Restless Leg Syndrome in Peripheral Artery Disease: Prevalence among Patients with Claudication and Benefits from Low-Intensity Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Settings
2.2. Variables Assessed upon Enrollment
2.3. Outcome Measures
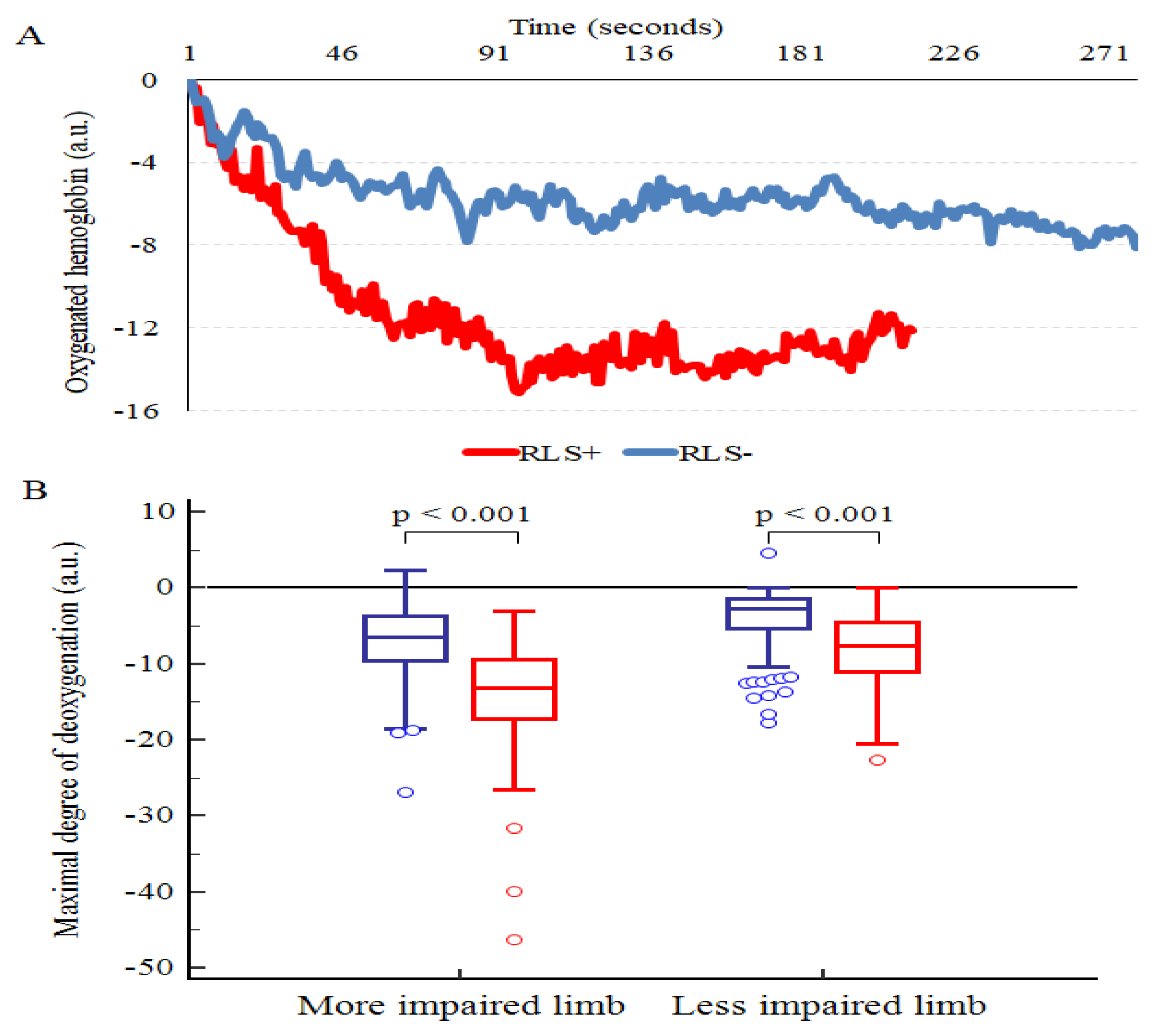
2.4. Exercise Program
2.5. RLS Progression throughout the Program
2.6. Statistical Analysis
3. Results
3.1. RLS Prevalence
3.2. RLS after the Exercise Program
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia-Borreguero, D.; Stillman, P.; Benes, H.; Buschmann, H.; Chaudhuri, K.R.; Gonzalez Rodríguez, V.M.; Högl, B.; Kohnen, R.; Monti, G.C.; Stiasny-Kolster, K.; et al. Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol. 2011, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Johansen, K.L.; Grimes, B.; Kaysen, G.A.; Dalrymple, L.S.; Kutner, N.G.; Chertow, G.M. Physical activity and self-reported symptoms of insomnia; restless legs syndrome; and depression: The comprehensive dialysis study. Hemodial. Int. 2013, 17, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.Y.; Kim, M.S.; Lee, S.M.; Hong, J.M.; Yoon, J.H. Impaired vascular endothelial function in patients with restless legs syndrome: A new aspect of the vascular pathophysiology. J. Neurol. Sci. 2015, 359, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, J.; Jiang, H.; Han, C.; Li, J.; Xu, X.; Zhang, G.; Lin, Z.; Xiong, N.; Wang, T. Restless Legs Syndrome: From Pathophysiology to Clinical Diagnosis and Management. Front. Aging Neurosci. 2017, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Borreguero, D.; Larrosa, O.; de la Llave, Y. Circadian aspects in the pathophysiology of the restless legs syndrome. Sleep Med. 2002, 3, S17–S21. [Google Scholar] [CrossRef]
- Michaud, M.; Dumont, M.; Paquet, J.; Desautels, A.; Fantini, M.L.; Montplaisir, J. Circadian variation of the effects of immobility on symptoms of restless legs syndrome. Sleep 2005, 28, 843–846. [Google Scholar] [CrossRef][Green Version]
- Duffy, J.F.; Lowe, A.S.; Silva, E.J.; Winkelman, J.W. Periodic limb movements in sleep exhibit a circadian rhythm that is maximal in the late evening/early night. Sleep Med. 2011, 12, 83–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daniele, T.M.; de Bruin, V.M.; e Forte, A.C.; de Oliveira, D.S.; Pompeu, C.M.; de Bruin, P.F. The relationship between physical activity; restless legs syndrome; and health-related quality of life in type 2 diabetes. Endocrine 2013, 44, 125–131. [Google Scholar] [CrossRef]
- Cuellar, N.G.; Ratcliffe, S.J. A comparison of glycemic control; sleep; fatigue; and depression in type 2 diabetes with and without restless legs syndrome. J. Clin. Sleep Med. 2008, 4, 50–56. [Google Scholar]
- Allen, R.P.; Walters, A.S.; Montplaisir, J.; Hening, W.; Myers, A.; Bell, T.J.; Ferini-Strambi, L. Restless legs syndrome prevalence and impact: REST general population study. Arch. Intern. Med. 2005, 165, 1286–1292. [Google Scholar] [CrossRef]
