Smoking as an Independent Risk Factor for Hepatocellular Carcinoma Due to the α7-Nachr Modulating the JAK2/STAT3 Signaling Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients’ Tissue Specimens
2.3. Reagents
2.4. Cell Lines and Cell Culture
2.5. Small Hairpin (sh)RNA Transfection
2.6. Analyses of an Online Cancer Microarray Dataset
2.7. Sulforhodamine B (SRB) Cell Viability Assay
2.8. Immunoprecipitation and Western Blot Analysis
2.9. RT-PCR
2.10. Wound Healing Migration Assay
2.11. Matrigel Invasion Assay
2.12. Sphere Formation Assay
2.13. Immunohistochemistry (IHC) and Immunofluorescence (IFC) Staining
2.14. Statistical Analysis
3. Results
3.1. Patient Characteristics
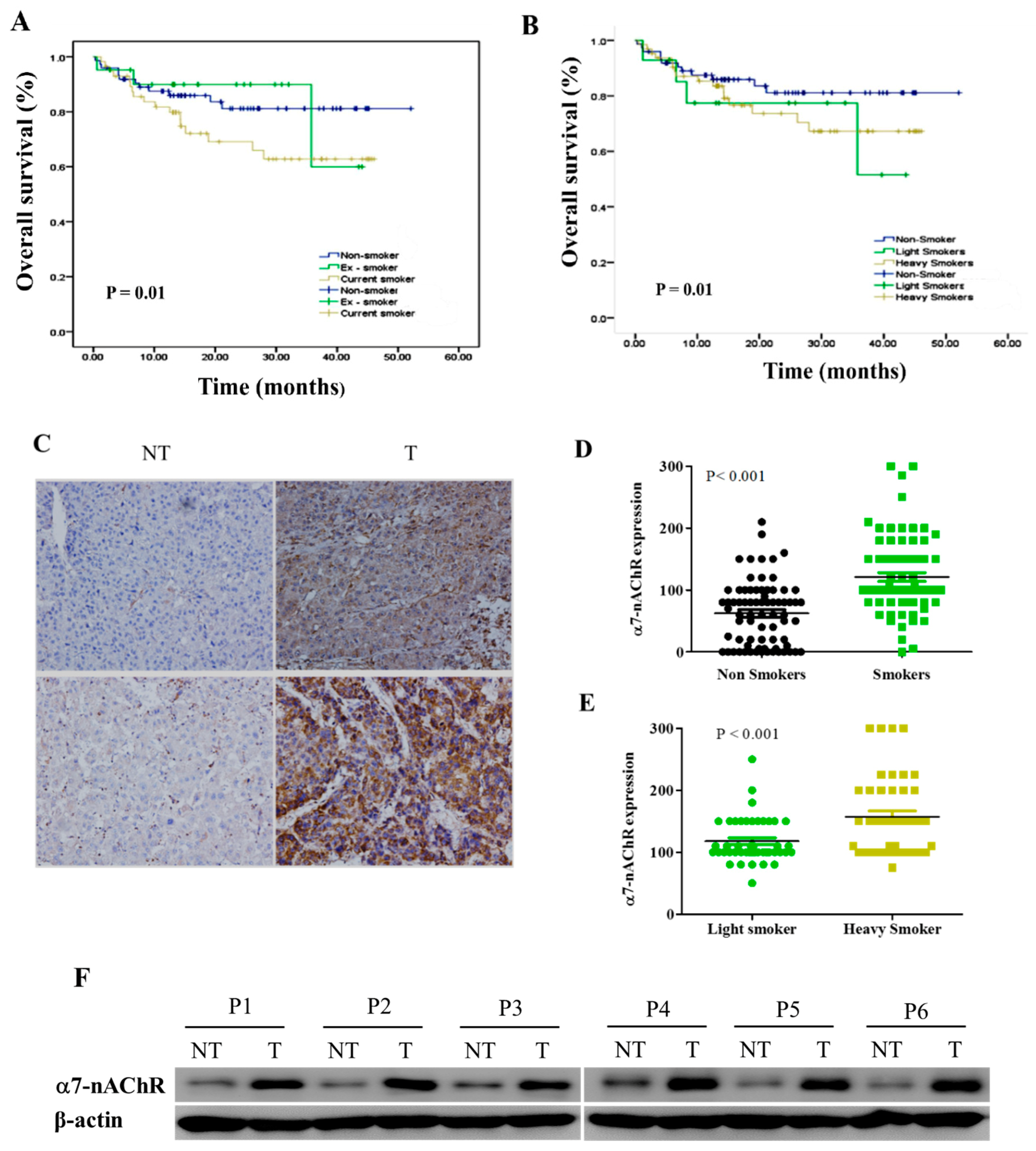
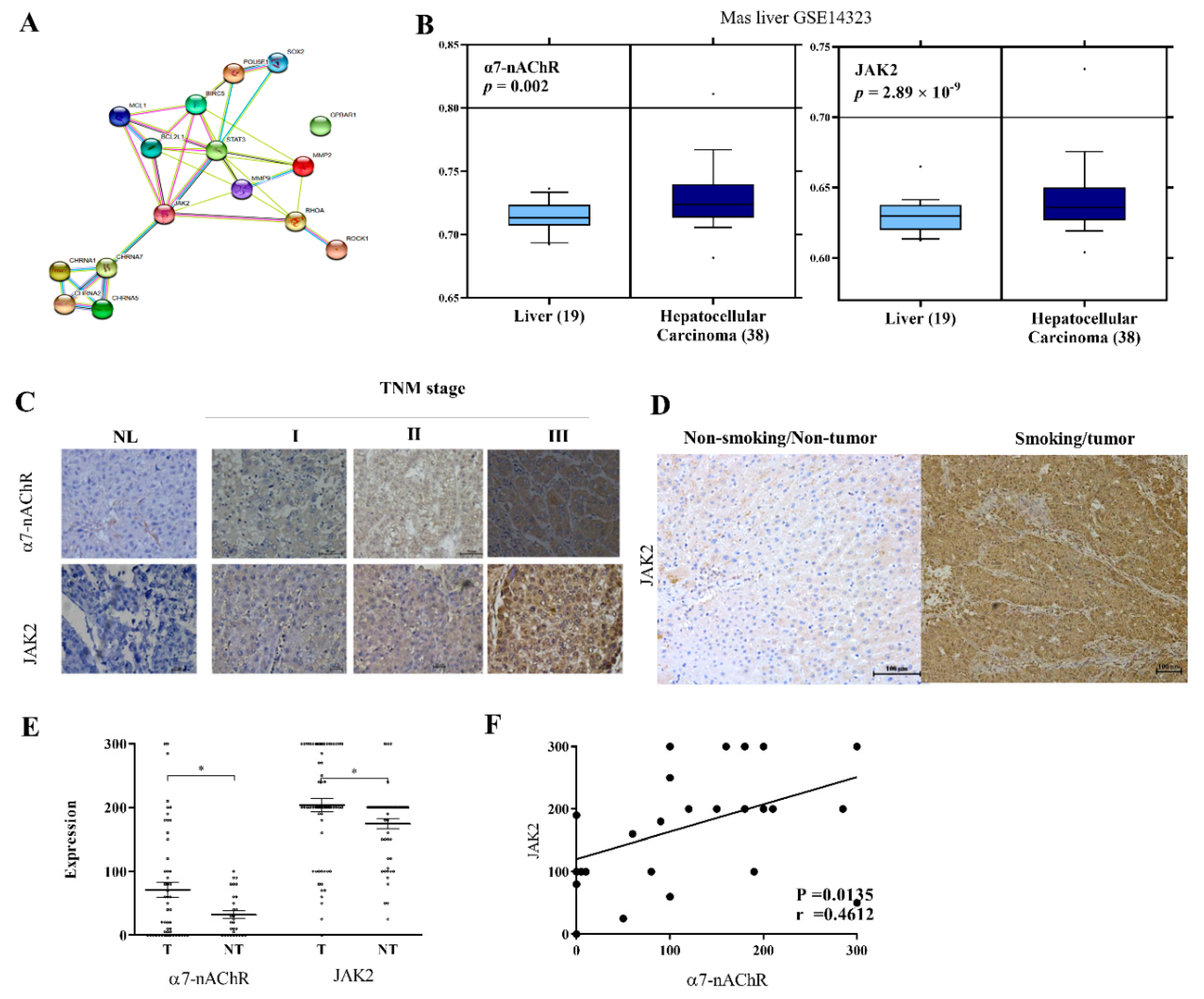
3.2. α7-nAChR Is Nicotine-Dependent and Deferentially Expressed in HCC and Non-Tumor Liver Tissues
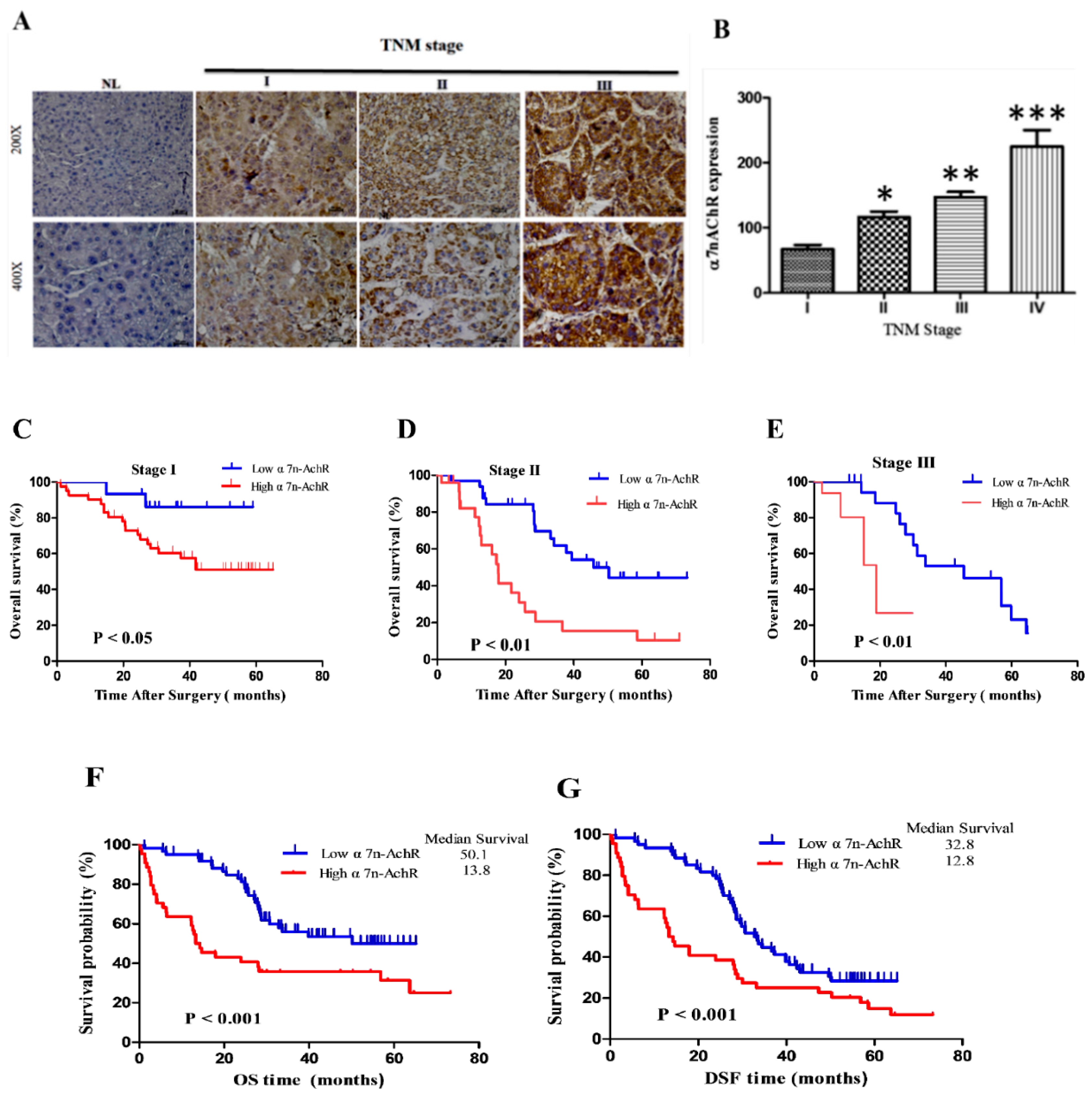
3.3. α7-nAChR Expression Is an Independent Prognosticator in Patients with HCC
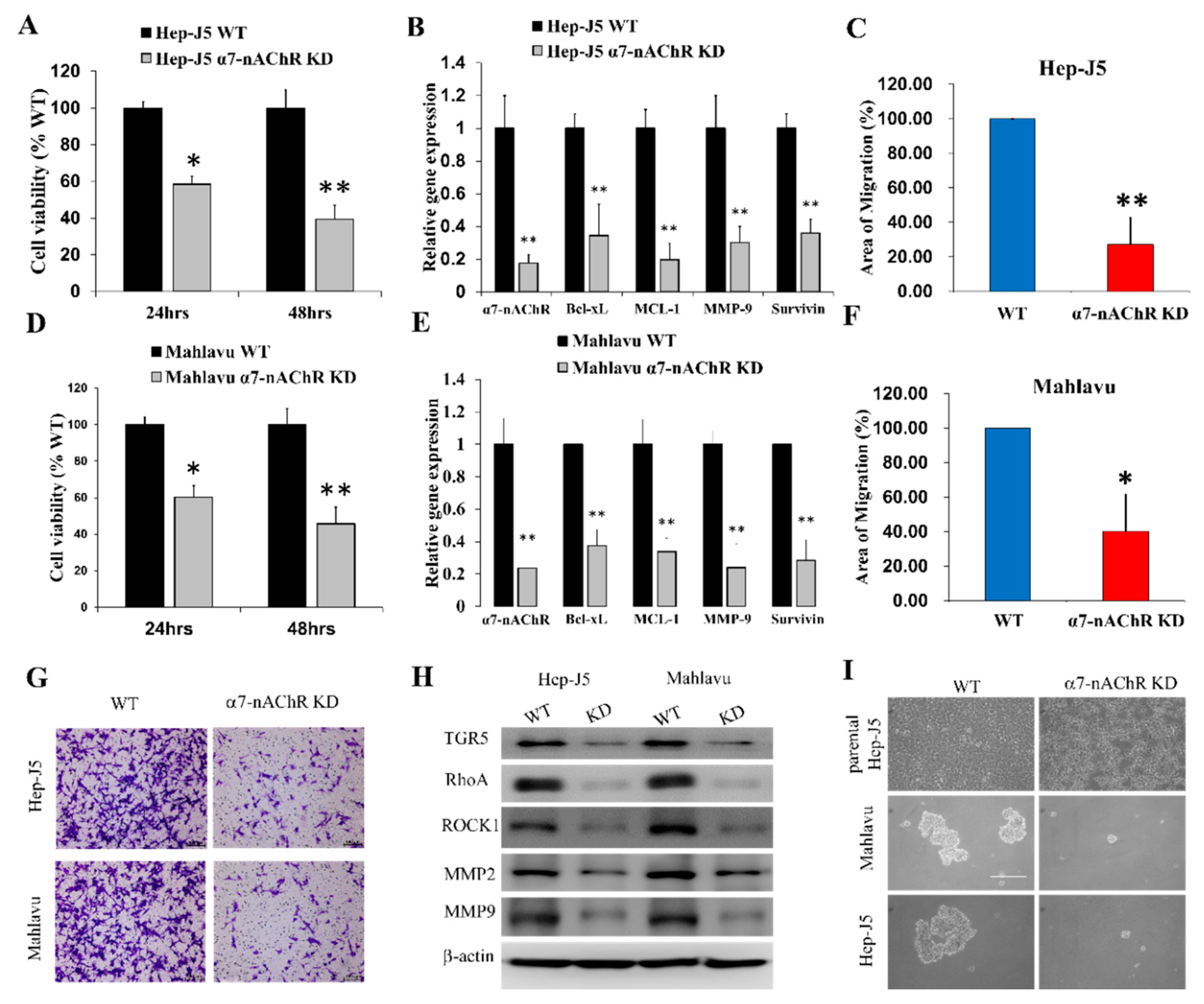
3.4. α7-nAChR Promotes Cell Viability, Metastasis, and CSCs-Like Phenotypes in HCC
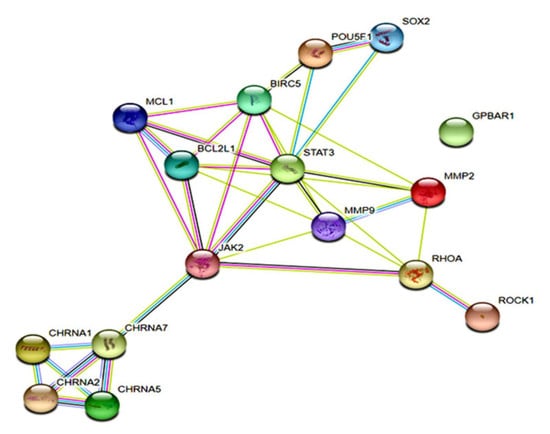
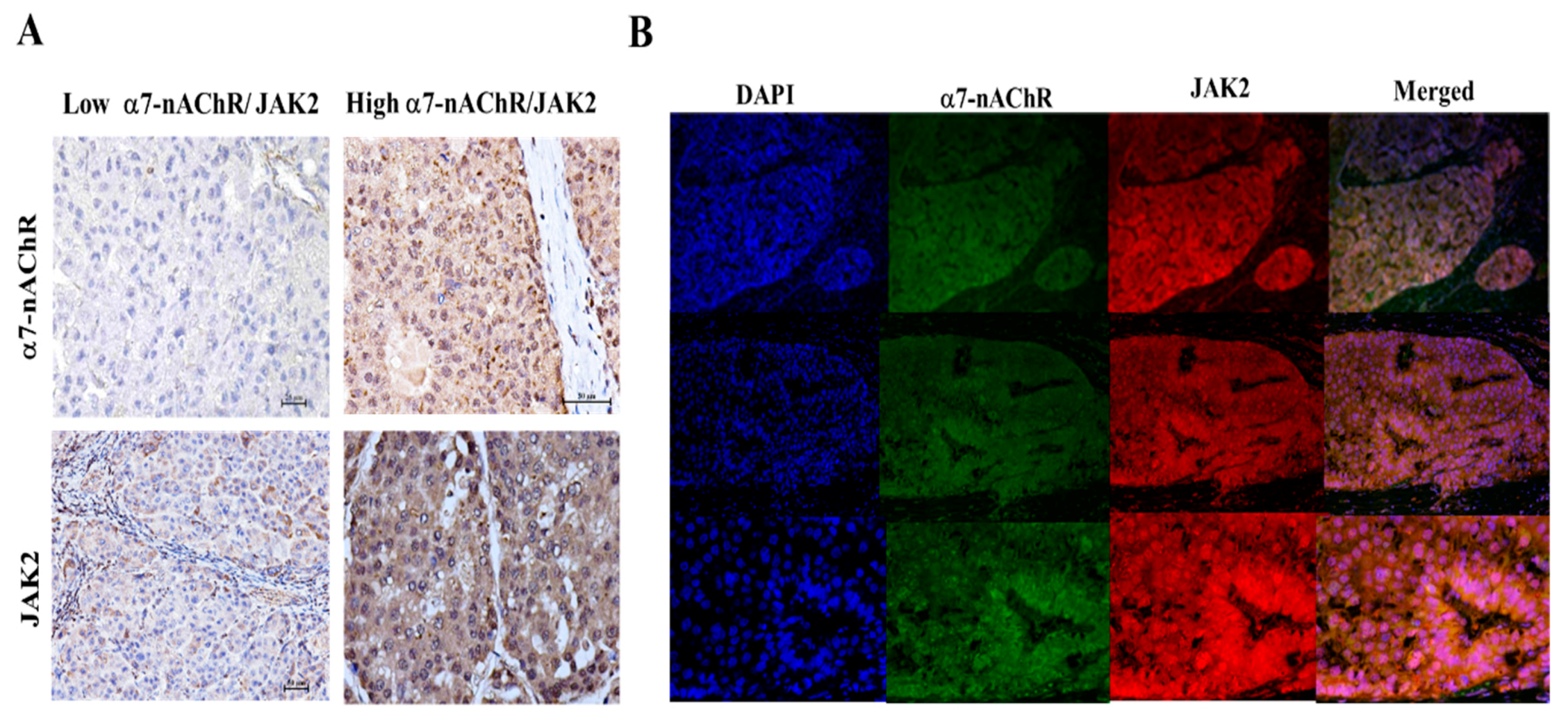
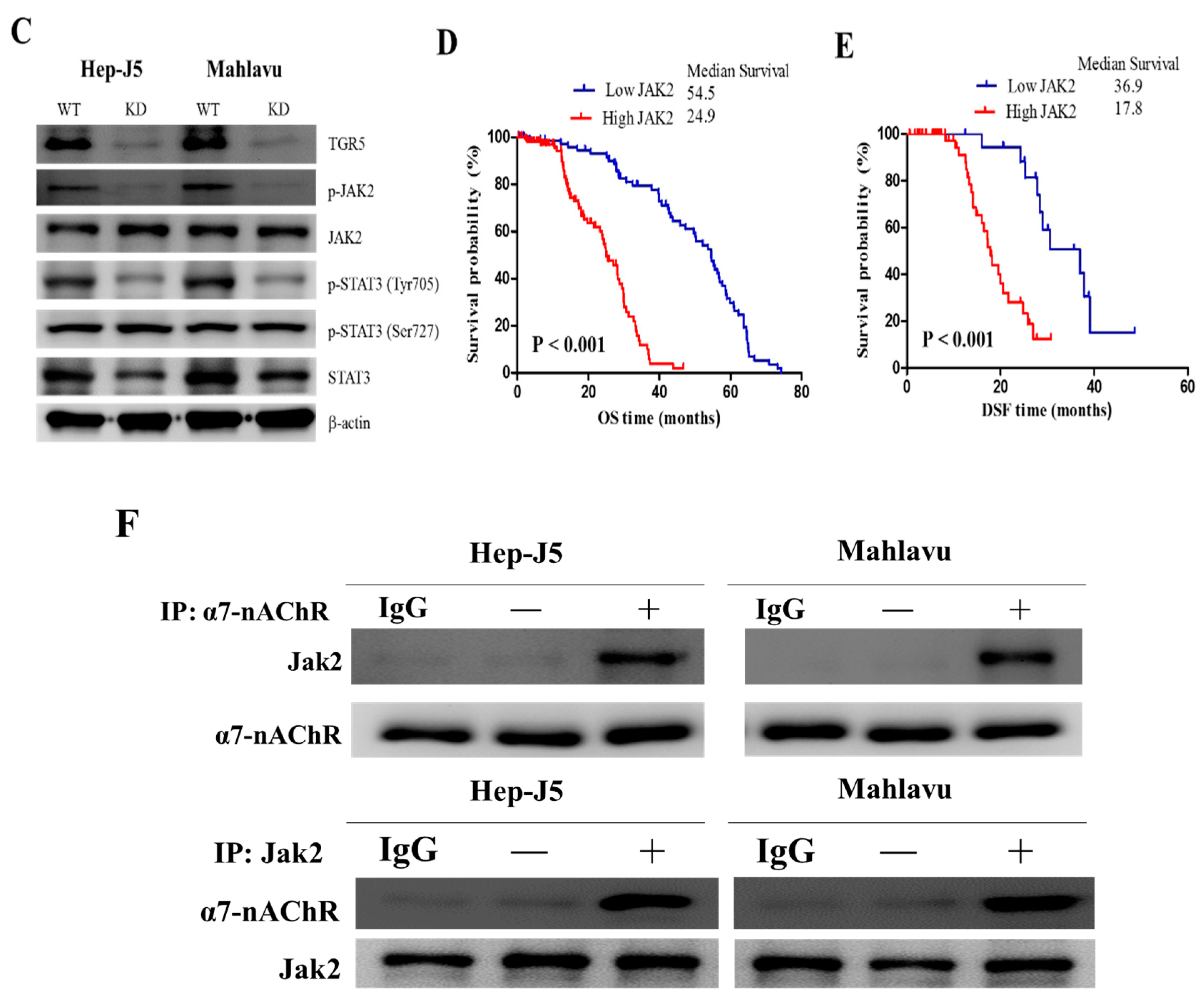
3.5. The α7-nAChR’s Oncogenic Role Is Mediated in Part by JAK2 Activation
3.6. The α7-nAChR Promotes the Recurrence of Human HCC by Modulating the TGR5/JAK2/STAT3 Signaling Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.; Zheng, H.; Liu, X.; Li, S.; Barber, T.; Gong, Z.; Gao, H.; Hao, K.; Willard, M.D.; Xu, J.; et al. Hepatocellular carcinoma genomic data from the Asian Cancer Research Group. GigaScience 2012. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Henley, S.J.; Cucinelli, J.E.; McGlynn, K.A. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am. J. Gastroenterol. 2014, 109, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Shimizu, T.; Hirokawa, F. Clinicopathological risk factors for recurrence within one year after initial hepatectomy for hepatocellular carcinoma. Am. Surg. 2011, 77, 572–578. [Google Scholar] [PubMed]
