Right Heart Catheterization—Background, Physiological Basics, and Clinical Implications
Abstract
1. Introduction
1.1. Aim of the Review
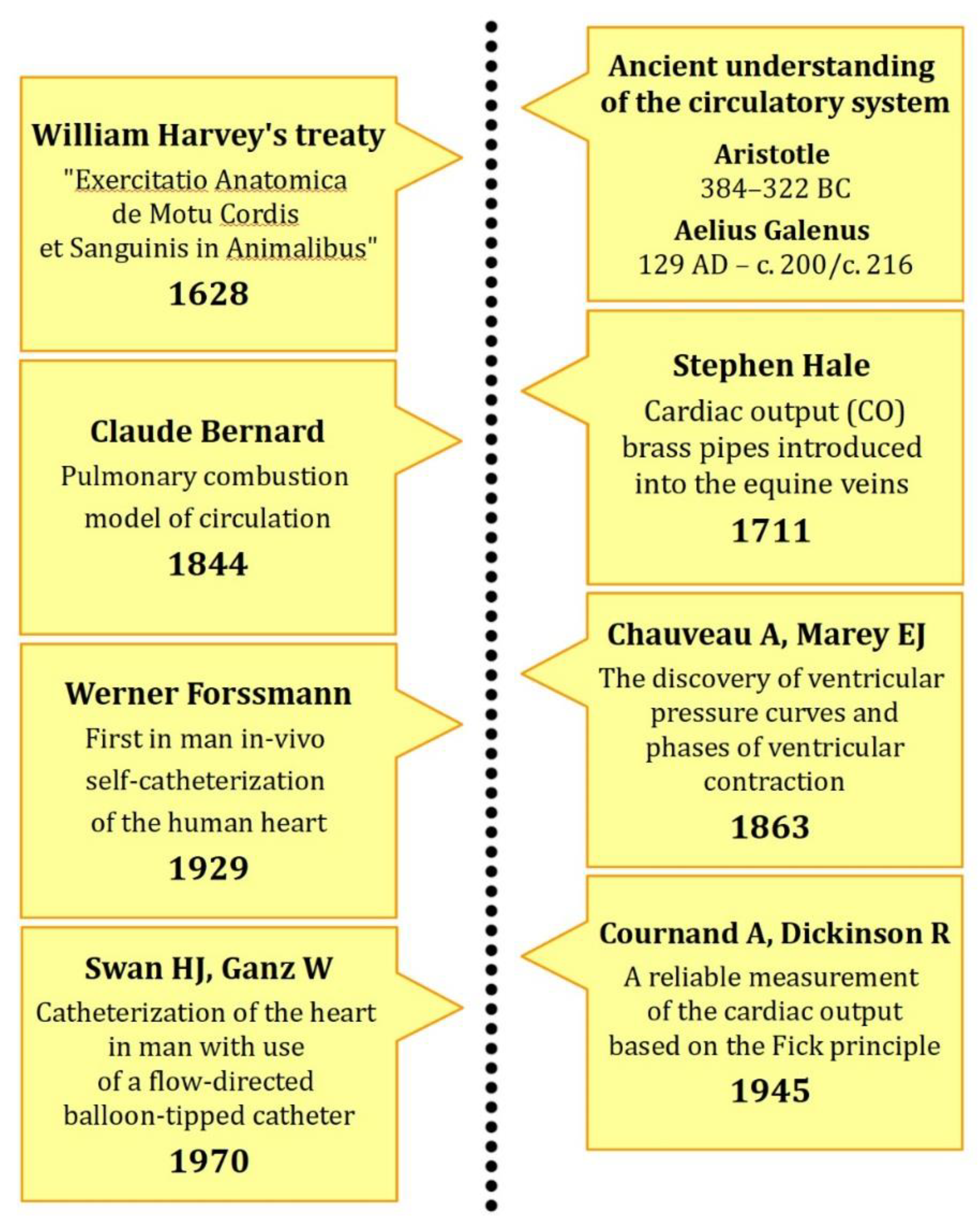
1.2. From Greek Philosophy into Modern Understanding
1.3. Pioneers of Right Heart Catheterization
1.4. Nobel Prize for the Famous Three
1.5. Jeremy Swan and William Ganz—Catheterization in Clinical Practice
1.6. Technique of the Measurement and Basic Definitions
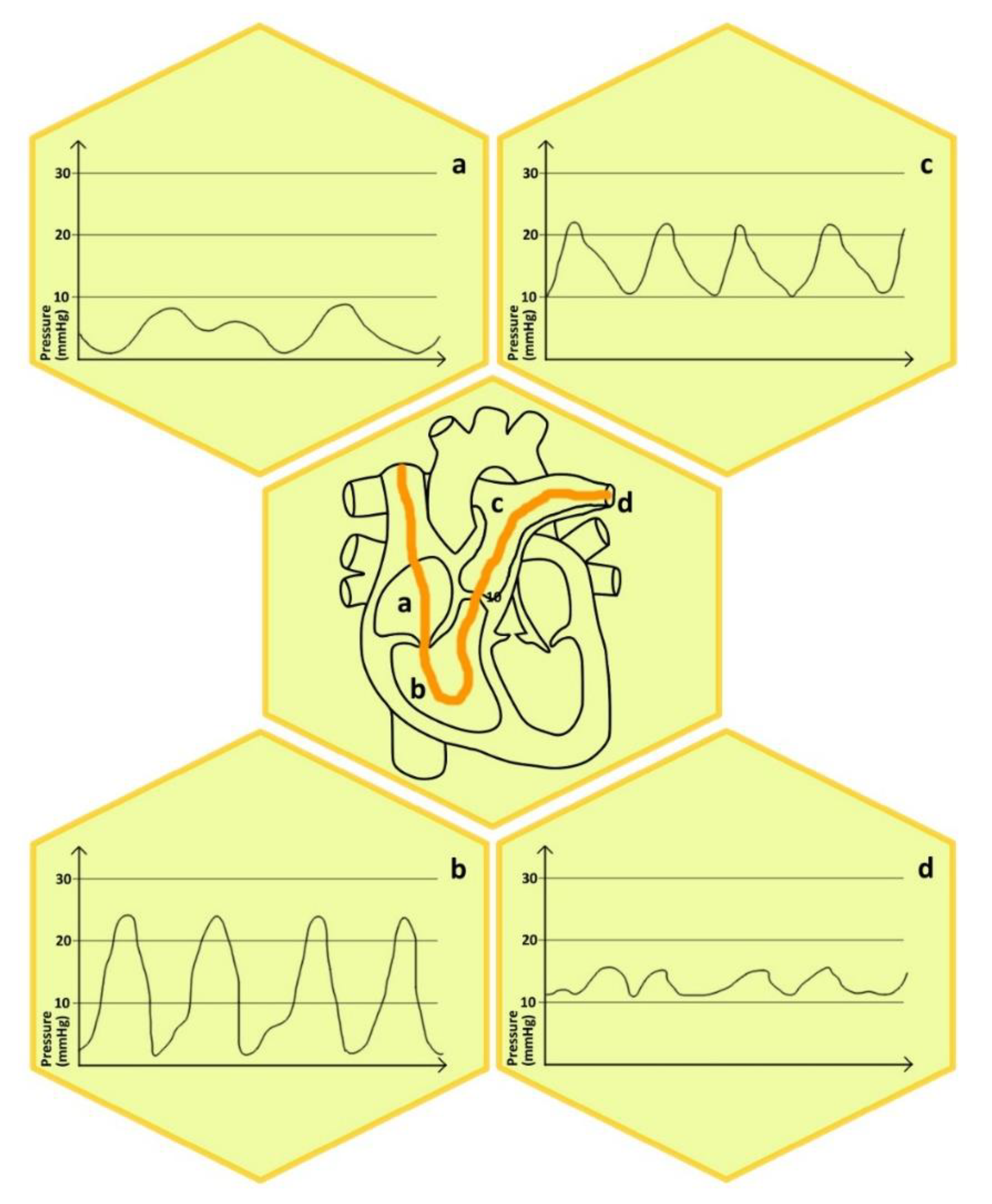
2. Physiological Aspects of Right Heart Catheterization
3. Clinical Practice Implications
3.1. Safety of Right Heart Catheterization—The Evaluation of Clinical Outcomes
3.2. The Position of Right Heart Catheterization in the Diagnostic and Management Algorithm of Heart Failure
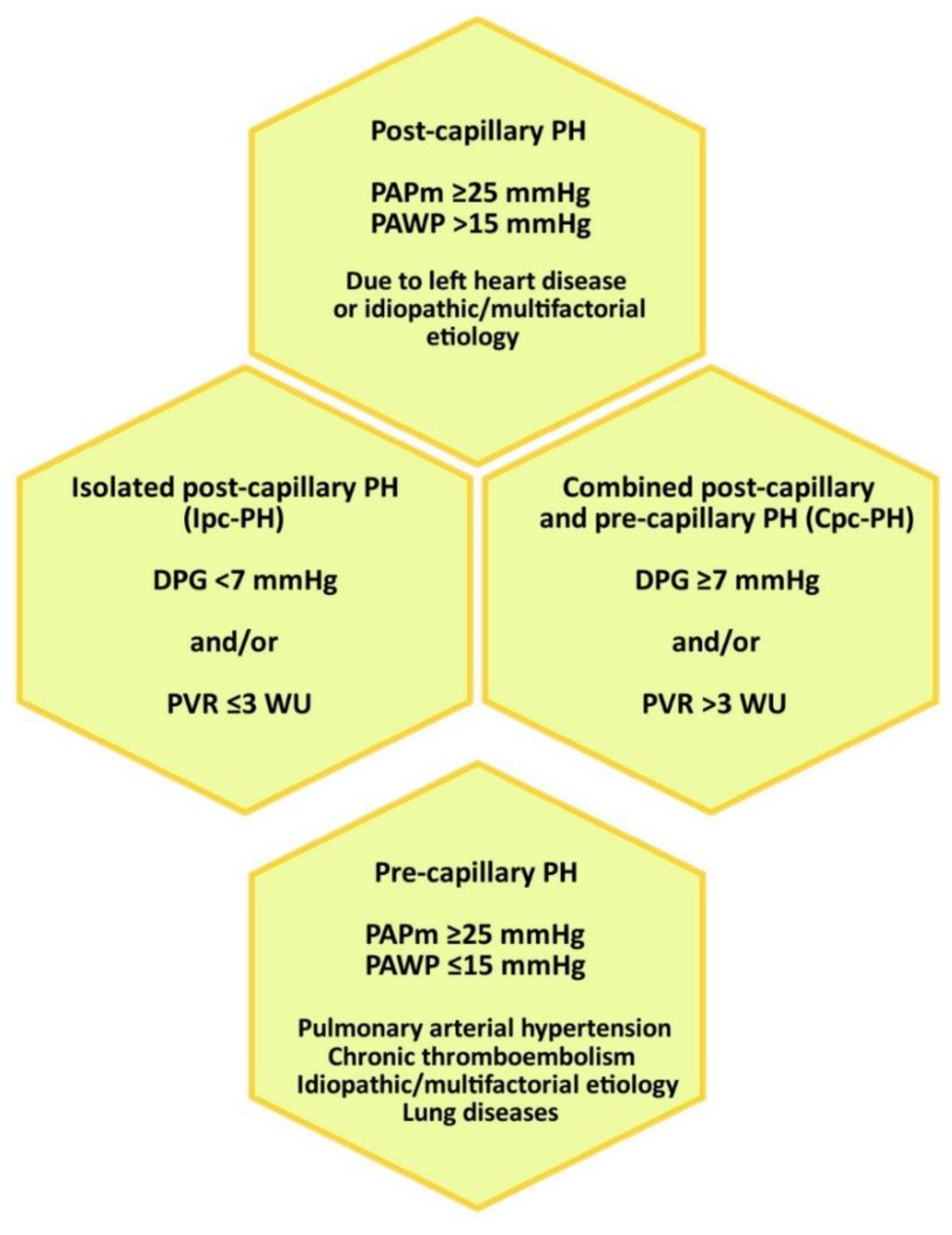
3.3. Position of the RHC in the Diagnostic Algorithm of Pulmonary Hypertension
4. Conclusions
Funding
Conflicts of Interest
References
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev. Esp. Cardiol. Engl. Ed. 2016, 69, 177. [Google Scholar] [PubMed]
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT); Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.-L.; Barbera, J.A.; Beghetti, M.; et al. Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Respir. J. 2009, 34, 1219–1263. [Google Scholar]
- Pasipoularides, A. Greek Underpinnings to His Methodology in Unraveling De Motu Cordis and What Harvey Has to Teach Us Still Today. Int. J. Cardiol. 2013, 168, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, R. The Theory of the Circulation of Blood and (Different) Paths of Aristotelianism. Girolamo Franzosi’s De Motu Cordis et Sanguinis in Animalibus pro Aristotele et Galeno Adversus Anatomicos Neotericos Libri Duo: Teleology versus Mechanism? Gesnerus 2014, 71, 271–289. [Google Scholar] [PubMed]
- Pasipoularides, A. Historical Perspective: Harvey’s Epoch-Making Discovery of the Circulation, Its Historical Antecedents, and Some Initial Consequences on Medical Practice. J. Appl. Physiol. 2013, 114, 1493–1503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Outram, Q. The Socio-Economic Relations of Warfare and the Military Mortality Crises of the Thirty Years’ War. Med. Hist. 2001, 45, 151–184. [Google Scholar] [CrossRef] [PubMed]
- Bruning, H. Life at German universities at the time of the Thirty Years War. Med. Monatsschr. 1954, 8, 830–832. [Google Scholar]
- Hoff, H.E.; Geddes, L.A.; McCrady, J.D. The Contributions of the Horse to Knowledge of the Heart and Circulation. 1. Stephen Hales and the Measurement of Blood Pressure. Conn. Med. 1965, 29, 795–800. [Google Scholar]
- Geddes, L.A.; Hoff, H.E.; Mccrady, J.D. Some aspects of the cardiovascular physiology of the horse. Cardiovasc. Res. Cent. Bull. 1965, 4, 80–95. [Google Scholar]
- Geddes, L.A.; McCrady, J.D.; Hoff, H.E. The Contribution of the Horse to Knowledge of the Heart and Circulation. II. Cardiac Catheterization and Ventricular Dynamics. Conn. Med. 1965, 29, 864–876. [Google Scholar]
- Braun, R. Voyaging in the Vein: Medical Experimentation with Heart Catheters in the Twentieth Century. Nuncius 2011, 26, 132–158. [Google Scholar] [CrossRef] [PubMed]
- Cournand, A. Historical Details of Claude Bernard’s Invention of a Technique for Measuring the Temperature and the Pressure of the Blood within the Cavities of the Heart. Trans. N. Y. Acad. Sci. 1980, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.G. The History of Cardiac Catheterization. Can. J. Cardiol. 2005, 21, 1011–1014. [Google Scholar] [PubMed]
