Contemporary Review of Borderline Resectable Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
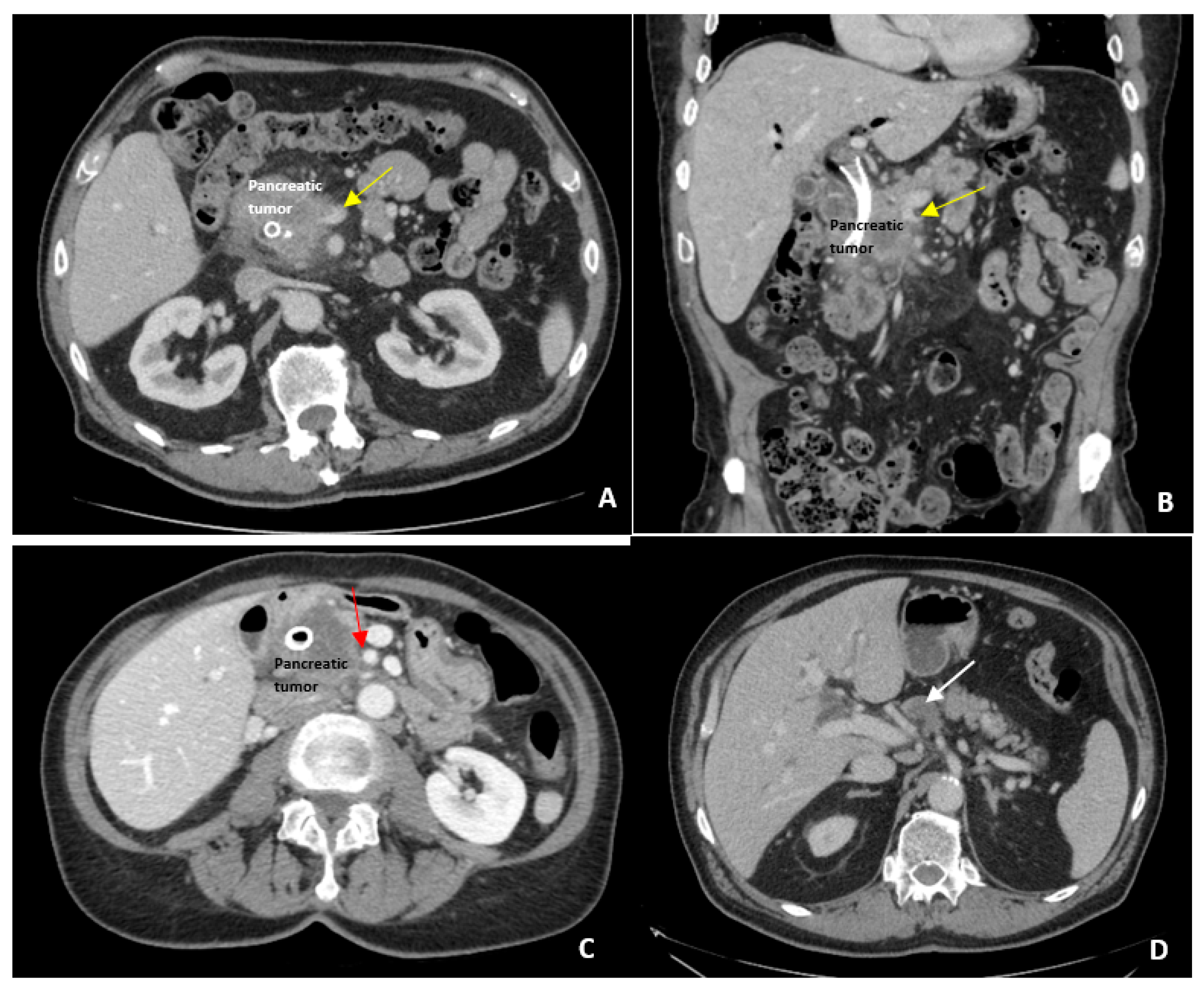
2. Definition of Borderline Resectable Disease
3. Current Treatment for Borderline Resectable Disease
3.1. Neoadjuvant Therapy
3.2. Radiation Therapy
3.3. Assessing Response to Neoadjuvant Therapy
3.4. Surgical Resection
4. Current Investigation in the Treatment of Borderline Resectable Disease
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Raut, C.P.; Tseng, J.F.; Sun, C.C.; Wang, H.; Wolff, R.A.; Crane, C.H.; Hwang, R.; Vauthey, J.N.; Abdalla, E.K.; Lee, J.E.; et al. Impact of Resection Status on Pattern of Failure and Survival after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma. Ann. Surg. 2007, 246, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Menon, K.V.; Gomez, D.; Smith, A.M.; Anthoney, A.; Verbeke, C.S. Impact of Margin Status on Survival Following Pancreateoduodenectomy for Cancer: The Leeds Pathology Protocol (LEEPP). HPB 2009, 11, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, W.; Hackert, T.; Hinz, U.; Gluth, A.; Bergmann, F.; Strobel, O.; Buchler, M.; Werner, J. Pancreatic Cancer Surgery in the New Millennium: Better Prediction of Outcome. Ann. Surg. 2011, 254, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.K.; Fisher, G.; Ford, J.A.; Poen, J.C.; Vierra, M.A.; Oberhelman, H.; Niederhuber, J.; Bastidas, J.A. Preoperative Chemoradiation for Marginally Resectable Adenocarcinoma of the Pancreas. J. Gastrointest. Surg. 2001, 5, 27–35. [Google Scholar] [CrossRef]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.T.; Evans, D.B.; Wolff, R.A. Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Buchler, M.; Charnley, R.M.; et al. Borderline Resectable Pancreatic Cancer: A Consensus Statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernandez-del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.; Kim, S.W.; Kishiwada, M.; et al. International Consensus on Definition and Criteria of Boderline Resectable Pancreatic Ductal Adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Callery, M.P.; Chang, K.J.; Fishman, E.K.; Talamonti, M.S.; Traverson, L.W.; Linehan, D.C. Pretreatment Assessment of Resectable and Borderline Resectable Pancreatic Cancer: Expert Consensus. Ann. Surg. Oncol. 2009, 16, 1727–1733. [Google Scholar] [CrossRef]
- Clanton, J.; Oh, S.; Kaplan, S.J.; Johnson, E.; Ross, A.; Kozarek, R.; Alseidi, A.; Biehl, T.; Picozzi, V.J.; Helton, W.S.; et al. Does Mesenteric Venous Imaging Assessment Accurately Predict Pathologic Invasion in Localized Pancreatic Ductal Adenocarcinoma? HPB 2018, 20, 925–931. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Pisters, P.W.T.; Evans, D.B.; Sun, C.C.; Lee, J.E.; Fleming, J.B.; Vauthey, J.N.; Abdalla, E.K.; Crane, C.H.; Wolff, R.A.; et al. Borderline Resectable Pancreatic Cancer: The Importance of this Emerging Stage of Disease. J. Am. Coll. Surg. 2008, 206, 833–848. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Marsh, R.; Herman, J.M.; Shi, Q.; Collison, E.; Venook, A.P.; Kindler, H.L.; Alberts, S.R.; Philip, P.; Lowy, A.M.; et al. Borderline Resectable Pancreatic Cancer: Need for Standardization and Methods for Optimal Clinical Trial Design. Ann. Surg. Oncol. 2013, 20, 2787–2795. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M. “NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma”. Version 2. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf (accessed on 6 June 2019).