- Katyal, S.; Oliver, J.H.; Peterson, M.S.; Ferris, J.V.; Carr, B.S.; Baron, R.L. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000, 216, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Natsuizaka, M.; Omura, T.; Akaike, T.; Kuwata, Y.; Yamazaki, K.; Sato, T.; Karino, Y.; Toyota, J.; Suga, T.; Asaka, M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J. Gastroenterol. Hepatol. 2005, 20, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; Kikuchi, E.; Tanaka, N.; Matsumoto, K.; Ide, H.; Miyajima, A.; Masuda, T.; Nakamura, S.; Oya, M. Impact of smoking status on bladder tumor recurrence after radical nephroureterectomy for upper tract urothelial carcinoma. J. Urol. 2013, 189, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Capanu, M.; Janjigian, Y.Y.; Kelsen, D.K.; Coit, D.; Strong, V.E.; Shah, M.A. Tobacco use is associated with increased recurrence and death from gastric cancer. Ann. Surg. Oncol. 2012, 19, 2088–2094. [Google Scholar] [CrossRef]
- Phipps, A.I.; Shi, Q.; Newcomb, P.A.; Nelson, G.D.; Sargent, D.J.; Alberts, S.R.; Limburg, P.J. Associations between cigarette smoking status and colon cancer prognosis among participants in North Central Cancer Treatment Group Phase III Trial N0147. J. Clin. Oncol. 2013, 31, 2016–2023. [Google Scholar] [CrossRef]
- Chuang, S.C.; Lee, Y.C.; Hashibe, M.; Dai, M.; Zheng, T.; Boffetta, P. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: A meta-analysis. Cancer Epidemiol. Biomark. 2010, 19, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.P.; Robien, K.; Wang, R.; Govindarajan, S.; Yuan, J.M.; Yu, M.C. Smoking as an independent risk factor for hepatocellular carcinoma: The Singapore Chinese Health Study. Br. J. Cancer 2011, 105, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Wei, T.; Liu, X.M.; Liu, C.; Lv, Y. Impact of Cigarette Smoking on Outcome of Hepatocellular Carcinoma after Surgery in Patients with Hepatitis B. PLoS ONE 2014, 9, e85077. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.L.; Limm, W.M.; Tsai, N.; Severino, R. Hepatitis B and alcohol affect survival of hepatocellular carcinoma patients. World J. Gastroenterol. 2005, 11, 3491–3497. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Koga, H.; Aishima, S.; Kawaguchi, A.; Yamaji, K.; Ide, T.; Ueda, J.; Noshiro, H. Impact of smoking habit on surgical outcomes in non-B non-C patients with curative resection for hepatocellular carcinoma. World J. Gastroenterol. 2017, 23, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Komukai, S.; Koga, H.; Yamaji, K.; Ide, T.; Kawaguchi, A.; Aishima, S.; Noshiro, H. Correlation between smoking habit and surgical outcomes on viral-associated hepatocellular carcinomas. World J. Gastroenterol. 2018, 24, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.Y.; Wu, Y.H.; Bamodu, O.A.; Chen, X.; Lin, Y.K.; Hu, P.; Chang, N.C.; Pang, J.H.; Yeh, C.T. Activation of the monocytic α7 nicotinic acetylcholine receptor modulates oxidative stress and inflammation-associated development of coronary artery spasm via a p38 MAP-kinase signaling-dependent pathway. Free Radic. Biol. Med. 2018, 120, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Machaalani, R.; Ghazavi, E.; Hinton, T.; Waters, K.A.; Hennessy, A. Cigarette smoking during pregnancy regulates the expression of specific nicotinic acetylcholine receptor (nAChR) subunits in the human placenta. Toxicol. Appl. Pharmacol. 2014, 276, 204–212. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Zhou, Z.; Liu, Y.; Bai, Y.; Xing, X.; Wang, X. Nicotine induces the production of IL-1β and IL-8 via the α7 nAChR/NF-κB pathway in human periodontal ligament cells: An in vitro study. Cell. Physiol. Biochem. 2014, 34, 423–431. [Google Scholar] [CrossRef]
- Cesario, A.; Russo, P.; Nastrucci, C.; Granone, P. Is α7-nAChR a possible target for lung cancer and malignant pleural mesothelioma treatment? Curr. Drug Targets 2012, 13, 688–694. [Google Scholar]