- Zhuang, S.; Na, M.; Winkelman, J.W.; Ba, D.; Liu, C.F.; Liu, G.; Gao, X. Association of Restless Legs Syndrome With Risk of Suicide and Self-harm. JAMA Netw. Open 2019, 2, e199966. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.; Hening, W.A.; Trenkwalder, C.; Walters, A.S.; Montplaisi, J.; Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group. Restless legs syndrome: Diagnostic criteria; special considerations; and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003, 4, 101–119. [Google Scholar] [CrossRef]
- Salminen, A.V.; Rimpilä, V.; Polo, O. Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease). Neurology 2014, 82, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G. Neurochemical features of idiopathic restless legs syndrome. Sleep Med. Rev. 2019, 45, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Trenkwalder, C.; Allen, R.; Högl, B.; Paulus, W.; Winkelmann, J. Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology 2016, 86, 1336–1343. [Google Scholar] [CrossRef]
- Han, S.H.; Park, K.Y.; Kim, J.M.; Youn, Y.C.; Shin, H.W. Restless legs syndrome is associated with arterial stiffness and clinical outcome in stroke patients. Sleep Med. 2019, 60, 219–223. [Google Scholar] [CrossRef]
- Szentkirályi, A.; Völzke, H.; Hoffmann, W.; Trenkwalder, C.; Berger, K. Multimorbidity and the risk of restless legs syndrome in 2 prospective cohort studies. Neurology 2014, 82, 2026–2033. [Google Scholar] [CrossRef]
- Calviño, J.; Cigarrán, S.; Lopez, L.M.; Martinez, A.; Sobrido, M.J. Restless legs syndrome in non-dialysis renal patients: Is it really that common? J. Clin. Sleep Med. 2015, 11, 57–60. [Google Scholar] [CrossRef][Green Version]
- Lanza, G.; Bachmann, C.G.; Ghorayeb, I.; Wang, Y.; Ferri, R.; Paulus, W. Central and peripheral nervous system excitability in restless legs syndrome. Sleep Med. 2017, 31, 49–60. [Google Scholar] [CrossRef]
- Butler, J.V.; Mulkerrin, E.C.; O’Keeffe, S.T. Nocturnal leg cramps in older people. Postgrad. Med. J. 2002, 78, 596–598. [Google Scholar] [CrossRef]
- King, A.C.; Pruitt, L.A.; Woo, S.; Castro, C.M.; Ahn, D.K.; Vitiello, M.V.; Woodward, S.H.; Bliwise, D.L. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- King, A.C.; Oman, R.F.; Brassington, G.S.; Bliwise, D.L.; Haskell, W.L. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA 1997, 277, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Aukerman, M.M.; Aukerman, D.; Bayard, M.; Tudiver, F.; Thorp, L.; Bailey, B. Exercise and restless legs syndrome: A randomized controlled trial. J. Am. Board Fam. Med. 2006, 19, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.J.; Jones, P.W.; Pearce, V.R. Leg cramps in the elderly: Prevalence; drug and disease associations. Int. J. Clin. Pract. 1999, 53, 494–496. [Google Scholar] [CrossRef]
