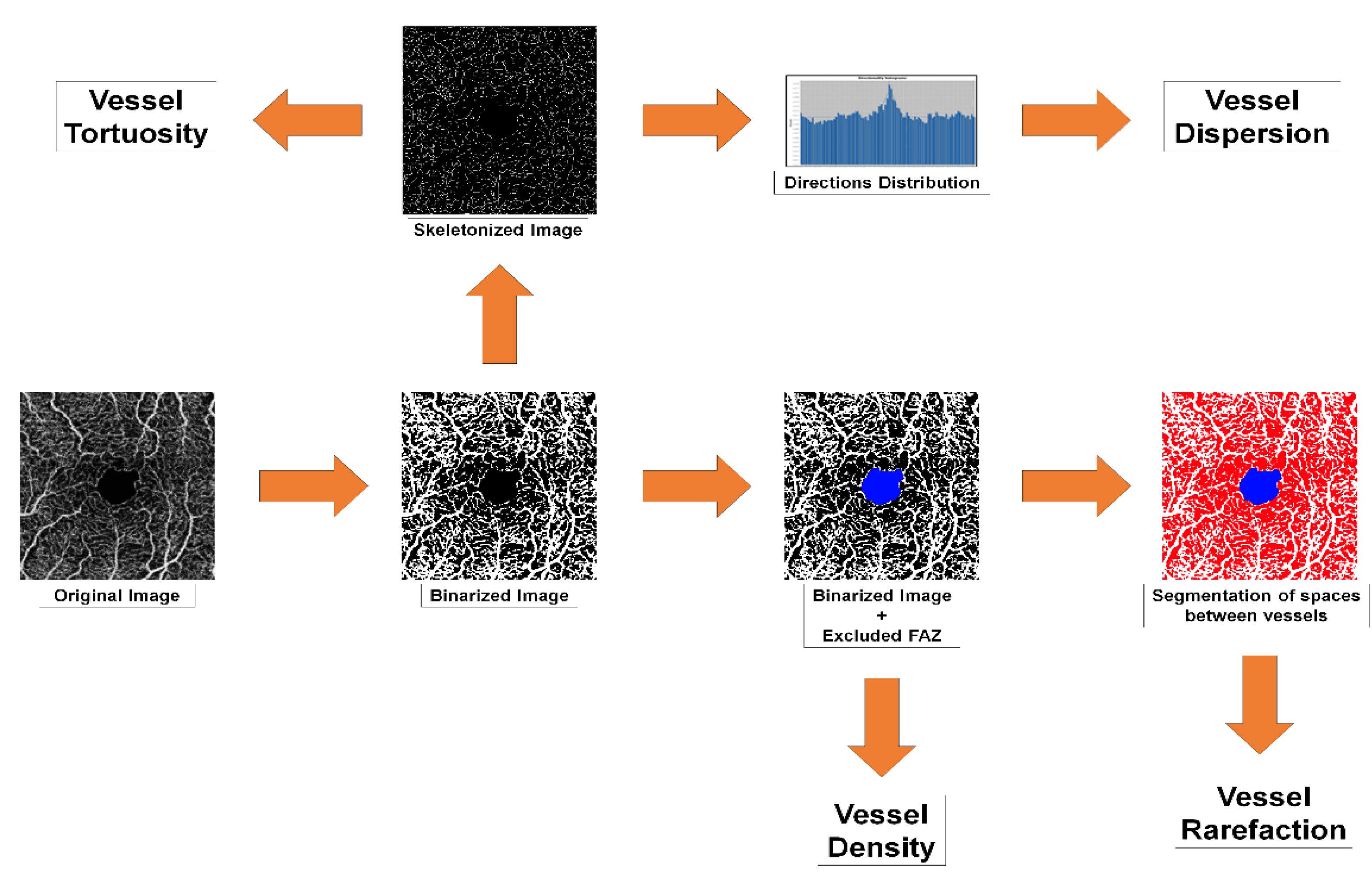
2.2. OCTA Quantitative Analyses
2.3. Statistical Analyses
3. Results
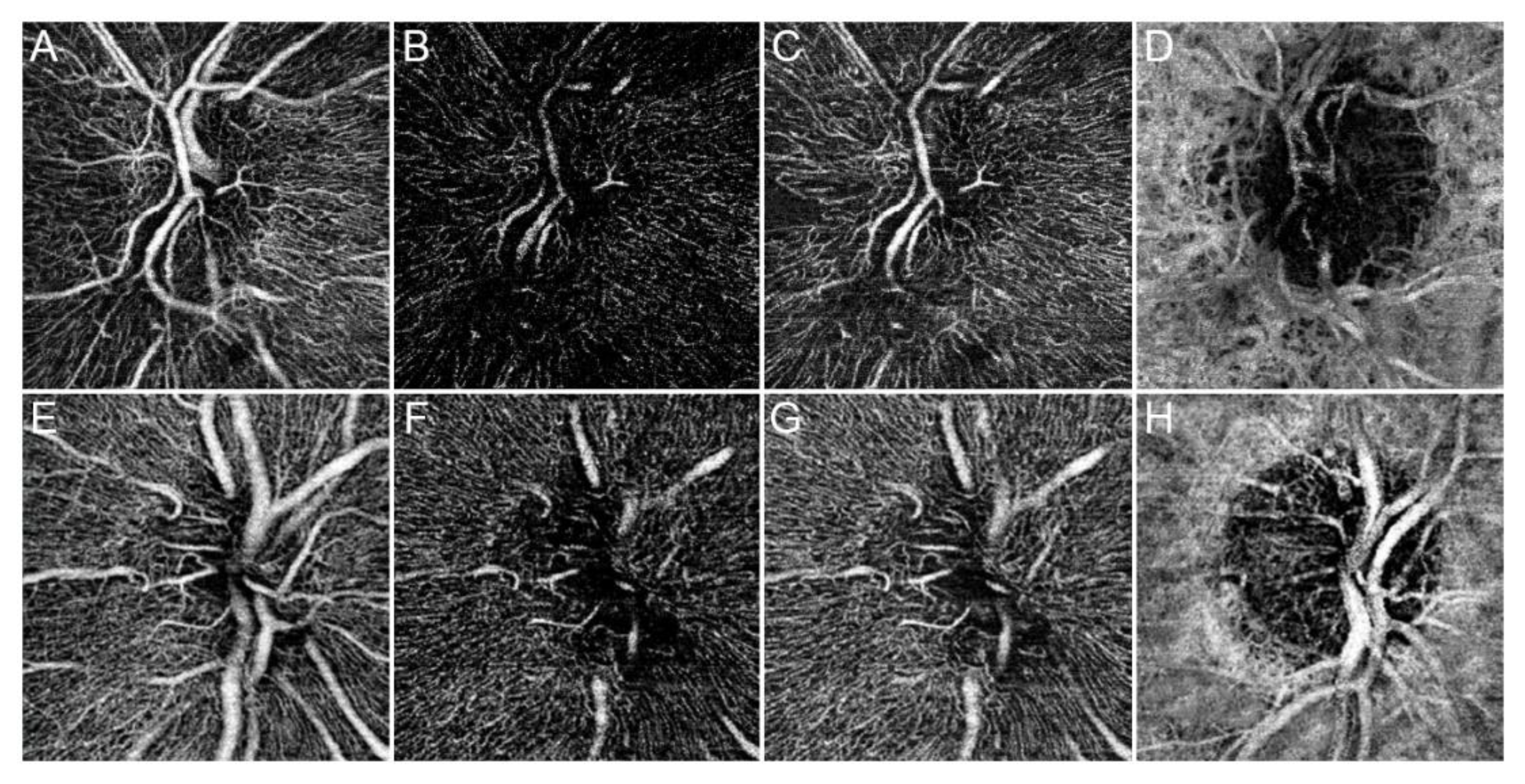
3.1. Main Results
3.2. Cut-Off Analysis to Identify Different RP Subgroups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Dyonne, T.; Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar]
- Marigo, V. Programmed cell death in retinal degeneration: Targeting apoptosis in photoreceptors as potential therapy for retinal degeneration. Cell Cycle 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Anselmi, G.M.; Marangoni, D.; D’Esposito, F.; Fadda, A.; Di Renzo, A.; Campos, E.C.; Riva, C.E. Subfoveal choroidal blood flow and central retinal function in retinitis pigmentosa. IOVS 2011, 5, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Iacono, P.; Battaglia Parodi, M.; La Spina, C.; Zerbini, G.; Bandello, F. Dynamic and static vessel analysis in patients with retinitis pigmentosa: A pilot study of vascular diameters and functionality. Retina 2017, 37, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Peng, J.; Ying, D.; Peng, Q.H. A Brief Review on the Pathological Role of Decreased Blood Flow Affected in Retinitis Pigmentosa. J. Ophthalmol. 2018, 2018, 3249064. [Google Scholar] [CrossRef] [PubMed]
- Battaglia Parodi, M.; Cicinelli, M.V.; Rabiolo, A.; Pierro, L.; Gagliardi, M.; Bolognesi, G.; Bandello, F. Vessel density analysis in patients with retinitis pigmentosa by means of optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 428–432. [Google Scholar] [CrossRef]
- Toto, L.; Borrelli, E.; Mastropasqua, R.; Senatore, A.; Di Antonio, L.; Di Nicola, M.; Carpineto, P.; Mastropasqua, L. Macular Features in Retinitis Pigmentosa: Correlations Among Ganglion Cell Complex Thickness, Capillary Density, and Macular Function. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6360–6366. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Borrelli, E.; Agnifili, L.; Toto, L.; Di Antonio, L.; Senatore, A.; Palmieri, M.; D’Uffizi, A.; Carpineto, P. Radial Peripapillary Capillary Network in Patients with Retinitis Pigmentosa: An Optical Coherence Tomography Angiography Study. Front. Neurol. 2017, 8, 572. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Ghasemi Falavarjani, K.; Akil, H.; Sadda, S.R. Impact of image quality on OCT angiography based quantitative measurements. Int. J. Retina Vitreous 2017, 13. [Google Scholar] [CrossRef]
- Bengtsson, B.; Heijl, A. A visual field index for calculation of glaucoma rate of progression. Am. J. Ophthalmol. 2008, 145, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Fernández-González, R.; Muñoz-Barrutia, A.; Ortiz-De-Solorzano, C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 2010, 73, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Grisan, E.; Foracchia, M.; Ruggeri, A. A novel method for the automatic grading of retinal vessel tortuosity. IEEE Trans. Med. Imaging 2008, 27, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S. Measures of dispersion. J. Pharmacol. Pharmacother. 2011, 2, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q. Scale space approach to directional analysis of images. Appl. Opt. 1991, 30, 369–373. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C.G., Jr. DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef]
- D’Amore, A.; Stella, J.A.; Wagner, W.R.; Sacks, M.S. Characterization of the complete fiber network topology of planar fibrous tissues and scaffolds. Biomaterials 2010, 31, 5345–5354. [Google Scholar] [CrossRef]
- Tomba, E.; Facco, P.; Roso, M.; Modesti, M.; Bezzo, F.; Barolo, M. Artificial Vision System for the Automatic Measurement of Interfiber Pore Characteristics and Fiber Diameter Distribution in Nanofiber Assemblies. Ind. Eng. Chem. Res. 2010, 49, 2957–2968. [Google Scholar] [CrossRef]