- Mei, D.; Lin, Z.; Fu, J.; He, B.; Gao, W.; Ma, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; et al. The use of α-conotoxin ImI to actualize the targeted delivery of paclitaxel micelles to α7 nAChR-overexpressing breast cancer. Biomaterials 2015, 42, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Hirata, N.; Sekino, Y.; Kanda, Y. Role of α7-nicotinic acetylcholine receptor in normal and cancer stem cells. Curr. Drug Targets 2012, 13, 656–665. [Google Scholar] [PubMed]
- Guo, C.; Chen, W.D.; Wang, Y.D. TGR5, Not Only a Metabolic Regulator. Front. Physiol. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Kida, T.; Tsubosaka, Y.; Hori, M.; Ozaki, H.; Murata, T. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Liu, N.; Fu, X.; Wang, X.; Wang, Y.D.; Chen, W.D.; Zhang, L.; Forman, B.M.; Huang, W. Insufficient bile acid signaling impairs liver repair in CYP27(-/-) mice. J. Hepatol. 2011, 55, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Huang, B.Q.; Radtke, B.N.; Gajdos, G.B.; Splinter, P.L.; Masyuk, T.V.; Gradilone, S.A.; LaRusso, N.F. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1013–G1024. [Google Scholar] [CrossRef]
- Duboc, H.; Tache, Y.; Hofmann, A.F. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig. Liver Dis. 2014, 46, 302–312. [Google Scholar] [CrossRef]
- Karin, M.; He, G. NF-kB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar]
- Chen, W.D.; Yu, D.; Forman, B.M.; Huang, W.; Wang, Y.D. Deficiency of G-Protein-Coupled Bile Acid Receptor Gpbar1 (TGR5) enhances chemically induced liver carcinogenesis. Hepatology 2013, 57, 656–666. [Google Scholar] [CrossRef]
- Wang, R.C.; Huang, C.Y.; Pan, T.L.; Chen, W.Y.; Ho, C.T.; Liu, T.Z.; Chang, Y.J. Proteomic Characterization of Annexin I (ANX1) and Heat Shock Protein 27 (HSP27) as Biomarkers for Invasive Hepatocellular Carcinoma Cells. PLoS ONE 2015, 10, e0139232. [Google Scholar] [CrossRef]
- Li, F.; Guo, Z.; Wang, H. Influencing elements and treatment strategies associated with the relapse of hepatocellular carcinoma after surgery. Hepatogastroenterology 2013, 60, 1148–1155. [Google Scholar] [PubMed]