- Sette, P.; Dorizzi, R.M.; Azzini, A.M. Vascular Access: An Historical Perspective from Sir William Harvey to the 1956 Nobel Prize to André F. Cournand, Werner Forssmann, and Dickinson W. Richards. J. Vasc. Access. 2012, 13, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Fye, W.B. Jean-Baptiste Auguste Chauveau. Clin. Cardiol. 2003, 26, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Barold, S.S. Willem Einthoven and the Birth of Clinical Electrocardiography a Hundred Years Ago. Card. Electrophysiol. Rev. 2003, 7, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.E. Etienne-Jules Marey: 19th Century Cardiovascular Physiologist and Inventor of Cinematography. Clin. Cardiol. 1996, 19, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Cournand, A. Cardiac Catheterization; Development of the Technique, Its Contributions to Experimental Medicine, and Its Initial Applications in Man. Acta Med. Scand. Suppl. 1975, 579, 3–32. [Google Scholar] [PubMed]
- Forssmann, W. Die Sondierung des rechten Herzens [Probing of the right heart]. Klin. Wochenschr. 1929, 8, 2085–2087. [Google Scholar] [CrossRef]
- Forssmann, W. Ueber Kontrastdarstellung der Hohlen des lebenden rechten Herzens und der Lungenschlagader [On contrast demonstration of the chambers of the living right heart and the pulmonary artery]. Miinchener Med. Wochenschr. 1931, 78, 489–492. [Google Scholar]
- Meyer, J.A. Werner Forssmann and Catheterization of the Heart, 1929. Ann. Thorac. Surg. 1990, 49, 497–499. [Google Scholar] [CrossRef]
- Hansson, N.; Packy, L.-M.; Halling, T.; Groß, D.; Fangerau, H. From Nobody to Nobel laureate? The case of Werner Forßmann. Urologe A 2015, 54, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, W. Werner Forssmann, Eberswalde, the 1956 Nobel Prize for Medicine. Eur. J. Med. Res. 2006, 11, 409–412. [Google Scholar] [PubMed]
- Forssmann-Falck, R. Werner Forssmann: A Pioneer of Cardiology. Am. J. Cardiol. 1997, 79, 651–660. [Google Scholar] [CrossRef]
- Packy, L.-M.; Krischel, M.; Gross, D. Werner Forssmann—A Nobel Prize Winner and His Political Attitude before and after 1945. Urol. Int. 2016, 96, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Cournand, A.; Riley, R.L.; Breed, E.S.; Baldwin, E.D.; Richards, D.W.; Lester, M.S.; Jones, M. Measurement of cardiac output in man using the technique of catheterization of the right auricle or ventricle. J. Clin. Investig. 1945, 24, 106–116. [Google Scholar] [CrossRef]
- Cournand, A.; Noble, R.P.; Breed, E.S.; Lauson, H.D.; Baldwin, E.d.F.; Pinchot, G.B.; Richards, D.W. Chemical, clinical, and immunological studies on the products of human plasma fractionation. viii. clinical use of concentrated human serum albumin in shock, and comparison with whole blood and with rapid saline infusion. J. Clin. Investig. 1944, 23, 491–505. [Google Scholar] [CrossRef]
- Kubiak, G.M.; Tomasik, A.R.; Bartus, K.; Olszanecki, R.; Ceranowicz, P. Lactate in Cardiogenic Shock—Current Understanding and Clinical Implications. J. Physiol. Pharmacol. 2018, 69, 15–21. [Google Scholar]
- Kubiak, G.M.; Jacheć, W.; Wojciechowska, C.; Traczewska, M.; Kolaszko, A.; Kubiak, L.; Jojko, J.; Nowalany-Kozielska, E. Handheld Capillary Blood Lactate Analyzer as an Accessible and Cost-Effective Prognostic Tool for the Assessment of Death and Heart Failure Occurrence during Long-Term Follow-Up. Dis. Markers 2016, 2016, 5965782. [Google Scholar] [CrossRef]
- Cournand, A.; Ranges, H.A.; Riley, R.L. Comparison of results of the normal ballistocardiogram and a direct fick method in measuring the cardiac output in man. J. Clin. Investig. 1942, 21, 287–294. [Google Scholar] [CrossRef]
- Hellems, H.K.; Haynes, F.W.; Dexter, L. Pulmonary Capillary Pressure in Man. J. Appl. Physiol. 1949, 2, 24–29. [Google Scholar] [PubMed]