- Arrigo, A.; Bandello, F.; Battaglia Parodi, M.; IRCCS Ospedale San Raffaele, University Vita-Salute, Milan, Italy. Reproducibility and repeatability optical coherence tomography angiography coefficients. 2019; Unpublished work. [Google Scholar]
- Battaglia Parodi, M.; La Spina, C.; Triolo, G.; Riccieri, F.; Pierro, L.; Gagliardi, M.; Bandello, F. Correlation of SD-OCT findings and visual function in patients with retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1275–1279. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Capone, L.; Pierro, L.; Romano, F.; Bandello, F.; Parodi, M.B. Advanced Optical Coherence Tomography Angiography Analysis of Age-related Macular Degeneration Complicated by Onset of Unilateral Choroidal Neovascularization. Am. J. Ophthalmol. 2018, 195, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kylstra, J.A.; Wierzbicki, T.; Wolbarsht, M.L.; Landers, M.B., 3rd; Stefansson, E. The relationship between retinal vessel tortuosity, diameter, and transmural pressure. Graefes Arch. Clin. Exp. Ophthalmol. 1986, 224, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Wang, J.J.; Cheung, N.; Zhang, Y.P.; Hsu, W.; Lee, M.L.; Mitchell, P.; Tikellis, G.; Taylor, B.; Wong, T.Y. The retinal vasculature as a fractal: Methodology, reliability, and relationship to blood pressure. Ophthalmology 2008, 115, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Massof, R.W.; Finkelstein, D. A two-Stage Hypothesis for the Natural Course of Retinitis Pigmentosa. In Research in Retinitis Pigmentosa; Zrenner, E., Krastel, H., Goebel, H.-H., Eds.; Advances in the Biosciences; Pergamon Press: Oxford, UK, 1987; Volume 62, pp. 29–58. [Google Scholar]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in optical coherence tomography angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Clinical Features | ||
|---|---|---|
| Parameter | Retinitis Pigmentosa Patients | Controls |
| Sex (M/F) | 16/16 | 16/16 |
| Age | 43.2 ± 12.5 | 42.8 ± 11.2 |
| BCVA | 0.21 ± 0.34 | 0.0 ± 0.0 |
| CMT | 231.49 ± 28.22 | 301.52 ± 18.55 |