- Shiohira, S.; Yoshida, T.; Sugiura, H.; Yoshida, S.; Mitobe, M.; Shimada, K.; Ohba, T.; Tsuchiya, K.; Kabaya, T.; Nitta, K. Effect of the antiplatelet agent cilostazol on endovascular inflammatory biochemical parameters and the clinical symptoms of peripheral artery disease and restless legs syndrome in hemodialysis patients. Clin. Exp. Nephrol. 2011, 15, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; et al. ACC/AHA Task Force Members. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary. Vasc. Med. 2017, 22, NP1–NP43. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A. Sleep disorders; nocturnal blood pressure; and cardiovascular risk: A translational perspective. Auton. Neurosci. 2019, 218, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Zanigni, S.; Calandra-Buonaura, G.; Giannini, G.; Tonon, C.; Cortelli, P.; Provini, F. The association between restless legs syndrome; cardiovascular and metabolic diseases: Hypotheses and evidence from the literature. Arch. Ital. Biol. 2015, 153, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Malagoni, A.M.; Mascoli, F.; Mandini, S.; Taddia, M.C.; Basaglia, N.; Manfredini, R.; Conconi, F.; Zamboni, P. Training rather than walking: The test in -train out program for home-based rehabilitation in peripheral arteriopathy. Circ. J. 2008, 72, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Malagoni, A.M.; Vagnoni, E.; Felisatti, M.; Mandini, S.; Heidari, M.; Mascoli, F.; Basaglia, N.; Manfredini, R.; Zamboni, P.; Manfredini, F. Evaluation of patient compliance; quality of life impact and cost-effectiveness of a “test in-train out” exercise-based rehabilitation program for patients with intermittent claudication. Circ. J. 2011, 75, 2128–2134. [Google Scholar] [CrossRef]
- Malagoni, A.M.; Cavazza, S.; Ferraresi, G.; Grassi, G.; Felisatti, M.; Lamberti, N.; Basaglia, N.; Manfredini, F. Effects of a “test in-train out” walking program versus supervised standard rehabilitation in chronic stroke patients: A feasibility and pilot randomized study. Eur. J. Phys. Rehabil. Med. 2016, 52, 279–287. [Google Scholar] [PubMed]
- Lamberti, N.; Straudi, S.; Malagoni, A.M.; Argirò, M.; Felisatti, M.; Nardini, E.; Zambon, C.; Basaglia, N.; Manfredini, F. Effects of low-intensity endurance and resistance training on mobility in chronic stroke survivors: A pilot randomized controlled study. Eur. J. Phys. Rehabil. Med. 2017, 53, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Malagoni, A.M.; Catizone, L.; Mandini, S.; Soffritti, S.; Manfredini, R.; Boari, B.; Russo, G.; Basaglia, N.; Zamboni, P.; Manfredini, F. Acute and long-term effects of an exercise program for dialysis patients prescribed in hospital and performed at home. J. Nephrol. 2008, 21, 871–878. [Google Scholar] [PubMed]
- Manfredini, F.; Mallamaci, F.; D’Arrigo, G.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-Rey, L.; et al. Exercise in Patients on Dialysis: A Multicenter; Randomized Clinical Trial. J. Am. Soc. Nephrol. 2017, 28, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Malagoni, A.M.; Mandini, S.; Felisatti, M.; Mascoli, F.; Basaglia, N.; Manfredini, R.; Mikhailidis, D.P.; Zamboni, P. Near-infrared spectroscopy assessment following exercise training in patients with intermittent claudication and in untrained healthy participants. Vasc. Endovascular. Surg. 2012, 46, 315–324. [Google Scholar] [CrossRef]