- Ho, C.M.; Lee, P.H.; Shau, W.Y.; Ho, M.C.; Wu, Y.M.; Hu, R.H. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: Comparative effectiveness of treatment modalities. Surgery 2012, 151, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, P.; Becchetti, A. Targeting neuronal nicotinic receptors in cancer: New ligands and potential side effects. Recent Pat. Anti-Cancer Drug Discov. 2013, 8, 38–52. [Google Scholar] [CrossRef]
- Russo, P.; Taly, A. α7-Nicotinic acetylcholine receptors: An old actor for new different roles. Curr. Drug Targets 2012, 13, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Perry, H.E.; Lau, J.K.; Jones, D.V.; Pulliam, J.F.; Thornhill, B.A.; Crabtree, C.M.; Luo, H.; Chen, Y.C.; Dasgupta, P. Nicotine induces the up-regulation of the α7-nicotinic receptor (α7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA protein pathway. J. Biol. Chem. 2013, 288, 33049–33059. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pillai, S.; Chellappan, S. Nicotinic Acetylcholine Receptor Signaling in Tumor Growth and Metastasis. J. Oncol. 2011, 2011, 456743. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ogunwobi, O.O.; Liu, C. Survivin Inhibition Is Critical for Bcl-2 Inhibitor-Induced Apoptosis in Hepatocellular Carcinoma Cells. PLoS ONE 2011, 6, e21980. [Google Scholar] [CrossRef]
- Priya, R.; Liang, X.; Teo, J.L.; Duszyc, K.; Yap, A.S.; Gomez, G.A. ROCK1 but not ROCK2 contributes to RhoA signaling and NMIIA-mediated contractility at the epithelial zonula adherens. Mol. Biol. Cell 2017, 28, 12–20. [Google Scholar] [CrossRef]
- Lien, Y.C.; Wang, W.; Kuo, L.J.; Liu, J.J.; Wei, P.L.; Ho, Y.S.; Ting, W.C.; Wu, C.H.; Chang, Y.J. Nicotine promotes cell migration through alpha7 nicotinic acetylcholine receptor in gastric cancer cells. Ann. Surg. Oncol. 2011, 18, 2671–2679. [Google Scholar] [CrossRef]