| CT | 214.31 ± 41.03 | 255.85 ± 25.79 |
| RNFL | 82.53 ± 19.77 | 101.21 ± 9.15 |
| Genetic Analysis in Retinitis Pigmentosa | ||
|---|---|---|
| Gene | Number of Patients | % |
| ABCA4 | 6 | 18.75 |
| USH2A | 8 | 25 |
| PROM1 | 3 | 9.375 |
| CYP4V2 | 2 | 6.25 |
| NR2E3 | 1 | 3.125 |
| PDE6A | 1 | 3.125 |
| RP1L1 | 1 | 3.125 |
| RPGR | 1 | 3.125 |
| CNGA1 | 1 | 3.125 |
| CNGB1 | 1 | 3.125 |
| FSCN2 | 1 | 3.125 |
| BBS1 | 1 | 3.125 |
| C2ORF71 | 1 | 3.125 |
| MYO7A | 1 | 3.125 |
| CEPB90 | 1 | 3.125 |
| EYE | 1 | 3.125 |
| EYS | 1 | 3.125 |
| OCTA Parameters in Retinitis Pigmentosa | ||||||
|---|---|---|---|---|---|---|
| Vessel Density Analysis | ||||||
| Vascular Plexus | mSCP | p Value | mDCP | p Value | mCC | p Value |
| RP | 0.39 ± 0.02 | p < 0.01 | 0.36 ± 0.03 | p < 0.01 | 0.49 ± 0.01 | p < 0.01 |
| Controls | 0.41 ± 0.01 | 0.43 ± 0.01 | 0.50 ± 0.01 | |||
| Vessel Dispersion Analysis | ||||||
| Vascular Plexus | mSCP | p Value | mDCP | p Value | ||
| RP Patients | 24 ± 15 | p < 0.01 | 16 ± 12 | p < 0.01 | ||
| Controls | 11 ± 4 | 11 ± 3 | ||||
| Vessel Tortuosity Analysis | ||||||
| Vascular Plexus | mSCP | p Value | mDCP | p Value | ||
| RP Patients | 4.80 ± 0.29 | p < 0.01 | 4.42 ± 0.49 | p < 0.01 | ||
| Controls | 7.20 ± 0.31 | 7.84 ± 0.34 | ||||
| Vessel Rarefaction Analysis | ||||||
| Vascular Plexus | mSCP | p Value | mDCP | p Value | ||
| RP Patients | 0.66 ± 0.04 | p < 0.01 | 0.62 ± 0.03 | p < 0.01 | ||
| Controls | 1.80 ± 0.32 | 1.09 ± 0.20 | ||||
| Correlation Analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VD Mean | Vdisp Mean | |||||||||||||
| AGE | Tau Coeff. | −0.282 | 0.286 | |||||||||||
| p value | 0.02 | 0.02 | ||||||||||||
| CMT | BCVA (logMAR) | VD mSCP | VD mDCP | VD mCC | VD Mean | Vdisp Mean | VT mSCP | VT mDCP | VT Mean | VR mSCP | VR mDCP | VR Mean | ||
| RNFL | Tau Coeff. | 0.375 | −0.548 | 0.529 | 0.255 | 0.44 | 0.578 | −0.368 | 0.287 | 0.376 | 0.448 | −0.392 | −0.396 | −0.481 |