- Manfredini, F.; Lamberti, N.; Malagoni, A.M.; Felisatti, M.; Zuccalà, A.; Torino, C.; Tripepi, G.; Catizone, L.; Mallamaci, F.; Zoccali, C. The role of deconditioning in the end-stage renal disease myopathy: Physical exercise improves altered resting muscle oxygen consumption. Am. J. Nephrol. 2015, 41, 329–336. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases; in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral; mesenteric; renal; upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Manfredini, F.; Conconi, F.; Malagoni, A.M.; Manfredini, R.; Mascoli, F.; Liboni, A.; Zamboni, P. Speed rather than distance: A novel graded treadmill test to assess claudication. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 303–309. [Google Scholar] [CrossRef]
- Manfredini, F.; Malagoni, A.M.; Felisatti, M.; Mandini, S.; Mascoli, F.; Manfredini, R.; Basaglia, N.; Zamboni, P. A dynamic objective evaluation of peripheral arterial disease by near-infrared spectroscopy. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 441–448. [Google Scholar] [CrossRef][Green Version]
- Montgomery, P.S.; Gardner, A.W. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J. Am. Geriatr. Soc. 1998, 46, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Szentkirályi, A.; Völzke, H.; Hoffmann, W.; Dörr, M.; Hense, H.W.; Berger, K. Ankle-brachial index and peripheral artery disease are not related to restless legs syndrome. Sleep Med. 2017, 35, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, M.; Sharma, S.K.; Chimoriya, R.; Das, G.C. Intradialytic Muscle Cramp and its Association with Peripheral Arterial Disease in End Stage Renal Disease Patients on Hemodialysis. JNMA J. Nepal. Med. Assoc. 2014, 52, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.P.; Adler, S.; Sietsema, K.E.; Amato, A.; Esler, A.; Hiatt, W.R. Peripheral arterial disease is not associated with an increased prevalence of intradialytic cramps in patients on maintenance hemodialysis. Am. J. Nephrol. 2002, 22, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, I.; Vainas, A.; Dardiotis, E.; Giannaki, C.D.; Gourli, P.; Papadopoulou, D.; Vakianis, P.; Patsidis, E.; Eleftheriadis, T.; Liakopoulos, V.; et al. Restless legs syndrome in hemodialysis patients: An epidemiologic survey in Greece. Sleep Med. 2013, 14, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.; Lins Cde, M.; Adeodato, V.G.; Quental, D.P.; de Bruin, P.F.; Montenegro, R.M., Jr.; de Bruin, V.M. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care 2005, 28, 2633–2636. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, J.A.; Abuaisha, F.; Geoghegan, M. Prevalence and forms of neuropathic morbidity in 800 diabetics. Ir. J. Med. Sci. 1994, 163, 132–135. [Google Scholar] [CrossRef]
- Merlino, G.; Fratticci, L.; Valente, M.; Del Giudice, A.; Noacco, C.; Dolso, P.; Cancelli, I.; Scalise, A.; Gigli, G.L. Association of restless legs syndrome in type 2 diabetes: A case-control study. Sleep 2007, 30, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Skomro, R.P.; Ludwig, S.; Salamon, E.; Kryger, M.H. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med. 2001, 2, 417–422. [Google Scholar] [CrossRef]
