A Novel Application of Buprenorphine Transdermal Patch to Relieve Pain in the Knee Joint of Knee Osteoarthritis Patients: A Retrospective Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
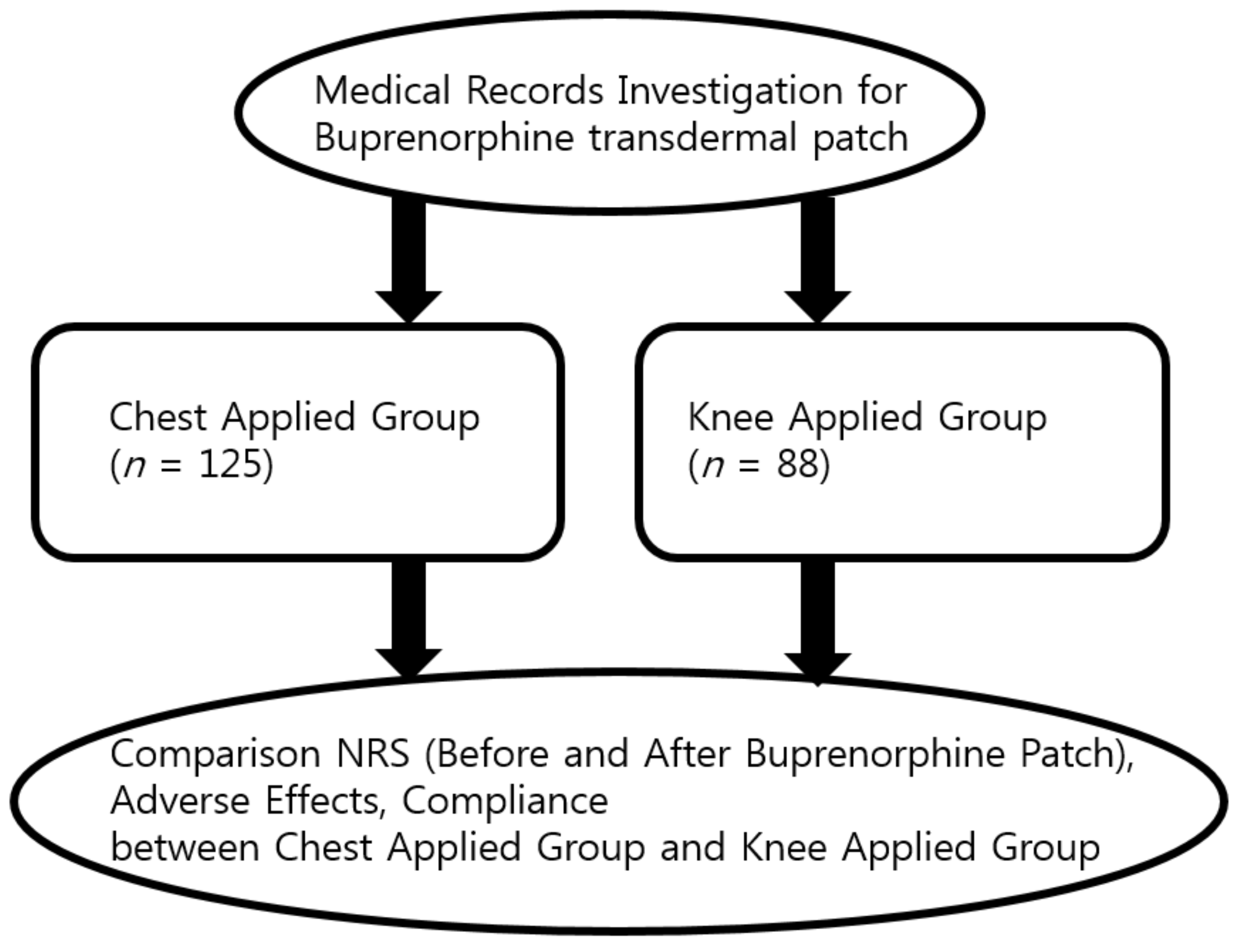
2.2. Participants
2.3. Clinical Evaluations
2.4. Statistical Analysis