| p value | <0.01 | <0.01 | <0.01 | 0.04 | <0.01 | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| BCVA (logMAR) | VD mSCP | VD mDCP | VD mCC | VD Mean | Vdisp mDCP | Vdisp Mean | VT mSCP | VT mDCP | VT Mean | VR mSCP | VR mDCP | VR Mean | ||
| CMT | Tau Coeff. | −0.673 | 0.52 | 0.313 | 0.516 | 0.479 | −0.451 | −0.447 | 0.568 | 0.354 | 0.576 | −0.564 | −0.601 | −0.625 |
| p value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| VD mSCP | VD mDCP | VD mCC | VD Mean | Vdisp mDCP | Vdisp Mean | VT mSCP | VT mDCP | VT Mean | VR mSCP | VR mDCP | VR Mean | |||
| BCVA (logMAR) | Tau Coeff. | −0.443 | −0.463 | −0.592 | −0.573 | 0.563 | 0.563 | −0.621 | −0.463 | −0.712 | 0.645 | 0.573 | 0.721 | |
| p value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||
| OCTA Cut-off Analysis | ||||
|---|---|---|---|---|
| Parameter | Mean ± STD | p Values | ||
| RNFL | RP1 | 96 ± 10 | RP1 vs. RP2 | <0.01 |
| RP2 | 62 ± 10 | RP1 vs. Controls | 0.286 | |
| Controls | 101 ± 9 | RP2 vs. Controls | <0.01 | |
| CMT | RP1 | 247 ± 21 | RP1 vs. RP2 | <0.01 |
| RP2 | 209 ± 23 | RP1 vs. Controls | <0.01 | |
| Controls | 302 ± 19 | RP2 vs. Controls | <0.01 | |
| BCVA (logMAR) | RP1 | 0.01 ± 0.04 | RP1 vs. RP2 | <0.01 |
| RP2 | 0.49 ± 0.38 | RP1 vs. Controls | 0.94 | |
| Controls | 0 ± 0 | RP2 vs. Controls | <0.01 | |
| VD mSCP | RP1 | 0.41 ± 0.02 | RP1 vs. RP2 | <0.01 |
| RP2 | 0.38 ± 0.01 | RP1 vs. Controls | 0.976 | |
| Controls | 0.41 ± 0.01 | RP2 vs. Controls | <0.01 | |
| VD mDCP | RP1 | 0.37 ± 0.03 | RP1 vs. RP2 | <0.01 |
| RP2 | 0.35 ± 0.02 | RP1 vs. Controls | <0.01 | |
| Controls | 0.43 ± 0.01 | RP2 vs. Controls | <0.01 | |
| VD mCC | RP1 | 0.50 ± 0.02 | RP1 vs. RP2 | <0.01 |
| RP2 | 0.47 ± 0.01 | RP1 vs. Controls | 0.768 | |
| Controls | 0.50 ± 0.01 | RP2 vs. Controls | <0.01 | |
| Vdisp mSCP | RP1 | 12.76 ± 3.71 | RP1 vs. RP2 | <0.01 |
| RP2 | 21.42 ± 15.77 | RP1 vs. Controls | 0.92 | |
| Controls | 10.72 ± 4.15 | RP2 vs. Controls | <0.01 | |