- Piagnerelli, R.; Marrelli, D.; Roviello, G.; Ferrara, F.; Di Mare, G.; Voglino, C.; Petrioli, R.; Marini, M.; Macchiarelli, R.; Roviello, F. Clinical Value and Impact on Prognosis of Peri-operative CA 19-9 Serum Levels in Stage I and II Adenocarcinoma of the Pancreas. Tumor Biol. 2016, 37, 1959–1966. [Google Scholar] [CrossRef]
- Bergquist, J.R.; Puig, C.A.; Shubert, C.R.; Groeschl, R.T.; Habermann, E.B.; Kendrick, M.L.; Nagorney, D.M.; Smoot, R.L.; Farnell, M.B.; Truty, M.J. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J. Am. Coll. Surg. 2016, 223, 52–65. [Google Scholar] [CrossRef]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McCallister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef]
- Mokdad, A.A.; Minter, R.M.; Zhu, H.; Augustine, M.M.; Porembka, M.R.; Wang, S.C.; Yopp, A.C.; Mansour, J.C.; Choti, M.A.; Polanco, P.M. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J. Clin. Oncol. 2016, 35, 515–522. [Google Scholar] [CrossRef]
- Aldakkak, M.; Christians, K.K.; Krepline, A.N.; George, B.; Ritch, P.S.; Erickson, B.A.; Johnston, F.M.; Evans, D.B.; Tsai, S. Pre-treatment carbohydrate antigen 19-9 does not predict the response to neoadjuvant therapy in patients with localized pancreatic cancer. HPB 2015, 17, 942–952. [Google Scholar] [CrossRef]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncologic Benefits of Neoadjuvant Chemoradiation with Gemcitabine Versus Upfront Surgery in Patients with Borderline Resectable Pancreatic Cancer: A Prospective Randomized Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Rose, J.B.; Rocha, F.G.; Alseidi, A.; Biehl, T.; Moonka, R.; Ryan, J.A.; Lin, B.; Picozzi, V.; Helton, S. Extended Neoadjuvant Chemotherapy for Borderline Resectable Pancreatic Cancer Demonstrates Promising Postoperative Outcomes and Survival. Ann. Surg. Oncol. 2014, 21, 1530–1537. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Takahashi, S.; Sueda, T. Survival Impact of Neoadjuvant Gemcitabine Plus S-1 Chemotherapy for Patients with Borderline Resectable Pancreatic Carcinoma with Arterial Contact. Cancer Chemother. Pharmacol. 2017, 79, 37–47. [Google Scholar] [CrossRef]
- Ferrone, C.R.; Marchegiani, G.; Hong, T.S.; Ryan, D.P.; Deshpande, V.; McDonnell, E.I.; Sabbatino, F.; Santos, D.D.; Allen, J.N.; Blaszkowsky, L.S.; et al. Radiological and Surgical Implications of Neoadjuvant Treatment With FOLFIRINOX for Locally Advanced and Borderline Resectable Pancreatic Cancer. Ann. Surg. 2015, 261, 12–17. [Google Scholar] [CrossRef]
- Michelakos, T.; Pergolini, I.; Castillo, C.F.D.; Honselmann, K.C.; Cai, L.; Deshpande, V.; Wo, J.Y.; Ryan, D.P.; Allen, J.N.; Blaszkowsky, L.S.; et al. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment With Folfirinox. Ann. Surg. 2019, 269, 733–740. [Google Scholar] [CrossRef]
- Franko, J.; Hsu, H.; Thirunavukarasu, P.; Frankova, D.; Goldman, C. Chemotherapy and radiation components of neoadjuvant treatment of pancreatic head adenocarcinoma: Impact on perioperative mortality and long-term survival. Eur. J. Surg. Oncol. 2017, 43, 351–357. [Google Scholar] [CrossRef]
