Impact of Oxidative Stress and Protein S-Glutathionylation in Aortic Valve Sclerosis Patients with Overt Atherosclerosis
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. Echocardiographic Evaluation
2.3. Blood Sampling and Biochemical Measurements
2.4. Glutathione Measurements
2.5. Asymmetric Dimethylarginine Measurements
2.6. Dot-Blot, Western Blot and Immunohistochemistry
2.7. In Vitro Model of Protein S-Glutathionylation
2.8. Immunoprecipitation
2.9. Reverse Transcription and Real-Time PCR
2.10. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Oxidative Stress and Endothelial Dysfunction
3.3. Systemic Inflammation Status
3.4. Aortic Valve Protein S-Glutathionylation
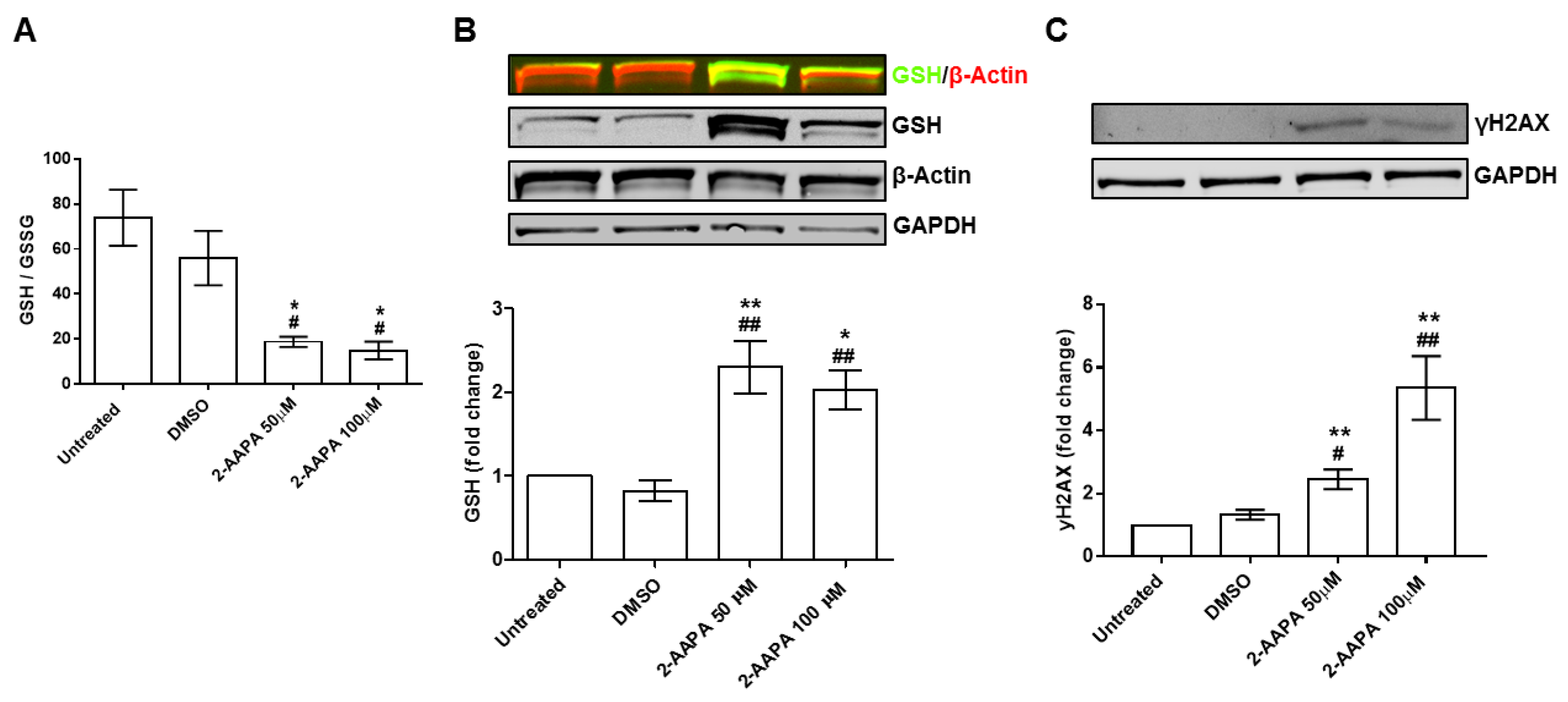
3.5. In Vitro Model of Protein S-Glutathionylation
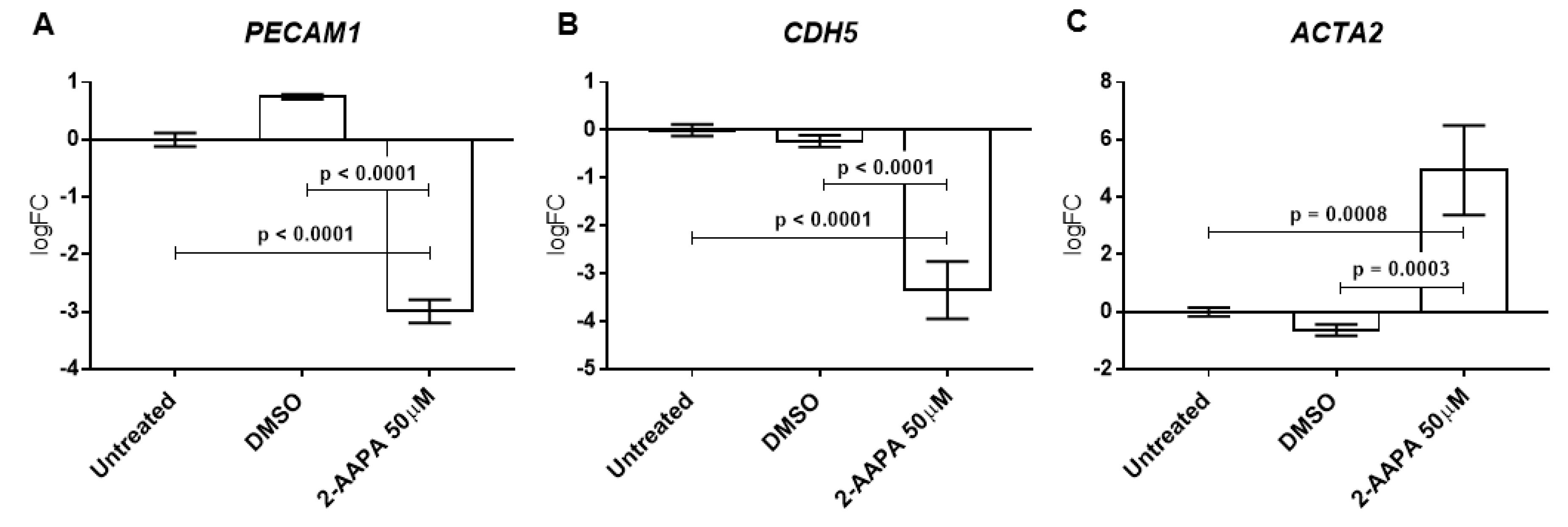
3.6. Protein S-Glutathionylation and Endothelial-to-Mesenchymal Transition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic stenosis working group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.V.; Otto, C.M. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression and treatment strategies. Circulation 2005, 111, 3316–3326. [Google Scholar] [CrossRef]
- Faggiano, P.; Antonini-Canterin, F.; Erlicher, A.; Romeo, C.; Cervesato, E.; Pavan, D.; Piazza, R.; Huang, G.; Nicolosi, G.L. Progression of Aortic valve sclerosis to Aortic stenosis. Am. J. Cardiol. 2003, 91, 99–101. [Google Scholar] [CrossRef]
- Coffey, S.; Cox, B.; Williams, M.J. The prevalence, incidence, progression and risks of Aortic valve sclerosis: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2014, 63, 2852–2861. [Google Scholar] [CrossRef]
- Beckmann, E.; Grau, J.B.; Sainger, R.; Poggio, P.; Ferrari, G. Insights into the use of biomarkers in calcific aortic valve disease. J. Heart Valve Dis. 2010, 19, 441–452. [Google Scholar]
- Di Minno, M.N.D.; Di Minno, A.; Ambrosino, P.; Songia, P.; Pepi, M.; Tremoli, E.; Poggio, P. Cardiovascular morbidity and mortality in patients with aortic valve sclerosis: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 260, 138–144. [Google Scholar] [CrossRef]
- Di Minno, M.N.; Di Minno, A.; Songia, P.; Ambrosino, P.; Gripari, P.; Ravani, A.; Pepi, M.; Rubba, P.O.; Medda, E.; Tremoli, E.; et al. Markers of subclinical atherosclerosis in patients with aortic valve sclerosis: A meta-analysis of literature studies. Int. J. Cardiol. 2016, 223, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Vallejo, S.; Sanchez, F.J.; Sandoval, E.; Blanco, E.; Cannata, P.; Peiro, C.; Sanchez-Ferrer, C.F.; Lamas, S. Role of glutathione biosynthesis in endothelial dysfunction and fibrosis. Redox Biol. 2018, 14, 88–99. [Google Scholar] [CrossRef]