3. Results
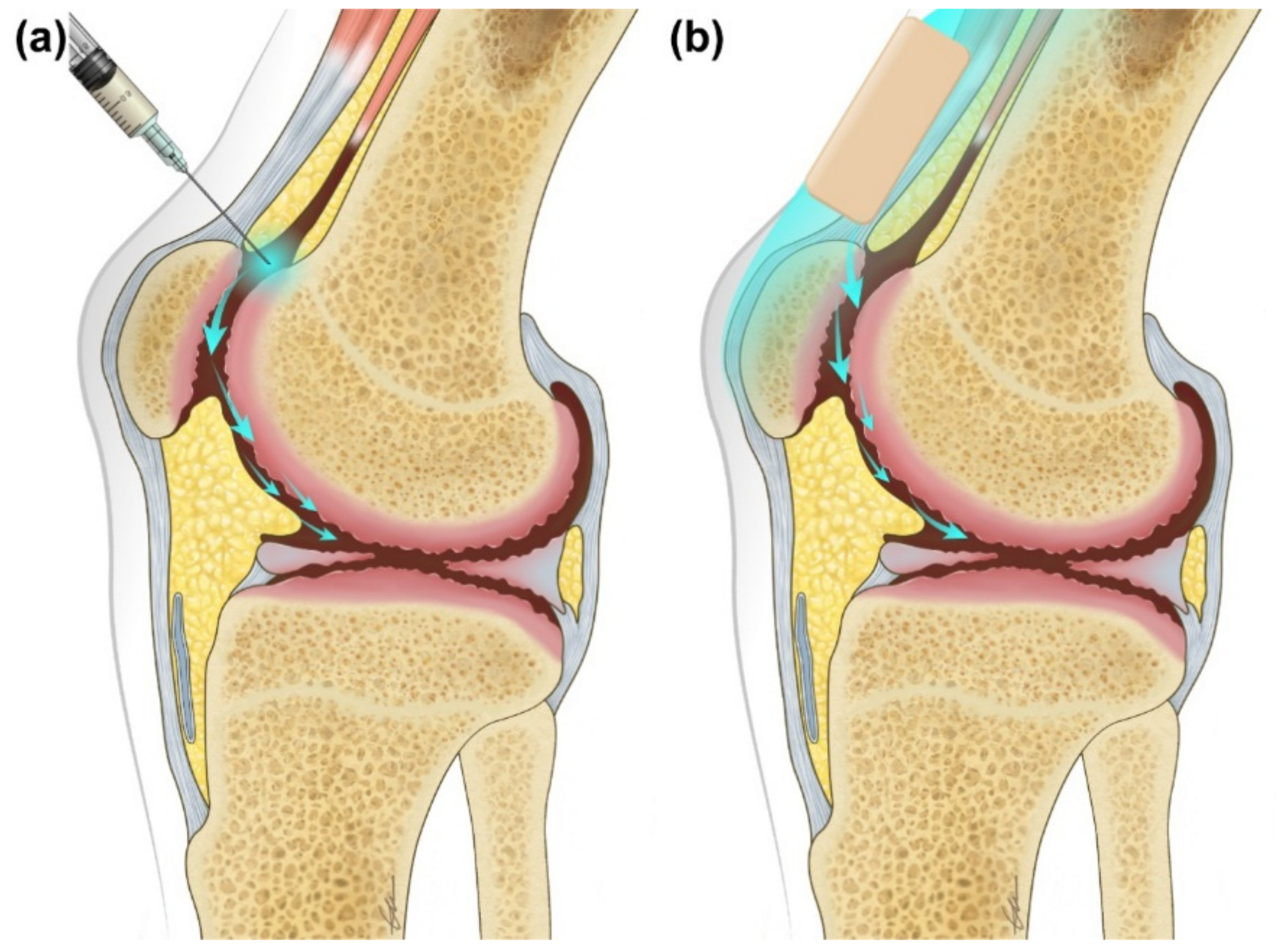
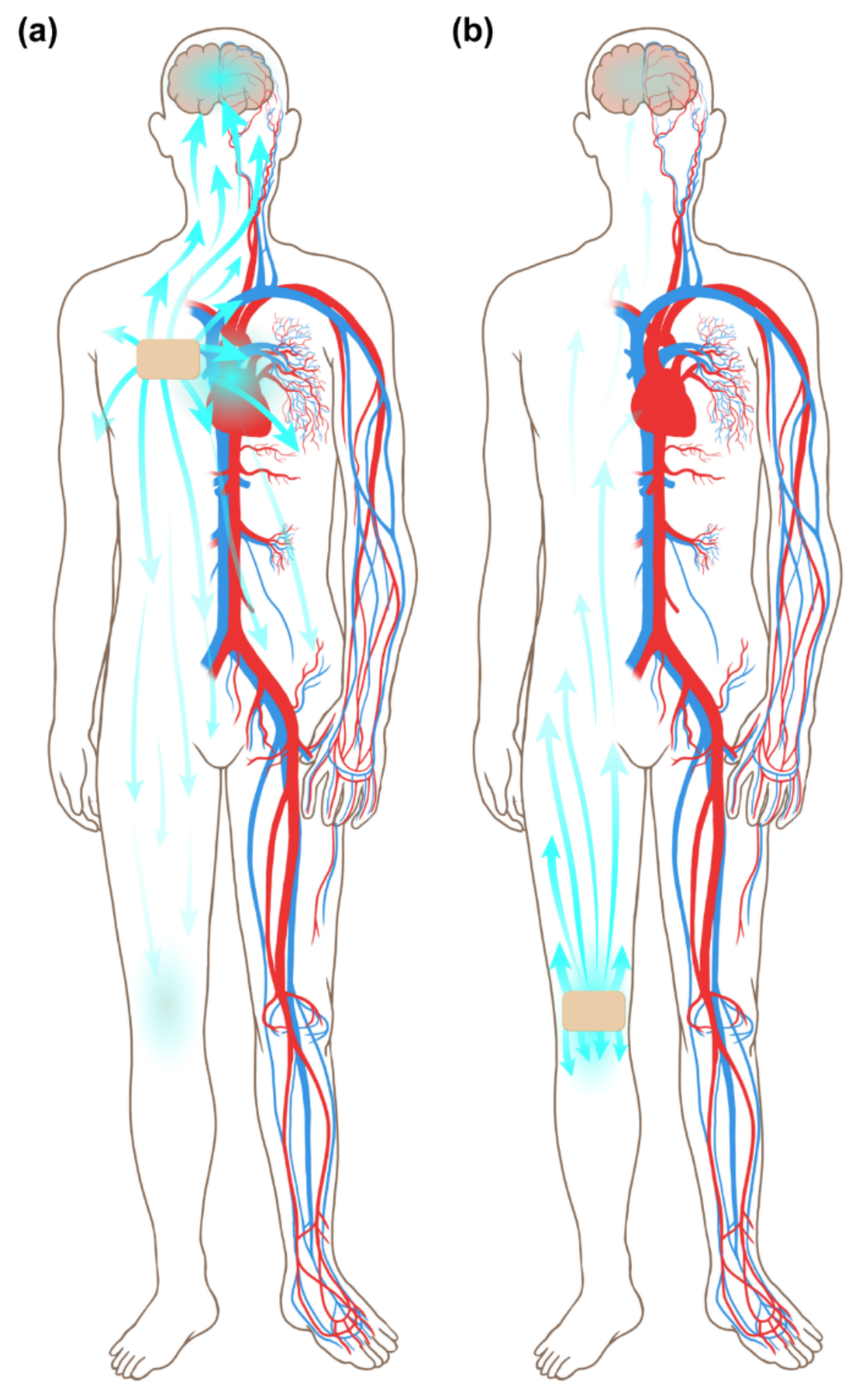
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chronic Rheumatic Conditions. Available online: https://www.who.int/chp/topics/rheumatic/en/ (accessed on 20 April 2019).
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J. Am. Geriatr. Soc. 2009, 57, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- The 2008 European Guidelines for Primary Care Management of Chronic Osteoarthritis Pain. Available online: http://www.eGuidelines.co.uk (accessed on 20 April 2019).