- Swan, H.J.; Ganz, W.; Forrester, J.; Marcus, H.; Diamond, G.; Chonette, D. Catheterization of the Heart in Man with Use of a Flow-Directed Balloon-Tipped Catheter. N. Engl. J. Med. 1970, 283, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Lategola, M.; Rahn, H. A Self-Guiding Catheter for Cardiac and Pulmonary Arterial Catheterization and Occlusion. Proc. Soc. Exp. Biol. Med. 1953, 84, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Ganz, W.; Donoso, R.; Marcus, H.S.; Forrester, J.S.; Swan, H.J. A New Technique for Measurement of Cardiac Output by Thermodilution in Man. Am. J. Cardiol. 1971, 27, 392–396. [Google Scholar] [CrossRef]
- Ganz, W.; Tamura, K.; Marcus, H.S.; Donoso, R.; Yoshida, S.; Swan, H.J. Measurement of Coronary Sinus Blood Flow by Continuous Thermodilution in Man. Circulation 1971, 44, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Saugel, B.; Scheeren, T.W.L.; Teboul, J.-L. Ultrasound-Guided Central Venous Catheter Placement: A Structured Review and Recommendations for Clinical Practice. Crit. Care 2017, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Brass, P.; Hellmich, M.; Kolodziej, L.; Schick, G.; Smith, A.F. Ultrasound Guidance versus Anatomical Landmarks for Internal Jugular Vein Catheterization. Cochrane Database Syst. Rev. 2015, 1, CD006962. [Google Scholar] [CrossRef]
- Brass, P.; Hellmich, M.; Kolodziej, L.; Schick, G.; Smith, A.F. Ultrasound Guidance versus Anatomical Landmarks for Subclavian or Femoral Vein Catheterization. Cochrane Database Syst. Rev. 2015, 1, CD011447. [Google Scholar] [CrossRef]
- Parienti, J.-J.; Mongardon, N.; Mégarbane, B.; Mira, J.-P.; Kalfon, P.; Gros, A.; Marqué, S.; Thuong, M.; Pottier, V.; Ramakers, M.; et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N. Engl. J. Med. 2015, 373, 1220–1229. [Google Scholar] [CrossRef]
- Ge, X.; Cavallazzi, R.; Li, C.; Pan, S.M.; Wang, Y.W.; Wang, F.-L. Central Venous Access Sites for the Prevention of Venous Thrombosis, Stenosis and Infection. Cochrane Database Syst. Rev. 2012, 3, CD004084. [Google Scholar] [CrossRef]
- Hsu, C.C.-T.; Kwan, G.N.C.; Evans-Barns, H.; Rophael, J.A.; van Driel, M.L. Venous Cutdown versus the Seldinger Technique for Placement of Totally Implantable Venous Access Ports. Cochrane Database Syst. Rev. 2016, 8, CD008942. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, K. Left Internal versus Right Internal Jugular Vein Access to Central Venous Circulation Using the Seldinger Technique. J. Cardiothorac. Vasc. Anesth. 1995, 9, 115–116. [Google Scholar] [CrossRef]
- Meyer, P.; Cronier, P.; Rousseau, H.; Vicaut, E.; Choukroun, G.; Chergui, K.; Chevrel, G.; Maury, E. Difficult Peripheral Venous Access: Clinical Evaluation of a Catheter Inserted with the Seldinger Method under Ultrasound Guidance. J. Crit. Care 2014, 29, 823–827. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; Dimopoulos, K.; Coghlan, J.G.; Kovacs, G.; Rosenkranz, S.; Naeije, R. Right Heart Catheterization for the Diagnosis of Pulmonary Hypertension: Controversies and Practical Issues. Heart Fail. Clin. 2018, 14, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Preston, I.R. Right heart catheterisation: Best practice and pitfalls in pulmonary hypertension. Eur. Respir. Rev. 2015, 24, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Avian, A.; Pienn, M.; Naeije, R.; Olschewski, H. Reading Pulmonary Vascular Pressure Tracings. How to Handle the Problems of Zero Leveling and Respiratory Swings. Am. J. Respir. Crit. Care Med. 2014, 190, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Taquini, A.C.; Fermoso, J.D.; Aramendia, P. Behaviour of the right ventricle following acute constriction of the pulmonary artery. Circ. Res. 1960, 8, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sagawa, K.; Shoukas, A.A. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ. Res. 1973, 32, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Ditchey, R.F. Human right ventricular end-systolic pressure-volume relation defined by maximal elastance. Circulation 1988, 78, 81–91. [Google Scholar] [CrossRef]