- Cho, Y.W.; Na, G.Y.; Lim, J.G.; Kim, S.H.; Kim, H.S.; Earley, C.J.; Allen, R.P. Prevalence and clinical characteristics of restless legs syndrome in diabetic peripheral neuropathy: Comparison with chronic osteoarthritis. Sleep Med. 2013, 14, 1387–1392. [Google Scholar] [CrossRef]
- Manfredini, F.; Lamberti, N.; Rossi, T.; Mascoli, F.; Basaglia, N.; Zamboni, P. A Toe Flexion NIRS assisted Test for Rapid Assessment of Foot Perfusion in Peripheral Arterial Disease: Feasibility, Validity, and Diagnostic Accuracy. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Roth, T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J. Psychosom. Res. 2002, 53, 547–554. [Google Scholar] [CrossRef]
- Bertisch, S.M.; Muresan, C.; Schoerning, L.; Winkelman, J.W.; Taylor, J.A. Impact of Restless Legs Syndrome on Cardiovascular Autonomic Control. Sleep 2016, 39, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Giannaki, C.D.; Sakkas, G.K.; Karatzaferi, C.; Maridaki, M.D.; Koutedakis, Y.; Hadjigeorgiou, G.M.; Stefanidis, I. Combination of Exercise Training and Dopamine Agonists in Patients with RLS on Dialysis: A Randomized; Double-Blind Placebo-Controlled Study. ASAIO J. 2015, 61, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.; Vahdatpour, B.; Ghasempour, A.; Taheri, D.; Shahidi, S.; Moeinzadeh, F.; Dolatkhah, B.; Dolatkhah, S. Aerobic exercise improves signs of restless leg syndrome in end stage renal disease patients suffering chronic hemodialysis. Sci. World J. 2013, 2013, 628142. [Google Scholar] [CrossRef] [PubMed]
- Gopaluni, S.; Sherif, M.; Ahmadouk, N.A. Interventions for chronic kidney disease-associated restless legs syndrome. Cochrane Database Syst. Rev. 2016, 11, CD010690. [Google Scholar] [CrossRef]
- Driver, H.S.; Taylor, S.R. Exercise and sleep. Sleep Med. Rev. 2000, 4, 387–402. [Google Scholar] [CrossRef]
- Phillips, B.; Young, T.; Finn, L.; Asher, K.; Hening, W.A.; Purvis, C. Epidemiology of restless legs symptoms in adults. Arch. Intern. Med. 2000, 160, 2137–2141. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Sakkas, G.K.; Karatzaferi, C.; Hadjigeorgiou, G.M.; Lavdas, E.; Kyriakides, T.; Koutedakis, Y.; Stefanidis, I. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: A six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol. 2013, 14, 194. [Google Scholar] [CrossRef]
- Forbes, S.C.; Little, J.P.; Candow, D.G. Exercise and nutritional interventions for improving aging muscle health. Endocrine 2012, 42, 29–38. [Google Scholar] [CrossRef]
- Von Spiczak, S.; Whone, A.L.; Hammers, A.; Asselin, M.C.; Turkheimer, F.; Tings, T.; Happe, S.; Paulus, W.; Trenkwalder, C.; Brooks, D.J. The role of opioids in restless legs syndrome: An [11C] diprenorphine PET study. Brain 2005, 128, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.S.; Winkelmann, J.; Trenkwalder, C.; Fry, J.M.; Kataria, V.; Wagner, M.; Sharma, R.; Hening, W.; Li, L. Long-term follow-up on restless legs syndrome patients treated with opioids. Mov. Disord. 