- Lutfi, W.; Talamonti, M.S.; Kantor, O.; Wang, C.H.; Stocker, S.J.; Bentrem, D.J.; Roggin, K.K.; Winchester, D.J.; Marsh, R.; Prinz, R.A.; et al. Neoadjuvant external beam radiation is associated with No benefit in overall survival for early stage pancreatic cancer. Am. J. Surg. 2017, 213, 521–525. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Chen, H.C.; Wang, X.; Tzeng, C.W.; Kim, M.P.; Aloia, T.A.; Vauthey, J.N.; Lee, J.E.; Katz, M.H.G. Chemotherapy Versus Chemoradiation as Preoperative Therapy for Resectable Pancreatic Ductal Adenocarcinoma: A Propensity Score Adjusted Analysis. Pancreas 2019, 48, 216–222. [Google Scholar] [CrossRef]
- Chuong, M.D.; Springett, G.M.; Freilich, J.M.; Park, C.K.; Weber, J.M.; Mellon, E.A.; Hodul, P.J.; Malafa, M.P.; Meredith, K.L.; Hoffe, S.E.; et al. Stereotactic Body Radiation Therapy for Locally Advanced and Borderline Resectable Pancreatic Cancer Is Effective and Well Tolerated. Int. J. Radiat. Oncol. 2013, 86, 516–522. [Google Scholar] [CrossRef]
- Mellon, E.A.; Hoffe, S.E.; Springett, G.M.; Hodul, P.J.; Malafa, M.P.; Frakes, J.M.; Chuong, M.D.; Strom, T.J.C.; Shridhar, R. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015, 54, 979–985. [Google Scholar] [CrossRef]
- Lin, C.; Verma, V.; Ly, Q.P.; Lazenby, A.; Sasson, A.; Schwarz, J.K.; Meza, J.L.; Are, C.; Li, S.; Wang, S.; et al. Phase I trial of concurrent stereotactic body radiotherapy and nelfinavir for locally advanced borderline or unresectable pancreatic adenocarcinoma. Radiother. Oncol. 2019, 132, 55–62. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Fleming, J.B.; Bhosale, P.; Varadhachary, G.; Lee, J.E.; Wolff, R.; Wang, H.; Abbruzzese, J.; Pisters, P.W.T.; Vauthey, J.N.; et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012, 118, 5749–5756. [Google Scholar] [CrossRef]
- Van Veldhuisen, E.; Vogel, J.A.; Klompmaker, S.; Busch, O.R.; van Laarhoven, H.W.M.; van Lienden, K.P.; Wilmink, J.W.; Marsman, H.A.; Besselink, M.G. Added Value of CA 19-9 Response in Predicting Resectability of Locally Advanced Pancreatic Cancer Following Induction Chemotherapy. HPB 2018, 20, 605–611. [Google Scholar] [CrossRef]
- Akita, H.; Takahashi, H.; Ohigashi, H.; Tomokuni, A.; Kobayashi, S.; Sugimura, K.; Miyoshi, N.; Moon, J.H.; Yasui, M.; Omori, T.; et al. FDG-PET predicts treatment efficacy and surgical outcome of pre-operative chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Eur. J. Surg. Oncol. 2017, 43, 1061–1067. [Google Scholar] [CrossRef]
- Fuhrman, G.M.; Leach, S.D.; Staley, C.A.; Cusack, J.C.; Charnsangavej, C.; Cleary, K.R.; El-Naggar, A.K.; Fenoglio, C.J.; Lee, J.E.; Evans, D.B. Rationale for En Bloc Vein Resection in the Treatment of Pancreatic Adenocarcinoma Adherent to the Superior Mesenteric-Portal Vein Confluence. Ann. Surg. 1996, 223, 154–162. [Google Scholar] [CrossRef]
- Tseng, J.F.; Raut, C.P.; Lee, J.E.; Pisters, P.W.T.; Vauthey, J.N.; Abdalla, E.K.; Gomez, H.F.; Sun, C.C.; Crane, C.H.; Wolff, R.A.; et al. Pancreaticoduodenectomy with Vascular Resection: Margin Status and Survival. J. Gastrointest. Surg. 2004, 8, 935–950. [Google Scholar] [CrossRef]
- Adham, M.; Mirz, D.F.; Chapuis, F.; Mayer, A.D.; Bramhall, S.R.; Coldham, C.; Baulieux, J.; Buckels, J. Results of Vascular Resections During Pancreatectomy from Two European Centres: An Analysis of Survival and Disease-free Survival Explicative Factors. HPB 2006, 8, 465–473. [Google Scholar] [CrossRef][Green Version]