- Lambermont, B.; Ghuysen, A.; Kolh, P.; Tchana-Sato, V.; Segers, P.; Gérard, P.; Morimont, P.; Magis, D.; Dogné, J.M.; Masereel, B.; et al. Effects of endotoxic shock on right ventricular systolic function and mechanical efficiency. Cardiovasc. Res. 2003, 59, 412–418. [Google Scholar] [CrossRef]
- Kuehne, T.; Yilmaz, S.; Steendijk, P.; Moore, P.; Groenink, M.; Saaed, M.; Weber, O.; Higgins, C.B.; Ewert, P.; Fleck, E.; et al. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: In vivo validation and clinical application in patients with pulmonary hypertension. Circulation 2004, 110, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Dell’Italia, L.J.; Walsh, R.A. Application of a time-varying elastance model to right ventricular performance in man. Cardiovasc. Res. 1988, 22, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Tedford, R.J.; Mudd, J.O.; Girgis, R.E.; Mathai, S.C.; Zaiman, A.L.; Housten-Harris, T.; Boyce, D.; Kelemen, B.W.; Bacher, A.C.; Shah, A.A.; et al. Right ventricular dysfunction in systemic sclerosis associated pulmonary arterial hypertension. Circ. Heart Fail. 2013, 6, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Rondelet, B.; Dewachter, C.; Kerbaul, F.; Kang, X.; Fesler, P.; Brimioulle, S.; Naeije, R.; Dewachter, L. Prolonged overcirculation-induced pulmonary arterial hypertension as a cause of right ventricular failure. Eur. Heart J. 2012, 33, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.J.; Dalinghaus, M.; Lankhuizen, I.M.; Steendijk, P.; Hop, W.C.; Schoemaker, R.G.; Duncker, D.J.; Lamers, J.M.; Helbing, W.A. Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure-volume loops. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1580–H1586. [Google Scholar] [CrossRef] [PubMed]
- Rex, S.; Missant, C.; Segers, P.; Rossaint, R.; Wouters, P.F. Epoprostenol treatment of acute pulmonary hypertension is associated with a paradoxical decrease in right ventricular contractility. Intensive Care Med. 2008, 34, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Brimioulle, S.; Wauthy, P.; Ewalenko, P.; Rondelet, B.; Vermeulen, F.; Kerbaul, F.; Naeije, R. Single-Beat Estimation of Right Ventricular End-Systolic Pressure-Volume Relationship. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1625–H1630. [Google Scholar] [CrossRef]
- Sunagawa, K.; Yamada, A.; Senda, Y.; Kikuchi, Y.; Nakamura, M.; Shibahara, T.; Nose, Y. Estimation of the Hydromotive Source Pressure from Ejecting Beats of the Left Ventricle. IEEE Trans. Biomed. Eng. 1980, 27, 299–305. [Google Scholar] [CrossRef]
- Trip, P.; Kind, T.; van de Veerdonk, M.C.; Marcus, J.T.; de Man, F.S.; Westerhof, N.; Vonk-Noordegraaf, A. Accurate Assessment of Load-Independent Right Ventricular Systolic Function in Patients with Pulmonary Hypertension. J. Heart Lung Transplant. 2013, 32, 50–55. [Google Scholar] [CrossRef]
- Katz, R.W.; Pollack, M.M.; Weibley, R.E. Pulmonary artery catheterization in pediatric intensive care. Adv. Pediatr. 1983, 30, 169–190. [Google Scholar]
- Monnet, X.; Richard, C.; Teboul, J.L. The pulmonary artery catheter in critically ill patients. Does it change outcome? Minerva Anestesiol. 2004, 70, 219–224. [Google Scholar] [PubMed]