2001, 16, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.S.; Ondo, W.G.; Zhu, W.; Le, W. Does the endogenous opiate system play a role in the Restless Legs Syndrome? A pilot post-mortem study. J. Neurol. Sci. 2009, 279, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, U.H. Nondrug-related aspect of treating Ekbom disease; formerly known as restless legs syndrome. Neuropsychiatr. Dis. Treat. 2011, 7, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.; Schredl, M.; Strobl, P.; Deuschle, M. Restless legs syndrome: Evidence for nocturnal hypothalamic-pituitary-adrenal system activation. Mov. Disord. 2010, 25, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Sieminski, M.; Partinen, M. Nocturnal systolic blood pressure is increased in restless legs syndrome. Sleep Breath. 2016, 20, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Ulu, S.M.; Ahsen, A.; Akcı, Ö.; Yaman, F.; Demir, K.; Yaman, G.; Yüksel, Ş.; Acartürk, G. The relationship between dipping-non-dipping arterial blood pressure pattern and frequency of restless leg syndrome with related factors. Anatol. J. Cardiol. 2015, 15, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Somers, V.K.; Punjabi, N.M.; Winkelman, J.W. Restless legs syndrome and cardiovascular disease: A research roadmap. Sleep Med. 2017, 31, 10–17. [Google Scholar] [CrossRef] [PubMed]
| Whole Population (n = 286) | |
|---|---|
| Age, years | 71 ± 9 |
| Males, n (%) | 219 (77) |
| BMI (kg·m−2) | 28 ± 5 |
| Risk factors, n (%) | |
| Smoking | 239 (84) |
| Hypertension | 246 (86) |
| Dyslipidemia | 215 (75) |
| Diabetes | 138 (48) |
| Familiarity for CVD | 26 (9) |
| Comorbidities, n (%) | |
| Coronary artery disease | 111 (39) |
| Lung disease | 49 (17) |
| Osteoarticular disorders | 71 (25) |
| Chronic kidney disease | 51 (18) |
| Cerebrovascular disease | 29 (10) |
| Lower limbs revascularizations | 78 (27) |
| Charlson Index | 6.3 ± 2.0 |
| Therapy, n (%) | |
| Anticoagulants | 32 (11) |
| Antiplatelet | 261 (91) |
| Anti-hypertensive | 246 (86) |
| Diuretics | 90 (31) |
| Statins | 212 (74) |
| Hypoglycemic agents and/or insulin | 138 (48) |
| RLS-treatment drugs | 18 (6) |
| Levodopa | 1 (0.00) |
| Ropinirole | 0 (0) |
| Pramipexole | 2 (0.01) |
| Rotigotine | 0 (0) |
| Pregabalin | 6 (2) |
| Clonazepam | 3 (0.01) |
| Gabapentin | 2 (0.01) |
| Peripheral artery disease | |
| Duration, years | 4 ± 7 |
| ABI more diseased limb | 0.68 ± 0.21 |
| ABI less diseases limb | 0.84 ± 0.19 |
| Speed at symptoms (km·h−1) | 2.7 ± 0.9 |
| Maximal speed (km·h−1) | 3.3 ± 1.0 |
| Pain-free walking distance (m) | 142 ± 83 |
| 6-min walking distance (m) | 313 ± 81 |
| RLS+ (n = 101) | RLS− (n = 185) | p | |
|---|---|---|---|
| Age, years | 71 ± 9 | 72 ± 9 | 0.51 |
| Males, n (%) | 70 (69) | 149 (81) | 0.032 |
| BMI (kg·m−2) | 28 ± 5 | 27 ± 5 | 0.76 |
| Risk factors, n (%) | |||
| Smoking | 82 (81) | 157 (85) | 0.42 |
| Hypertension | 74 (73) | 141 (76) | 0.58 |
| Dyslipidemia | 74 (73) | 141 (76) | 0.58 |
| Diabetes | 52 (52) | 86 (47) | 0.42 |
| Familiarity for CVD | 8 (8) | 18 (10) | 0.61 |
| Comorbidities, n (%) | |||
| Coronary artery disease | 36 (36) | 75 (41) | 0.42 |