- Kalso, E.; Edwards, J.E.; Moore, R.A.; McQuay, H.J. Opioids in chronic non-cancer pain: Systematic review of efficacy and safety. Pain 2004, 112, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.; Treadwell, J.R.; Tregear, S.J.; Coates, V.H.; Wiffen, P.J.; Akafomo, C.; Schoelles, K.M. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst. Rev. 2010, 1, CD006605. [Google Scholar] [CrossRef] [PubMed]
- Khoury, G.F.; Chen, A.C.; Garland, D.E.; Stein, C. Intraarticular morphine, bupivacaine, and morphine/bupivacaine for pain control after knee videoarthroscopy. Anesthesiology 1992, 77, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Comisel, K.; Haimerl, E.; Yassouridis, A.; Lehrberger, K.; Herz, A.; Peter, K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N. Engl. J. Med. 1991, 325, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Kalso, E.; Tramer, M.R.; Carroll, D.; McQuay, H.J.; Moore, R.A. Pain relief from intra-articular morphine after knee surgery: A qualitative systematic review. Pain 1997, 71, 127–134. [Google Scholar] [CrossRef]
- Likar, R.; Sittl, R.; Gragger, K.; Pipam, W.; Blatnig, H.; Breschan, C.; Schalk, H.V.; Stein, C.; Schafer, M. Peripheral morphine analgesia in dental surgery. Pain 1998, 76, 145–150. [Google Scholar] [CrossRef]
- Stein, C. The control of pain in peripheral tissue by opioids. N. Engl. J. Med. 1995, 332, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Yassouridis, A.; Szopko, C.; Helmke, K.; Stein, C. Intraarticular morphine versus dexamethasone in chronic arthritis. Pain 1999, 83, 525–532. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Osteo-arthrosis and disk degeneration in an urban population. Ann. Rheum. Dis. 1958, 17, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Bin, S.I.; Chan, S.K.; Chung, C.K.; In, Y.; Kim, H.; Lichauco, J.J.; Mok, C.C.; Moon, Y.W.; Ng, T.K.; et al. Effectiveness and tolerability of transdermal buprenorphine patches: A multicenter, prospective, open-label study in Asian patients with moderate to severe chronic musculoskeletal pain. BMC Musculoskelet. Disord. 2017, 18, 337. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; McCarroll, S.M.; Cooney, C.M.; Blunnie, W.P.; O’Brien, T.M.; Lawrence, A.J. Intra-articular morphine for pain relief after knee arthroscopy. J. Bone Jt. Surg. Br. 1992, 74, 749–751. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Ngai, S.H.; Yang, J.C.; Hempstead, J.; Spector, S. The diposition of morphine in surgical patients. Clin. Pharmacol. Ther. 1975, 17, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Nayman, J. Measurement and control of postoperative pain. Ann. R. Coll. Surg. Engl. 1979, 61, 419–426. [Google Scholar] [PubMed]