- Aikawa, N.; Martyn, J.A.; Burke, J.F. Pulmonary artery catheterization and thermodilution cardiac output determination in the management of critically burned patients. Am. J. Surg. 1978, 135, 811–817. [Google Scholar] [CrossRef]
- Moser, K.M.; Spragg, R.G. Use of the balloon-tipped pulmonary artery catheter in pulmonary disease. Ann. Intern. Med. 1983, 98, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, A.A. Pulmonary artery catheterization and wedge pressure measurement in the general intensive therapy unit. Br. J. Anaesth. 1974, 46, 97–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Risk, S.C.; Brandon, D.; D’Ambra, M.N.; Koski, E.G.; Hoffman, W.J.; Philbin, D.M. Indications for the use of pacing pulmonary artery catheters in cardiac surgery. J. Cardiothorac. Vasc. Anesth. 1992, 6, 275–279. [Google Scholar] [CrossRef]
- McGee, W.T.; Mailloux, P.; Jodka, P.; Thomas, J. The pulmonary artery catheter in critical care. Semin. Dial. 2006, 19, 480–491. [Google Scholar] [CrossRef]
- Mora, C.T.; Seltzer, J.L.; McNulty, S.E. Evaluation of a new design pulmonary artery catheter for intraoperative ventricular pacing. J. Cardiothorac. Anesth. 1988, 2, 303–308. [Google Scholar] [CrossRef]
- Zaidan, J.R.; Freniere, S. Use of a pacing pulmonary artery catheter during cardiac surgery. Ann. Thorac Surg. 1983, 35, 633–636. [Google Scholar] [CrossRef]
- McNabb, T.G.; Green, L.H.; Parker, F.L. A Potentially Serious Complication with Swan-Ganz Catheter Placement by the Percutaneous Internal Jugular Route. Br. J. Anaesth. 1975, 47, 895–897. [Google Scholar] [CrossRef]
- Colvin, M.P.; Savege, T.M.; Lewis, C.T. Pulmonary Damage from a Swan-Ganz Catheter. Br. J. Anaesth. 1975, 47, 1107–1110. [Google Scholar]
- Katz, S.A.; Cohen, E.L. Urologic Complication Associated with Swan-Ganz Catheter. Urology 1975, 6, 716–718. [Google Scholar] [CrossRef]
- Sink, J.D.; Comer, P.B.; James, P.M.; Loveland, S.R. Evaluation of Catheter Placement in the Treatment of Venous Air Embolism. Ann. Surg. 1976, 183, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.R.; Glauser, F.L.; Jemison, P. Ruptured Chordae of the Tricuspid Valve. The Consequence of Flow-Directed Swan-Ganz Catheterization. Chest 1976, 70, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.A.; Puckett, R.P. Pulmonary Artery—Bronchial Fistula: A New Complication of Swan-Ganz Catheterization. Chest 1979, 75, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.P.; Sandza, J.G.; Shaw, R.C.; Wolff, G.A.; Lombardo, J.A. Lobar Pulmonary Hemorrhage. An Unusual Complication of Swan-Ganz Catheterization. Arch. Surg. 1980, 115, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Connors, A.F.; Speroff, T.; Dawson, N.V.; Thomas, C.; Harrell, F.E., Jr.; Wagner, D.; Desbiens, N.; Goldman, L.; Wu, A.W.; Califf, R.M.; et al. The Effectiveness of Right Heart Catheterization in the Initial Care of Critically III Patients. JAMA 1996, 276, 889–897. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Schoenfeld, D.; Wiedemann, H.P.; deBoisblanc, B.; Connors, A.F., Jr.; Hite, R.D.; Harabin, A.L. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N. Engl. J. Med. 2006, 354, 2213–2224. [Google Scholar]
- Harvey, S.; Harrison, D.A.; Singer, M.; Ashcroft, J.; Jones, C.M.; Elbourne, D.; Brampton, W.; Williams, D.; Young, D.; Rowan, K.; et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): A randomised controlled trial. Lancet 2005, 366, 472–477. [Google Scholar] [CrossRef]