- Ouaissi, M.; Hubert, C.; Verhelst, R.; Astarci, P.; Sempoux, C.; Jouret-Mourin, A.; Loundou, A.; Gigot, J.F. Vascular Reconstruction During Pancreatoduodenectomy for Ductal Adenocarcinoma of the Pancreas Improves Resectability but Does Not Achieve Cure. World J. Surg. 2010, 34, 2648–2661. [Google Scholar] [CrossRef]
- Worni, M.; Castleberry, A.W.; Clary, B.M.; Gloor, B.; Carvalho, E.; Jacobs, D.O.; Pietrobon, R.; Scarborough, J.E.; White, R.R. Concomitant Vascular Reconstruction During Pancreatectomy for Malignant Disease. JAMA Surg. 2013, 148, 331. [Google Scholar] [CrossRef]
- Alemi, F.; Rocha, F.G.; Helton, W.S.; Biehl, T.; Alseidi, A. Classification and Techniques of en bloc Venous Reconstruction for Pancreaticoduodenectomy. HPB 2016, 18, 827–834. [Google Scholar] [CrossRef]
- Van Tienhoven, G.; Versteijne, E.; Suker, M.; Groothuis, K.B.C.; Busch, O.R.; Bonsing, B.A.; de Hingh, I.H.J.T.; Festen, S.; Patijn, G.A.; de Vos-Geelen, J.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer (PREOPANC-1): A Randomized, Controlled, Multicenter Phase III Trial. J. Clin. Oncol. 2018, 36, LBA4002. [Google Scholar] [CrossRef]
- Sohal, D.; McDonough, S.; Ahmad, S.A.; Gandhi, N.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Guthrie, K.A.; Lowy, A.M.; Philip, P.A.; et al. SWOG S1505, Initial Findings on Eligibility and Neoadjuvant Chemotherapy Experience with mFOLFIRINOX Versus Gemcitabine/nab-Paclitaxel for Resectable Pancreatic Adenocarcinoma. J. Clin. Oncol. 2018, 37, 414. [Google Scholar] [CrossRef]
- Takahashi, S.; Ohno, I.; Ikeda, M.; Konishi, M.; Kobayashi, T.; Akimoto, T.; Kojima, M.; Morinaga, S.; Toyama, H.; Shimizu, Y.; et al. Final results of JASPAC05: Phase II trial of neoadjuvant S-1 and concurrent radiotherapy followed by surgery in borderline resectable pancreatic cancer. J. Clin. Oncol. 2019, 37, 4127. [Google Scholar] [CrossRef]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; Yoshitomi, H.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J. Clin. Oncol. 2019, 37, 189. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Shi, Q.; Ahmad, S.A.; Herman, J.M.; de WMarsh, R.; Collisson, E.; Schwartz, L.; Frankel, W.; Martin, R.; Conway, W.; et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016, 151, e161137. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Ou, F.S.; Herman, J.M.; Ahmad, S.A.; Wolpin, B.; Marsh, R.; Behr, S.; Shi, Q.; Chuong, M.; Schwartz, L.H.; et al. Alliance for Clinical Trials in Oncology (ALLIANCE) Trial A021501: Preoperative Extended Chemotherapy vs. Chemotherapy Plus Hypofractionated Radiation Therapy for Borderline Resectable Adenocarcinoma of the Head of the Pancreas. BMC Cancer 2017, 17, 505–512. [Google Scholar] [CrossRef]

| NCCN Definition | AHPBA/SSO/SSAT Consensus Definition | MD Anderson Modified Definition | IAP Consensus Definition | |
|---|---|---|---|---|
| Venous Involvement | Involvement of SMV or PV that distorts, narrows, or occludes the vein with suitable vessel proximal and distal allowing resection and replacement | Involvement of the SMV or PV with or without narrowing, or encasement of the SMV or PV without encasement of nearby arteries, or short segment occlusion from tumor encasement or thrombus allowing resection and reconstruction | Short segment occlusion of SMV, PV, or SMV-PV confluence amenable to vascular resection and reconstruction | Tumor contact of 180 degrees or more circumference or occlusion of the SMV, PV, or SMV-PV confluence that does not exceed the inferior border of the duodenum |