- Dahlstrom, B.; Tamsen, A.; Paalzow, L.; Hartvig, P. Patient-controlled analgesic therapy, Part IV: Pharmacokinetics and analgesic plasma concentrations of morphine. Clin. Pharmacokinet. 1982, 7, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Yassen, A.; Romberg, R.; Sarton, E.; Teppema, L.; Olofsen, E.; Danhof, M. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br. J. Anaesth. 2006, 96, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.; Aloisi, A.M.; Dahan, A.; Filitz, J.; Langford, R.; Likar, R.; Mercadante, S.; Morlion, B.; Raffa, R.B.; Sabatowski, R.; et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract. 2010, 10, 428–450. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.; Jüni, P.; Da Costa, B.R.; Trelle, S.; Nüesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Wandel, S.; Jüni, P.; Tendal, B.; Nüesch, E.; Villiger, P.M.; Welton, N.J.; Reichenbach, S.; Trelle, S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: Network meta-analysis. BMJ 2010, 341, c4675. [Google Scholar] [CrossRef] [PubMed]
| Variable | Chest Applied Group (n = 125) | Knee Applied Group (n = 88) | p-Value |
|---|---|---|---|
| Sex | 1.000 1 | ||
| Male | 63 (50.40) | 44 (50.00) | |
| Female | 62 (49.60) | 44 (50.00) | |
| Age, years | 62.25 ± 15.44 | 57.00 ± 15.78 | 0.018 2,* |
| Height, cm | 161.89 ± 9.46 | 164.15 ± 10.45 | 0.180 3 |
| Weight, kg | 63.04 ± 13.18 | 64.82 ± 12.12 | 0.465 2 |
| BMI, kg/m2 | 23.86 ± 4.12 | 24.00 ± 3.39 | 0.826 3 |
| KL grade 4 | II:59, III:61, IV:5 | II:44, III:39, IV:5 | 0.892 |
| Malalignment (Varus, Valgus) | 95 (76.00) | 69 (78.41) | 0.681 |
| Variable | Chest Applied Group (n = 125) | Knee Applied Group (n = 88) | p-Value |
|---|---|---|---|
| NRS before | 0.195 | ||
| buprenorphine | |||
| application | |||
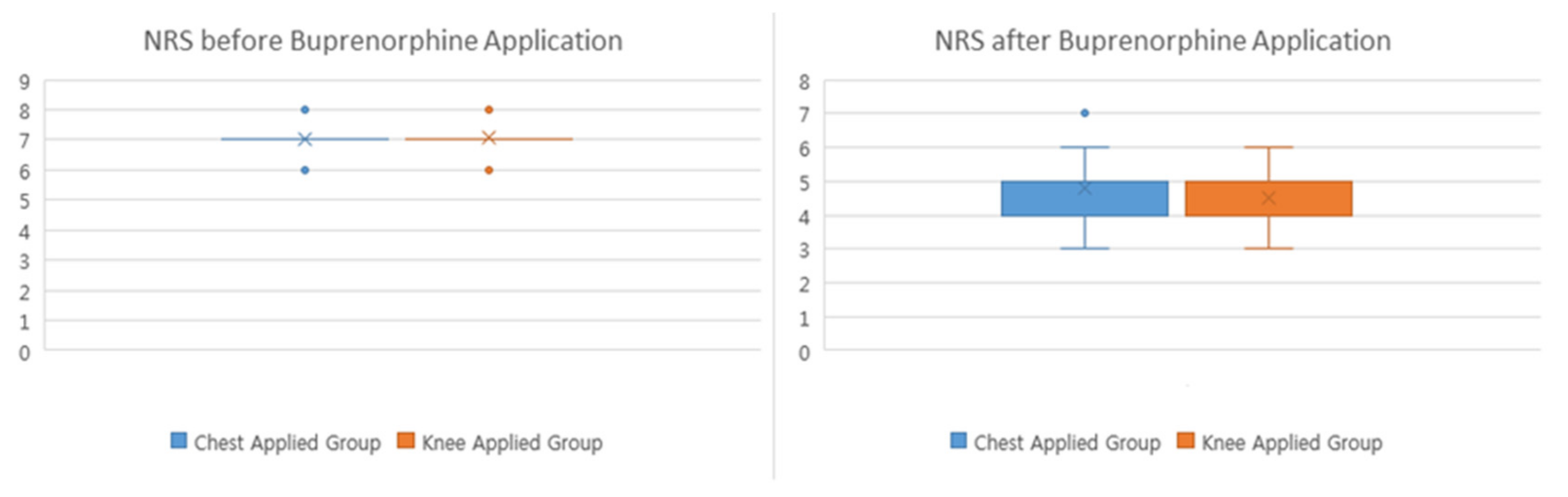
| Mean ± SD | 7.00 ± 0.31 | 7.06 ± 0.32 | |
| Median (Q1, Q3) | 7 (7.00, 7.00) | 7 (7.00, 7.00) | |
| NRS after | 0.007 * | ||
| buprenorphine | |||
| application | |||
| Mean ± SD | 4.79 ± 0.81 | 4.51 ± 0.69 | |
| Median (Q1, Q3) | 5 (4.00, 5.00) | 4 (4.00, 5.00) | |
| Side effect | <0.001 * | ||
| No | 45 (36.00) | 71 (80.68) | |
| Yes | 80 (64.00) | 17 (19.32) | |
| Maintenance | <0.001 * | ||
| No | 78 (62.40) | 15 (17.05) | |
| Yes | 47 (37.60) | 73 (82.95) |
| Variable | Chest Applied Group (n = 47) | Knee Applied Group (n = 73) | p-Value |
|---|---|---|---|
| NRS before buprenorphine application | 0.470 | ||
| 7.09 ± 0.35 | 7.04 ± 0.31 | ||
| 7 (7.00, 7.00) | 7 (7.00, 7.00) | ||
| NRS after buprenorphine application | 0.584 | ||
| 4.53 ± 0.75 | 4.45 ± 0.69 | ||
| 4 (4.00, 5.00) | 4 (4.00, 5.00) | ||
| Side effect | 0.644 | ||
| No | 45 (95.74%) | 71 (97.26%) | |
| Yes | 2 (4.26%) | 2 (2.74%) |