- Cohen, M.G.; Kelly, R.V.; Kong, D.F.; Menon, V.; Shah, M.; Ferreira, J.; Pieper, K.S.; Criger, D.; Poggio, R.; Ohman, E.M.; et al. Pulmonary artery catheterization in acute coronary syndromes: Insights from the GUSTO IIb and GUSTO III trials. Am. J. Med. 2005, 118, 482–488. [Google Scholar] [CrossRef]
- Richard, C.; Warszawski, J.; Anguel, N.; Deye, N.; Combes, A.; Barnoud, D.; Boulain, T.; Lefort, Y.; Fartoukh, M.; Baud, F.; et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: A randomized controlled trial. JAMA 2003, 290, 2713–2720. [Google Scholar] [CrossRef]
- Rhodes, A.; Cusack, R.J.; Newman, P.J.; Grounds, R.M.; Bennett, E.D. A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med. 2002, 28, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, S.D.; Saint, S.; Sullivan, S.D.; Dey, L.; Kelley, K.; Bowdle, A. Clinical and economic effects of pulmonary artery catheterization in nonemergent coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2000, 14, 113–118. [Google Scholar] [CrossRef]
- Wiener, R.S.; Welch, H.G. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA 2007, 298, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.; Wang, Y.; Robinson, A.; Ahmad, T.; Krumholz, H.M.; Desai, N.R. National Trends in Use and Outcomes of Pulmonary Artery Catheters Among Medicare Beneficiaries, 1999–2013. JAMA Cardiol. 2017, 2, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Francis, G.S.; Leier, C.V.; Miller, L.W.; et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness: The ESCAPE Trial. JAMA 2005, 294, 1625–1633. [Google Scholar]
- Sionis, A.; Rivas-Lasarte, M.; Mebazaa, A.; Tarvasmäki, T.; Sans-Roselló, J.; Tolppanen, H.; Varpula, M.; Jurkko, R.; Banaszewski, M.; Silva-Cardoso, J.; et al. Current Use and Impact on 30-Day Mortality of Pulmonary Artery Catheter in Cardiogenic Shock Patients: Results From the CardShock Study. J. Intensive Care Med. 2019. [Google Scholar] [CrossRef]
- Doshi, R.; Patel, H.; Shah, P. Pulmonary artery catheterization use and mortality in hospitalizations with HFrEF and HFpEF: A nationally representative trend analysis from 2005 to 2014. Int. J. Cardiol. 2018, 269, 289–291. [Google Scholar] [CrossRef]
- Hernandez, G.A.; Lemor, A.; Blumer, V.; Rueda, C.A.; Zalawadiya, S.; Stevenson, L.W.; Lindenfeld, J. Trends in Utilization and Outcomes of Pulmonary Artery Catheterization in Heart Failure with and Without Cardiogenic Shock. J. Card. Fail. 2019, 25, 364–371. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Sherwood, M.W.; Desai, S.; Tran, H.A.; Epps, K.C.; Singh, R.; Psotka, M.; Shah, P.; Cooper, L.B.; et al. Standardized Team-Based Care for Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 1659–1669. [Google Scholar] [CrossRef]
- Ma, T.S.; Paniagua, D.; Denktas, A.E.; Jneid, H.; Kar, B.; Chan, W.; Bozkurt, B. Usefulness of the Sum of Pulmonary Capillary Wedge Pressure and Right Atrial Pressure as a Congestion Index That Prognosticates Heart Failure Survival (from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Trial). Am. J. Cardiol. 2016, 118, 854–859. [Google Scholar]
- Uriel, N.; Sayer, G.; Addetia, K.; Fedson, S.; Kim, G.H.; Rodgers, D.; Kruse, E.; Collins, K.; Adatya, S.; Sarswat, N. Hemodynamic Ramp Tests in Patients with Left Ventricular Assist Devices. JACC Heart Fail. 2016, 4, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Estep, J.D.; Vivo, R.P.; Krim, S.R.; Cordero-Reyes, A.M.; Elias, B.; Loebe, M.; Bruckner, B.A.; Bhimaraj, A.; Trachtenberg, B.H.; Ashrith, G. Echocardiographic Evaluation of Hemodynamics in Patients with Systolic Heart Failure Supported by a Continuous-Flow LVAD. J. Am. Coll. Cardiol. 