- Wei, P.L.; Kuo, L.J.; Huang, M.T.; Ting, W.C.; Ho, Y.S.; Wang, W.; An, J.; Chang, Y.J. Nicotine enhances colon cancer cell migration by induction of fibronectin. Ann. Surg. Oncol. 2011, 18, 1782–1790. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B.; You, W.; Xue, S.; Qin, H.; Jiang, H. The membrane bile acid receptor TGR5 drives cell growth and migration via activation of the JAK2/STAT3 signaling pathway in non-small cell lung cancer. Cancer Lett. 2018, 412, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Momi, N.; Ponnusamy, M.P.; Kaur, S.; Rachagani, S.; Kunigal, S.S.; Chellappan, S.; Ouellette, M.M.; Batra, S.K. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through α7nAChR-mediated MUC4 upregulation. Oncogene 2013, 32, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Wittel, U.A.; Pandey, K.K.; Andrianifahanana, M.; Johansson, S.L.; Cullen, D.M.; Akhter, M.P.; Brand, R.E.; Prokopczyk, B.; Batra, S.K. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am. J. Gastroenterol. 2006, 101, 148–159. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Expression, n (%) | Spearman’s Correlation | 5-Year SR c | |||||
|---|---|---|---|---|---|---|---|---|
| Low | High | pa | r | pb value | Low | High | Pd | |
| 69 (38.5) | 110 (61.5) | |||||||
| Age (years) | ||||||||
| <60 | 51 (73.9) | 71 (64.5) | 0.13 | −0.10 | 0.39 | 55.6 | 9.1 | 0.28 |
| ≥60 | 18 (26.1) | 39 (35.5) | 39.8 | 14.2 | ||||
| Gender | ||||||||
| Male | 53 (76.8) | 90 (81.8) | 0.27 | −0.16 | 0.04 | 50.1 | 9.5 | 0.03 |
| Female | 16 (23.2) | 20 (18.2) | 28.6 | 3.7 | ||||
| Smoking Status | ||||||||
| smokers | 49 (71.0) | 41 (37.6) | <0.001 | 0.71 | 0.004 | 55.6 | 19.5 | <0.001 |
| Ex-smokers | 12 (17.4) | 24 (21.8) | 28.4 | 6.4 | ||||
| Current-smokers | 8 (11.6) | 45 (40.9) | 27.0 | 5.9 | ||||
| Smoking pack-years | ||||||||
| Non smokers | 50 (72.5) | 40 (36.4) | <0.001 | 0.67 | <0.001 | 50.3 | 8.6 | 0.001 |
| >0 and <20 | 10 (14.5) | 35 (31.8) | 29.5 | 6.8 | ||||
| ≥20 | 9 (13.0) | 35 (31.8) | 29.6 | 5.8 | ||||
| Alcohol consumption | ||||||||
| No | 46 (66.7) | 46 (41.8) | <0.001 | 0.56 | 0.03 | 55.6 | 14.8 | 0.02 |
| Low–Moderate | 17 (24.6) | 33 (30.0) | 39.4 | 6.8 | ||||
| Moderate–High | 6 (8.7) | 31 (28.2) | 34.5 | 6.1 | ||||
| Alpha-fetoprotein (ng/mL) | ||||||||