- Higashi, Y.; Maruhashi, T.; Noma, K.; Kihara, Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc. Med. 2014, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Sastre, J.; Pallardo, F.V.; Lloret, A.; Lehner, M.; Garcia-de-la Asuncion, J.; Vina, J. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999, 299, 267–276. [Google Scholar]
- Leopold, J.A.; Loscalzo, J. Oxidative enzymopathies and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1332–1340. [Google Scholar] [CrossRef]
- Emdin, M.; Pompella, A.; Paolicchi, A. Gamma-glutamyltransferase, atherosclerosis and cardiovascular disease: Triggering oxidative stress within the plaque. Circulation 2005, 112, 2078–2080. [Google Scholar] [CrossRef]
- Musthafa, Q.A.; Abdul Shukor, M.F.; Ismail, N.A.S.; Mohd Ghazi, A.; Mohd Ali, R.; IF, M.N.; Dimon, M.Z.; Wan Ngah, W.Z. Oxidative status and reduced glutathione levels in premature coronary artery disease and coronary artery disease. Free Radic. Res. 2017, 51, 787–798. [Google Scholar] [CrossRef]
- Widder, J.D.; Guzik, T.J.; Mueller, C.F.; Clempus, R.E.; Schmidt, H.H.; Dikalov, S.I.; Griendling, K.K.; Jones, D.P.; Harrison, D.G. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 762–768. [Google Scholar] [CrossRef]
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-glutathionylation: From molecular mechanisms to health outcomes. Antioxid. Redox Signal. 2011, 15, 233–270. [Google Scholar] [CrossRef]
- Popov, D. Protein S-glutathionylation: From current basics to targeted modifications. Arch. Physiol. Biochem. 2014, 120, 123–130. [Google Scholar] [CrossRef]
- Ghezzi, P. Protein glutathionylation in health and disease. Biochim. Biophys. Acta 2013, 1830, 3165–3172. [Google Scholar] [CrossRef]
- Pastore, A.; Piemonte, F. Protein glutathionylation in cardiovascular diseases. Int. J. Mol. Sci. 2013, 14, 20845–20876. [Google Scholar] [CrossRef]
- Otto, C.M.; Lind, B.K.; Kitzman, D.W.; Gersh, B.J.; Siscovick, D.S. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N. Engl. J. Med. 1999, 341, 142–147. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Ravani, A.L.; Frigerio, B.; Valerio, V.; Moschetta, D.; Songia, P.; Poggio, P. Novel pharmacological targets for calcific aortic valve disease: Prevention and treatments. Pharmacol. Res. 2018, 136, 74–82. [Google Scholar] [CrossRef]
- Kim, D.B.; Jung, H.O.; Jeon, D.S.; Park, C.S.; Jang, S.W.; Park, H.J.; Kim, P.J.; Baek, S.H.; Seung, K.B.; Rho, T.H.; et al. Aortic valve sclerosis on echocardiography is a good predictor of coronary artery disease in patients with an inconclusive treadmill exercise test. Korean Circ. J. 2009, 39, 275–279. [Google Scholar] [CrossRef]