2014, 64, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Uriel, N.; Levin, A.P.; Sayer, G.T.; Mody, K.P.; Thomas, S.S.; Adatya, S.; Yuzefpolskaya, M.; Garan, A.R.; Breskin, A.; Takayama, H. Left Ventricular Decompression During Speed Optimization Ramps in Patients Supported by Continuous-Flow Left Ventricular Assist Devices: Device-Specific Performance Characteristics and Impact on Diagnostic Algorithms. J. Card. Fail. 2015, 21, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.A.; Jia, C.X.; Cabreriza, S.E.; D’Alessandro, D.A.; Dickstein, M.L.; Sardo, M.J.; Chalik, N.; Spotnitz, H.M. Validation study of a new transit time ultrasonic flow probe for continuous great vessel measurements. ASAIO J. 1996, 42, M671–M676. [Google Scholar] [CrossRef] [PubMed]
- Salerno, C.T.; Sundareswaran, K.S.; Schleeter, T.P.; Moanie, S.L.; Farrar, D.J.; Walsh, M.N. Early elevations in pump power with the HeartMate II left ventricular assist device do not predict late adverse events. J. Heart Lung. Transplant. 2014, 33, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Rogers, J.G.; Warnica, J.W.; Disalvo, T.G.; Tasissa, G.; Binanay, C.; O’Connor, C.M.; Califf, R.M.; Leier, C.V.; Shah, M.R.; et al. High Mortality without ESCAPE: The Registry of Heart Failure Patients Receiving Pulmonary Artery Catheters without Randomization. J. Card. Fail. 2008, 14, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and Diagnosis of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. 25), D42–D50. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Dumitrescu, D.; Barner, A.; Greiner, S.; Grünig, E.; Hager, A.; Köhler, T.; Kozlik-Feldmann, R.; Kruck, I.; Lammers, A.E.; et al. Definition, clinical classification and initial diagnosis of pulmonary hypertension: Updated recommendations from the Cologne Consensus Conference 2018. Int. J. Cardiol. 2018, 272, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Dragu, R.; Hardak, E.; Ohanyan, A.; Adir, Y.; Aronson, D. Prognostic value and diagnostic properties of the diastolic pulmonary pressure gradient in patients with pulmonary hypertension and left heart disease. Int. J. Cardiol. 2019, 290, 138–143. [Google Scholar] [CrossRef]
- Rich, S.; Kaufmann, E.; Levy, P.S. The Effect of High Doses of Calcium-Channel Blockers on Survival in Primary Pulmonary Hypertension. N. Engl. J. Med. 1992, 327, 76–81. [Google Scholar] [CrossRef]
- Sitbon, O.; Humbert, M.; Jaïs, X.; Ioos, V.; Hamid, A.M.; Provencher, S.; Garcia, G.; Parent, F.; Hervé, P.; Simonneau, G. Long-Term Response to Calcium Channel Blockers in Idiopathic Pulmonary Arterial Hypertension. Circulation 2005, 111, 3105–3111. [Google Scholar] [CrossRef] [PubMed]
- Badesch, D.B.; Abman, S.H.; Ahearn, G.S.; Barst, R.J.; McCrory, D.C.; Simonneau, G.; McLaughlin, V.V.; American College of Chest Physicians. Medical Therapy for Pulmonary Arterial Hypertension: ACCP Evidence-Based Clinical Practice Guidelines. Chest 2004, 126 (Suppl. 1), 35S–62S. [Google Scholar] [CrossRef] [PubMed]
- Costard-Jäckle, A.; Fowler, M.B. Influence of Preoperative Pulmonary Artery Pressure on Mortality after Heart Transplantation: Testing of Potential Reversibility of Pulmonary Hypertension with Nitroprusside Is Useful in Defining a High Risk Group. J. Am. Coll. Cardiol. 1992, 19, 48–54. [Google Scholar] [CrossRef]
- Zakliczynski, M.; Zebik, T.; Maruszewski, M.; Swierad, M.; Zembala, M. Usefulness of Pulmonary Hypertension Reversibility Test with Sodium Nitroprusside in Stratification of Early Death Risk after Orthotopic Heart Transplantation. Transplant. Proc. 2005, 37, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Abbreviation | Normal Value | Definitions/Comments |
|---|---|---|---|