| Lung disease | 17 (17) | 32 (17) | 0.88 |
| Osteoarticular disorders | 23 (23) | 48 (26) | 0.55 |
| Chronic kidney disease | 24 (24) | 27 (15) | 0.06 |
| Cerebrovascular disease | 10 (10) | 19 (10) | 0.79 |
| Lower limbs revascularizations | 32 (32) | 46 (25) | 0.21 |
| Charlson Index | 6.4 ± 2.0 | 6.2 ± 2.0 | 0.41 |
| Therapy, n (%) | |||
| Anticoagulants | 10 (10) | 22 (11) | 0.67 |
| Antiplatelet | 90 (89) | 171 (92) | 0.55 |
| Anti-hypertensive | 74 (73) | 141 (76) | 0.58 |
| Diuretics | 38 (38) | 52 (28) | 0.10 |
| Statins | 74 (73) | 141 (76) | 0.58 |
| Hypoglycemic agents and/or insulin | 52 (52) | 86 (47) | 0.42 |
| RLS-treatment drugs | 14 (14) | 4 (2) | <0.001 |
| Peripheral artery disease | |||
| Duration, years | 4 ± 4 | 4 ± 7 | 0.81 |
| ABI more diseased limb | 0.69 ± 0.22 | 0.68 ± 0.20 | 0.83 |
| ABI less diseases limb | 0.83 ± 0.19 | 0.84 ± 0.20 | 0.77 |
| Speed at symptoms (km·h−1) | 2.5 ± 0.8 | 2.8 ± 1.0 | 0.018 |
| Maximal speed (km·h−1) | 3.1 ± 1.0 | 3.4 ± 1.0 | 0.032 |
| Pain-free walking distance (m) | 132 ± 90 | 148 ± 70 | 0.12 |
| 6-min walking distance (m) | 300 ± 75 | 320 ± 84 | 0.044 |
| RLS Remission (n = 83) | RLS Persistence (n = 18) | p | |
|---|---|---|---|
| Program duration (days) | 288 ± 121 | 304 ± 117 | 0.23 |
| Exercise sessions executed (%) | 94 | 71 | <0.001 |
| ∆ ABI more diseased limb | 0.05 ± 0.11 | 0.00 ± 0.12 | 0.041 |
| ∆ ABI less diseases limb | 0.03 ± 0.14 | 0.02 ± 0.12 | 0.43 |
| ∆ Pain-free walking distance (m) | 82 ± 79 | 40 ± 88 | 0.049 |
| ∆ 6-min walking distance (m) | 20 ± 43 | −11 ± 32 | 0.003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberti, N.; López-Soto, P.J.; Rodríguez-Borrego, M.A.; Straudi, S.; Basaglia, N.; Zamboni, P.; Manfredini, R.; Manfredini, F. Restless Leg Syndrome in Peripheral Artery Disease: Prevalence among Patients with Claudication and Benefits from Low-Intensity Exercise. J. Clin. Med. 2019, 8, 1403. https://doi.org/10.3390/jcm8091403
Lamberti N, López-Soto PJ, Rodríguez-Borrego MA, Straudi S, Basaglia N, Zamboni P, Manfredini R, Manfredini F. Restless Leg Syndrome in Peripheral Artery Disease: Prevalence among Patients with Claudication and Benefits from Low-Intensity Exercise. Journal of Clinical Medicine. 2019; 8(9):1403. https://doi.org/10.3390/jcm8091403
Chicago/Turabian StyleLamberti, Nicola, Pablo Jesús López-Soto, María Aurora Rodríguez-Borrego, Sofia Straudi, Nino Basaglia, Paolo Zamboni, Roberto Manfredini, and Fabio Manfredini. 2019. "Restless Leg Syndrome in Peripheral Artery Disease: Prevalence among Patients with Claudication and Benefits from Low-Intensity Exercise" Journal of Clinical Medicine 8, no. 9: 1403. https://doi.org/10.3390/jcm8091403
APA StyleLamberti, N., López-Soto, P. J., Rodríguez-Borrego, M. A., Straudi, S., Basaglia, N., Zamboni, P., Manfredini, R., & Manfredini, F. (2019). Restless Leg Syndrome in Peripheral Artery Disease: Prevalence among Patients with Claudication and Benefits from Low-Intensity Exercise. Journal of Clinical Medicine, 8(9), 1403. https://doi.org/10.3390/jcm8091403