- Sui, S.J.; Ren, M.Y.; Xu, F.Y.; Zhang, Y. A high association of aortic valve sclerosis detected by transthoracic echocardiography with coronary arteriosclerosis. Cardiology 2007, 108, 322–330. [Google Scholar] [CrossRef]
- Roy, G.C.; Rahman, F.; Hoque, M.H.; Habib, M.A.; Banerjee, S.K.; Siddique, M.A.; Barua, U.K.; Hossain, A.S.; Bhuiyan, G.R.; Haider, M.S. Aortic valve sclerosis is an indicator of coronary artery diseases. Mymensingh Med. J. MMJ 2012, 21, 226–232. [Google Scholar]
- Shah, S.J.; Ristow, B.; Ali, S.; Na, B.Y.; Schiller, N.B.; Whooley, M.A. Acute myocardial infarction in patients with versus without aortic valve sclerosis and effect of statin therapy (from the Heart and Soul Study). Am. J. Cardiol. 2007, 99, 1128–1133. [Google Scholar] [CrossRef][Green Version]
- Owens, D.S.; Budoff, M.J.; Katz, R.; Takasu, J.; Shavelle, D.M.; Carr, J.J.; Heckbert, S.R.; Otto, C.M.; Probstfield, J.L.; Kronmal, R.A.; et al. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC. Cardiovasc. Imaging 2012, 5, 619–625. [Google Scholar] [CrossRef]
- Chandra, H.R.; Goldstein, J.A.; Choudhary, N.; O’Neill, C.S.; George, P.B.; Gangasani, S.R.; Cronin, L.; Marcovitz, P.A.; Hauser, A.M.; O’Neill, W.W. Adverse outcome in Aortic sclerosis is associated with coronary artery disease and inflammation. J. Am. Coll. Cardiol. 2004, 43, 169–175. [Google Scholar] [CrossRef]
- Mazzone, A.; Venneri, L.; Berti, S. Aortic valve stenosis and coronary artery disease: Pathophysiological and clinical links. J. Cardiovasc. Med. (Hagerstown) 2007, 8, 983–989. [Google Scholar] [CrossRef]
- Soydinc, S.; Davutoglu, V.; Dundar, A.; Aksoy, M. Relationship between Aortic valve sclerosis and the extent of coronary artery disease in patients undergoing diagnostic coronary angiography. Cardiology 2006, 106, 277–282. [Google Scholar] [CrossRef]
- Milin, A.C.; Vorobiof, G.; Aksoy, O.; Ardehali, R. Insights into Aortic sclerosis and its relationship with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001111. [Google Scholar] [CrossRef]
- Poggianti, E.; Venneri, L.; Chubuchny, V.; Jambrik, Z.; Baroncini, L.A.; Picano, E. Aortic valve sclerosis is associated with systemic endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 41, 136–141. [Google Scholar] [CrossRef]