| Arterial Involvement | Gastroduodenal involvement up to the hepatic artery with short segment encasement or direct abutment of the hepatic artery without extension to the celiac access | Gastroduodenal artery encasement up to hepatic artery with short segment encasement or abutment of the hepatic artery without extension to the celiac access | 180 degree or less circumference involvement of the SMA or celiac access or short segment abutment/encasement of the hepatic artery (typically origin of gastroduodenal artery) | Tumor contact of 180 degrees or less circumference of the SMA or celiac access without deformity or tumor contact of the common hepatic artery without abutting the proper hepatic artery or celiac access |
| Biological | None | None | Concern for extrapancreatic disease (suspicious but non-diagnostic metastatic lesions or locoregional lymph node involvement) | Anatomically resectable PDAC suspicious for extrapancreatic disease (CA 19-9 of 500 units/mL or more or regional lymph node metastases on biopsy or PET-CT) |
| Performance Status | None | None | Poor performance status (ECOG 3 or more) or significant medical comorbidities that preclude immediate surgery | Anatomically resectable PDAC with poor performance status (ECOG 2 or more) |
| Study | Year | Pts | Status | Chemo | Resected (%) | Vein Resection (%) | Median Survival (months) All/R/UR | R0 (%) |
|---|---|---|---|---|---|---|---|---|
| Mehta | 2001 | 15 | Borderline | 5-FU | 60 | NA | NA/30/8 | 100 |
| Massuco | 2006 | 28 | Borderline Unresectable | GemOx | 39 | 38 | 15/21/10 | 87 |
| Small | 2008 | 39 | Resectable Borderline Unresectable | Gem/XRT | 33 | NA | 76% at 1 year | 94 |
| Katz | 2008 | 160 | Borderline | Gem/XRT | 41 | 27 | NA/40/13 | 94 |
| McClaine | 2010 | 29 | Borderline | Gem/XRT | 41 | 42 | NA/23.3/15.5 | 67 |
| Patel | 2011 | 17 | Borderline | Gem/Tax Cape 5-FU/XRT | 64 | 22 | 15/NA/NA | 89 |
| Stokes | 2011 | 40 | Borderline | Cape/XRT | 40 | 58 | 12/23/NA | 88 |
| Takahashi | 2013 | 80 | Borderline | Gem/XRT/LP | 51 | NR | 34% at 5 years | 100 |
| Christians | 2014 | 18 | Borderline | FOLFIRINOX Gem/XRT Cape/XRT | 67 | 83% | NA/NA/9.3 | 100 |
| Rose | 2014 | 64 | Borderline | Gem/Tax | 48 | 48 | 23.6/NA/15.4 | 87 |
| Blazer | 2015 | 43 | Borderline Unresectable | FOLFIRINOX GemOx/XRT | 51 | 18 | 21.2/NA/12.7 | 86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonds, M.; Rocha, F.G. Contemporary Review of Borderline Resectable Pancreatic Ductal Adenocarcinoma. J. Clin. Med. 2019, 8, 1205. https://doi.org/10.3390/jcm8081205
Bonds M, Rocha FG. Contemporary Review of Borderline Resectable Pancreatic Ductal Adenocarcinoma. Journal of Clinical Medicine. 2019; 8(8):1205. https://doi.org/10.3390/jcm8081205
Chicago/Turabian StyleBonds, Morgan, and Flavio G. Rocha. 2019. "Contemporary Review of Borderline Resectable Pancreatic Ductal Adenocarcinoma" Journal of Clinical Medicine 8, no. 8: 1205. https://doi.org/10.3390/jcm8081205
APA StyleBonds, M., & Rocha, F. G. (2019). Contemporary Review of Borderline Resectable Pancreatic Ductal Adenocarcinoma. Journal of Clinical Medicine, 8(8), 1205. https://doi.org/10.3390/jcm8081205