| Vdisp mDCP | RP1 | 13.66 ± 4.51 | RP1 vs. RP2 | <0.01 |
| RP2 | 34.75 ± 9.43 | RP1 vs. Controls | 0.53 | |
| Controls | 11.45 ± 3.48 | RP2 vs. Controls | <0.01 | |
| VT mSCP | RP1 | 5.16 ± 0.34 | RP1 vs. RP2 | <0.01 |
| RP2 | 4.56 ± 0.15 | RP1 vs. Controls | <0.01 | |
| Controls | 7.20 ± 0.31 | RP2 vs. Controls | <0.01 | |
| VT mDCP | RP1 | 4.86 ± 0.29 | RP1 vs. RP2 | <0.01 |
| RP2 | 4.23 ± 0.35 | RP1 vs. Controls | <0.01 | |
| Controls | 7.84 ± 0.34 | RP2 vs. Controls | <0.01 | |
| VR mSCP | RP1 | 0.62 ± 0.03 | RP1 vs. RP2 | <0.01 |
| RP2 | 0.70 ± 0.02 | RP1 vs. Controls | <0.01 | |
| Controls | 0.41 ± 0.01 | RP2 vs. Controls | <0.01 | |
| VR mDCP | RP1 | 0.59 ± 0.03 | RP1 vs. RP2 | <0.01 |
| RP2 | 0.65 ± 0.02 | RP1 vs. Controls | <0.01 | |
| Controls | 0.43 ± 0.01 | RP2 vs. Controls | <0.01 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrigo, A.; Romano, F.; Albertini, G.; Aragona, E.; Bandello, F.; Battaglia Parodi, M. Vascular Patterns in Retinitis Pigmentosa on Swept-Source Optical Coherence Tomography Angiography. J. Clin. Med. 2019, 8, 1425. https://doi.org/10.3390/jcm8091425
Arrigo A, Romano F, Albertini G, Aragona E, Bandello F, Battaglia Parodi M. Vascular Patterns in Retinitis Pigmentosa on Swept-Source Optical Coherence Tomography Angiography. Journal of Clinical Medicine. 2019; 8(9):1425. https://doi.org/10.3390/jcm8091425
Chicago/Turabian StyleArrigo, Alessandro, Francesco Romano, Giorgia Albertini, Emanuela Aragona, Francesco Bandello, and Maurizio Battaglia Parodi. 2019. "Vascular Patterns in Retinitis Pigmentosa on Swept-Source Optical Coherence Tomography Angiography" Journal of Clinical Medicine 8, no. 9: 1425. https://doi.org/10.3390/jcm8091425
APA StyleArrigo, A., Romano, F., Albertini, G., Aragona, E., Bandello, F., & Battaglia Parodi, M. (2019). Vascular Patterns in Retinitis Pigmentosa on Swept-Source Optical Coherence Tomography Angiography. Journal of Clinical Medicine, 8(9), 1425. https://doi.org/10.3390/jcm8091425