- Erdogan, T.; Cetin, M.; Kocaman, S.A.; Durakoglugil, M.E.; Ergul, E.; Canga, A. Aortic valve sclerosis is a high predictive marker of systemic endothelial dysfunction in hypertensive patients. Herz 2013, 38, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov, A.L.; Ngo, D.T.; Chan, W.P.; Chirkov, Y.Y.; Gersh, B.J.; McNeil, J.J.; Horowitz, J.D. Determinants of aortic sclerosis progression: Implications regarding impairment of nitric oxide signalling and potential therapeutics. Eur. Heart J. 2012, 33, 2419–2425. [Google Scholar] [CrossRef]
- Cavalca, V.; Tremoli, E.; Porro, B.; Veglia, F.; Myasoedova, V.; Squellerio, I.; Manzone, D.; Zanobini, M.; Trezzi, M.; Di Minno, M.N.; et al. Oxidative stress and nitric oxide pathway in adult patients who are candidates for cardiac surgery: Patterns and differences. Interac. Cardiovasc. Thorac. Surg. 2013, 17, 923–930. [Google Scholar] [CrossRef]
- Farrar, E.J.; Huntley, G.D.; Butcher, J. Endothelial-derived oxidative stress drives myofibroblastic activation and calcification of the aortic valve. PLoS ONE 2015, 10, e0123257. [Google Scholar] [CrossRef]
- Ngo, D.T.; Heresztyn, T.; Mishra, K.; Marwick, T.H.; Horowitz, J.D. Aortic stenosis is associated with elevated plasma levels of asymmetric dimethylarginine (ADMA). Nitric Oxide Biol. Chem. 2007, 16, 197–201. [Google Scholar] [CrossRef]
- Miller, J.D.; Chu, Y.; Brooks, R.M.; Richenbacher, W.E.; Pena-Silva, R.; Heistad, D.D. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific Aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 2008, 52, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Wang, T.Y.; Varadharaj, S.; Reyes, L.A.; Hemann, C.; Talukder, M.A.; Chen, Y.R.; Druhan, L.J.; Zweier, J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 2010, 468, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Hjortnaes, J.; Shapero, K.; Goettsch, C.; Hutcheson, J.D.; Keegan, J.; Kluin, J.; Mayer, J.E.; Bischoff, J.; Aikawa, E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis 2015, 242, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; El-Hamamsy, I.; Chen, S.; Sarang, Z.; Sarathchandra, P.; Yacoub, M.H.; Chester, A.H.; Butcher, J.T. Side-specific endothelial-dependent regulation of aortic valve calcification: Interplay of hemodynamics and nitric oxide signaling. Am. J. Pathol. 2013, 182, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Dahal, S.; Huang, P.; Murray, B.T.; Mahler, G.J. Endothelial to mesenchymal transformation is induced by altered extracellular matrix in aortic valve endothelial cells. J. Biomed. Mater. Res. Part A 2017, 105, 2729–2741. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Duffhues, G.; Garcia de Vinuesa, A.; Ten Dijke, P. Endothelial-to-mesenchymal transition in cardiovascular diseases: Developmental signaling pathways gone awry. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2018, 247, 492–508. [Google Scholar] [CrossRef]