| <20 | 43 (62.3) | 67 (60.9) | 0.44 | 0.09 | 0.23 | 29.7 | 20.5 | 0.29 |
| ≥20 | 26 (37.7) | 43 (39.1) | 28.4 | 16.5 | ||||
| Viral status | ||||||||
| Negative | 27 (39.1) | 39 (35.5) | 0.87 | 0.52 | 0.49 | 54.7 | 14.4 | 0.76 |
| HBV | 30 (43.5) | 54 (49.1) | 42.3 | 25.1 | ||||
| HCV | 10 (14.5) | 15 (13.6) | 42.4 | 17.8 | ||||
| HBV & HCV | 2 (2.9) | 2 (1.8) | 28.9 | 29.1 | ||||
| Liver cirrhosis | ||||||||
| Negative | 63 (91.3) | 36 (32.7) | <0.001 | 0.6 | 0.001 | 47.8 | 14.2 | |
| Positive | 6 (8.7) | 74 (67.3) | 47.5 | 28.1 | ||||
| Tumor size | ||||||||
| <5 cm | 54 (78.3) | 10 (9.1) | <0.001 | 0.46 | <0.001 | 46.6 | 21.1 | 0.002 |
| ≥5 cm | 15 (21.7) | 100 (90.9) | 47.2 | 18.1 | ||||
| Tumor number | ||||||||
| Solitary | 58 (84.1) | 52 (47.3) | <0.001 | 0.53 | 0.01 | 46.7 | 31.3 | 0.05 |
| Multiple | 11(15.9) | 58 (52.7) | 43.8 | 9.8 | ||||
| Vascular Invasion | ||||||||
| 0 | 50 (72.5) | 22 (20.0) | 0.001 | 0.6 | <0.001 | 46.6 | 31.5 | 0.03 |
| 1 | 12 (17.4) | 31 (28.2) | 59.1 | 23.6 | ||||
| 2 | 5 (7.2) | 42 (38.2) | 41.9 | 11.7 | ||||
| 3 | 1 (1.4) | 2 (1.8) | 33.2 | 11.1 | ||||
| 4 | 1 (1.4) | 13 (11.8) | 35.3 | 10.8 | ||||
| Recurrent | ||||||||
| No | 50 (72.5) | 54 (49.1) | 0.002 | 0.60 | 0.001 | 42.8 | 13.3 | <0.001 |
| Yes | 19 (27.5) | 56 (50.9) | 51.2 | 27.7 | ||||
| TNM stage | ||||||||
| I | 55 (79.9) | 10 (9.1) | <0.001 | 0.69 | <0.001 | 50.2 | 33.8 | 0.05 |
| II | 10 (14.5) | 49 (44.5) | 36.7 | 19.8 | 0.001 | |||
| III | 3 (4.3) | 43 (39.1) | 34.7 | 6.8 | 0.01 | |||
| IV | 1 (1.4) | 8 (7.3) | 3.5 | 2.3 | 0.02 | |||
| Parameters | Univariate | Overall Survival Multivariate | Univariate | Disease Free Survival Multivariate | ||
|---|---|---|---|---|---|---|
| p | HR (95% CI) | p | p | HR (95% CI) | p | |
| Age (<60 vs. ≥60) | 0.459 | 0.35 | ||||
| Gender (male vs. female) | 0.132 | 0.02 | ||||
| Smoking status (yes vs. no) | <0.001 | 2.02 (1.71–3.37) | <0.001 | 0.01 | 1.31 (1.32–2.66) | 0.025 |
| Smoking pack—years (light vs. heavy) | 0.001 | 2.19 (1.56–3.52) | 0.02 | 0.001 | 1.5 (1.51–2.05) | 0.023 |