| Variables | Chest Applied Group | Knee Applied Group | p-Value |
|---|---|---|---|
| Gastrointestinal system | 33 (41.25) | 2 (11.76) | |
| nausea | 26 | 1 | |
| vomiting | 1 | 0 | |
| constipation | 4 | 0 | |
| dry mouth | 2 | 1 | |
| Central nervous system | 24 (30.00) | 0 (0.00) | |
| dizziness | 18 | 0 | |
| somnolence | 6 | 0 | |
| Cardiovascular system | 0 (0.00) | 0 (0.00) | |
| Genitourinary system urinary retention | 0 (0.00) 0 | 1 (5.88) 1 | |
| Respiratory system | 0 (0.00) | 0 (0.00) | |
| Skin and appendages dermatitis irritation | 15 (18.75) 6 9 | 14 (82.35) 4 10 | |
| Special senses | 0 (0.00) | 0 (0.00) | |
| Body as a whole | 0 (0.00) | 0 (0.00) | |
| Metabolic and nutritional system | 0 (0.00) | 0 (0.00) | |
| Others general weakness | 8 (10.00) 8 | 0 (0.00) 0 | |
| Total | 80/125(64.00) | 17/88(19.32) | <0.001 * |
| Variable | Chest Applied Group | Knee Applied Group | p-Value |
|---|---|---|---|
| Buprenorphine dose of stopping group | 0.869 | ||
| n | 78 | 15 | |
| Mean ± SD | 6.99 ± 3.72 | 7.00 ± 4.14 | |
| Buprenorphine dose of maintenance group | 0.387 | ||
| n | 47 | 73 | |
| Mean ± SD | 7.66 ± 4.40 | 6.71 ± 2.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, H.Y.; Park, S.; Kim, N.E.; Choi, Y.H.; Kim, J.H.; Choi, S.; Kim, H.J.; Koh, J.C.; Lee, B.H.; Lee, S.Y.; et al. A Novel Application of Buprenorphine Transdermal Patch to Relieve Pain in the Knee Joint of Knee Osteoarthritis Patients: A Retrospective Case-Control Study. J. Clin. Med. 2019, 8, 1009. https://doi.org/10.3390/jcm8071009
Gil HY, Park S, Kim NE, Choi YH, Kim JH, Choi S, Kim HJ, Koh JC, Lee BH, Lee SY, et al. A Novel Application of Buprenorphine Transdermal Patch to Relieve Pain in the Knee Joint of Knee Osteoarthritis Patients: A Retrospective Case-Control Study. Journal of Clinical Medicine. 2019; 8(7):1009. https://doi.org/10.3390/jcm8071009
Chicago/Turabian StyleGil, Ho Young, Sungchul Park, Na Eun Kim, Yi Hwa Choi, Jae Hyung Kim, Sooil Choi, Hyun Joong Kim, Jae Chul Koh, Byung Ho Lee, Sook Young Lee, and et al. 2019. "A Novel Application of Buprenorphine Transdermal Patch to Relieve Pain in the Knee Joint of Knee Osteoarthritis Patients: A Retrospective Case-Control Study" Journal of Clinical Medicine 8, no. 7: 1009. https://doi.org/10.3390/jcm8071009
APA StyleGil, H. Y., Park, S., Kim, N. E., Choi, Y. H., Kim, J. H., Choi, S., Kim, H. J., Koh, J. C., Lee, B. H., Lee, S. Y., Min, S. K., Kim, B., Lee, H. S., Jeong, H. W., Park, J. H., Park, B., & Choi, J. B. (2019). A Novel Application of Buprenorphine Transdermal Patch to Relieve Pain in the Knee Joint of Knee Osteoarthritis Patients: A Retrospective Case-Control Study. Journal of Clinical Medicine, 8(7), 1009. https://doi.org/10.3390/jcm8071009