- Souilhol, C.; Harmsen, M.C.; Evans, P.C.; Krenning, G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc. Res. 2018, 114, 565–577. [Google Scholar] [CrossRef]
- Zhao, Y.; Seefeldt, T.; Chen, W.; Carlson, L.; Stoebner, A.; Hanson, S.; Foll, R.; Matthees, D.P.; Palakurthi, S.; Guan, X. Increase in thiol oxidative stress via glutathione reductase inhibition as a novel approach to enhance cancer sensitivity to X-ray irradiation. Free Radic. Biol. Med. 2009, 47, 176–183. [Google Scholar] [CrossRef]
- de Souza, L.F.; Schmitz, A.E.; da Silva, L.C.S.; de Oliveira, K.A.; Nedel, C.B.; Tasca, C.I.; de Bem, A.F.; Farina, M.; Dafre, A.L. Inhibition of reductase systems by 2-AAPA modulates peroxiredoxin oxidation and mitochondrial function in A172 glioblastoma cells. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2017, 42, 273–280. [Google Scholar] [CrossRef]
- Seefeldt, T.; Zhao, Y.; Chen, W.; Raza, A.S.; Carlson, L.; Herman, J.; Stoebner, A.; Hanson, S.; Foll, R.; Guan, X. Characterization of a novel dithiocarbamate glutathione reductase inhibitor and its use as a tool to modulate intracellular glutathione. J. Biol. Chem. 2009, 284, 2729–2737. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, A.; Epistolato, M.C.; De Caterina, R.; Storti, S.; Vittorini, S.; Sbrana, S.; Gianetti, J.; Bevilacqua, S.; Glauber, M.; Biagini, A.; et al. Neoangiogenesis, T-lymphocyte infiltration and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J. Am. Coll. Cardiol. 2004, 43, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, H.; Akdemir, R.; Binak, E.; Tamer, A.; Keser, N.; Uyan, C. Can serum lipid and CRP levels predict the “severity” of Aortic valve stenosis? Acta Cardiol. 2003, 58, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Galante, A.; Pietroiusti, A.; Vellini, M.; Piccolo, P.; Possati, G.; De Bonis, M.; Grillo, R.L.; Fontana, C.; Favalli, C. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J. Am. Coll. Cardiol. 2001, 38, 1078–1082. [Google Scholar] [CrossRef]
- Skowasch, D.; Schrempf, S.; Preusse, C.J.; Likungu, J.A.; Welz, A.; Luderitz, B.; Bauriedel, G. Tissue resident C reactive protein in degenerative aortic valves: Correlation with serum C reactive protein concentrations and modification by statins. Heart 2006, 92, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.L.; Mazzone, A.M. C-reactive protein in aortic valve disease. Cardiovascular ultrasound 2006, 4, 37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeevanantham, V.; Singh, N.; Izuora, K.; D’Souza, J.P.; Hsi, D.H. Correlation of high sensitivity C-reactive protein and calcific aortic valve disease. Mayo Clin. Proc. 2007, 82, 171–174. [Google Scholar] [CrossRef]