| Alcohol consumption (low vs. high) | 0.009 | 1.02 (1.14–2.62) | 0.01 | 0.001 | 1.6 (1.51–2.32) | 0.002 |
| AFP (<20 vs. ≥20 ng/mL) | 0.92 | 0.65 | ||||
| Viral status (yes vs. no) | 0.13 | 0.34 | ||||
| Liver cirrhosis (yes vs. no) | 0.02 | 1.51 (1.22–1.74) | 0.002 | 0.001 | 1.02 (1.14–2.62) | |
| Tumor size (<5 cm vs. ≥5 cm) | 0.04 | 1.23 (1.14–2.15) | <0.001 | 0.001 | 1.8 (1.73–2.05) | 0.028 |
| Tumor type (solitary vs. multiple) | <0.001 | 1.71 (1.74–2.13) | 0.05 | 0.001 | 1.3 (1.12–2.61) | <0.001 |
| Vascular Invasions (yes vs. no) | 0.008 | 1.59 (1.35–2.13) | 0.002 | 0.01 | 2.46 (1.32–3.84) | 0.008 |
| TNM stage (I/II vs. III/IV) | 0.01 | 1.01 (1.21–1.86) | <0.001 | 0.01 | 1.21 (1.81–2.01) | 0.012 |
| α7-nAChR (low vs. high) | <0.001 | 2.01 (1.76–3.42) | <0.001 | 0.001 | 2.62 (1.08–4.21) | 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-L.; Lin, Y.-K.; Chen, H.-A.; Huang, C.-Y.; Huang, M.-T.; Chang, Y.-J. Smoking as an Independent Risk Factor for Hepatocellular Carcinoma Due to the α7-Nachr Modulating the JAK2/STAT3 Signaling Axis. J. Clin. Med. 2019, 8, 1391. https://doi.org/10.3390/jcm8091391
Li C-L, Lin Y-K, Chen H-A, Huang C-Y, Huang M-T, Chang Y-J. Smoking as an Independent Risk Factor for Hepatocellular Carcinoma Due to the α7-Nachr Modulating the JAK2/STAT3 Signaling Axis. Journal of Clinical Medicine. 2019; 8(9):1391. https://doi.org/10.3390/jcm8091391
Chicago/Turabian StyleLi, Ching-Li, Yen-Kuang Lin, Hsin-An Chen, Chien-Yu Huang, Ming-Te Huang, and Yu-Jia Chang. 2019. "Smoking as an Independent Risk Factor for Hepatocellular Carcinoma Due to the α7-Nachr Modulating the JAK2/STAT3 Signaling Axis" Journal of Clinical Medicine 8, no. 9: 1391. https://doi.org/10.3390/jcm8091391
APA StyleLi, C.-L., Lin, Y.-K., Chen, H.-A., Huang, C.-Y., Huang, M.-T., & Chang, Y.-J. (2019). Smoking as an Independent Risk Factor for Hepatocellular Carcinoma Due to the α7-Nachr Modulating the JAK2/STAT3 Signaling Axis. Journal of Clinical Medicine, 8(9), 1391. https://doi.org/10.3390/jcm8091391