| Heart rate | HR | 60–100 bpm | number of beats per minute |
| Stroke volume | SV | 60–100 mL/beat | the amount of blood pumped by each ventricle of the heart during contraction |
| Cardiac output | CO | 4.0–8.0 L/min | volume of blood being pumped within the minute |
| Body surface area | BSA | 1.6–1.9 m2 | the measured or calculated surface area of a human body |
| Cardiac index | CI | 2.5–4.0 L/min/m2 | CO/BSA |
| Central Venous Pressure | CVP | 2–6 mmHg | superior vena cava pressure—might be used to estimate preload and right atrial pressure |
| Right atrial pressure | RAP | 2–6 mmHg | usually RAP~CVP |
| Pulmonary artery pressure (systolic/diastolic/mean) (s/d/m) | PAPs/d/m | 15–30/8–15/9–18 mmHg | assessed by a Swan-Ganz catheter placed in either RPA or LPA |
| Pulmonary artery wedge pressure | PAWP | 6–12 mmHg | the inflated balloon of the catheter tip passively transmits the pressure of LA |
| Transpulmonary gradient | TPG | ≤12 mm Hg | PAPm—PAWPm |
| Pulmonary vascular resistance | PVR | <3.125 WU | PAPm—PAWPm/CO |
| Diastolic pulmonary gradient | DPG | <7 mmHg | PAPd—PAWPm |
| Systemic vascular resistance | SVR | 10–15 WU | SBPm—RAPm/CO |
| Author, Reference Number, and the Year of Publication | Number of Patients and the Time of Observation | Primary Condition Diagnosed/Treated | Results and Main Findings |
|---|---|---|---|
| Connors [76], 1996 “SUPPORT” | 5735 pts, 180 days | Severe illness. RHC group versus no RHC group: Acute respiratory failure: 589 (27%) versus 1200 (34%) Multiorgan system failure: 1235 (57%) versus 1245 (35%) Congestive heart failure: 209 (10%) versus 247 (7%) Other: 151 (7%) versus 859 (24%) |
|
| Wheeler [77], 2006 | 1000 pts, 60 days | Acute lung injury (ALI) |
|
| Harvey [78], 2005 “PAC-Man” | 1014 pts, 90 days | Acute respiratory failure (13%), Multiorgan dysfunction (65–66%), Decompensated heart failure (11%), Other (10–11%) |
|
| Ramsey [82], 2004 | 13,907 pts, 7–8 days | Aortocoronary bypass for heart revascularization—any type |
|
| Binanay [85], 2005 “ESCAPE” | 433 pts, 180 days | Severe symptomatic heart failure despite recommended therapies |
|
| Sionis [86], 2019 “CardSchock” | 219 pts, 82 (37.4%) received PAC, 30 days | Patients with cardiogenic shock included in the CardShock Study |
|
| Doshi [87], 2018 | 6,645,363 pts, in hospital stay between 3–17 days | HFrEF—3,225,529 hospitalizations HFpEF—3,419,834 hospitalizations |
|
| Hernandez [88], 2019 | 9,431,944 pts, in hospital stay between 2–20 days | HF (n = 8,516,528) index manifestation CS (n = 915,416) developed during the index hospitalization |
|
| Tehrani [89], 2019 | 204 consecutive pts, 30-day survival | CS |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, G.M.; Ciarka, A.; Biniecka, M.; Ceranowicz, P. Right Heart Catheterization—Background, Physiological Basics, and Clinical Implications. J. Clin. Med. 2019, 8, 1331. https://doi.org/10.3390/jcm8091331
Kubiak GM, Ciarka A, Biniecka M, Ceranowicz P. Right Heart Catheterization—Background, Physiological Basics, and Clinical Implications. Journal of Clinical Medicine. 2019; 8(9):1331. https://doi.org/10.3390/jcm8091331
Chicago/Turabian StyleKubiak, Grzegorz M., Agnieszka Ciarka, Monika Biniecka, and Piotr Ceranowicz. 2019. "Right Heart Catheterization—Background, Physiological Basics, and Clinical Implications" Journal of Clinical Medicine 8, no. 9: 1331. https://doi.org/10.3390/jcm8091331
APA StyleKubiak, G. M., Ciarka, A., Biniecka, M., & Ceranowicz, P. (2019). Right Heart Catheterization—Background, Physiological Basics, and Clinical Implications. Journal of Clinical Medicine, 8(9), 1331. https://doi.org/10.3390/jcm8091331