- Novaro, G.M.; Katz, R.; Aviles, R.J.; Gottdiener, J.S.; Cushman, M.; Psaty, B.M.; Otto, C.M.; Griffin, B.P. Clinical factors but not C-reactive protein, predict progression of calcific aortic-valve disease: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2007, 50, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov, A.L.; Ngo, D.T.; Horowitz, J.D. Pathogenesis of Aortic sclerosis: Association with low BMI, tissue nitric oxide resistance but not systemic inflammatory activation. Am. J. Cardiovasc. Dis. 2012, 2, 43–49. [Google Scholar] [PubMed]


| Variable | CABG (n = 29) | CABG + AVSc (n = 29) | p Value |
|---|---|---|---|
| Age, years | 62.2 ± 6.2 | 65.2 ± 8.4 | 0.133 |
| Male sex, n (%) | 29 (100) | 29 (100) | 1.000 |
| Diabetes, n (%) | 7 (24) | 5 (17) | 0.525 |
| Hypertension, n (%) | 17 (59) | 22 (76) | 0.168 |
| Dyslipidemia, n (%) | 22 (76) | 19 (65.5) | 0.396 |
| Current Smoking, n (%) | 3 (10) | 7 (24) | 0.171 |
| Ex-Smokers, n (%) | 15 (52) | 13 (45) | 0.607 |
| Body mass index, kg/m2 | 26.7 ± 2.9 | 27.8 ± 3.6 | 0.156 |
| Creatinine, mg/dL | 0.91 ± 0.12 | 0.94 ± 0.17 | 0.411 |
| C-reactive protein, mg/L | 2.61 ± 2.56 | 2.73 ± 2.14 | 0.853 |
| New York Heart Association (NYHA) class | |||
| I | 10 (34) | 11 (38) | 1.000 |
| II | 16 (56) | 12 (41) | 0.593 |
| III | 3 (10) | 6 (21) | 0.470 |
| IV | - | - | - |
| 3-Vessels coronary disease, n (%) | 20 (69) | 19 (65.5) | 0.784 |
| Logistic EuroSCORE | 1.93 ± 1.79 | 2.68 ± 2.14 | 0.160 |
| Echocardiography | |||
| Left ventricle ejection fraction, n (%) | 61.3 ± 10.1 | 57.9 ± 10.1 | 0.210 |
| LV hypertrophy index, mm | 0.35 ± 0.13 | 0.41 ± 0.12 | 0.134 |
| Max. aortic velocity, m/s | 0.99 ± 0.54 | 1.23 ± 0.59 | 0.100 |
| Max. aortic gradient, mmHg | 5.14 ± 3.16 | 7.55 ± 6.79 | 0.090 |
| Therapies | |||
| Antiplatelets, n (%) | 21 (72) | 18 (62) | 0.410 |
| Angiotensin receptor blockers, n (%) | 5 (17) | 6 (21) | 0.743 |
| Converting enzyme inhibitors, n (%) | 8 (28) | 11 (38) | 0.410 |
| Calcium channel blockers, n (%) | 9 (31) | 9 (31) | 1.000 |
| Beta-blockers, n (%) | 19 (65.5) | 19 (65.5) | 1.000 |
| Nitrates, n (%) | 6 (21) | 10 (34.5) | 0.248 |
| Statins, n (%) | 18 (62) | 19 (65.5) | 0.789 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, V.; Myasoedova, V.A.; Moschetta, D.; Porro, B.; Perrucci, G.L.; Cavalca, V.; Cavallotti, L.; Songia, P.; Poggio, P. Impact of Oxidative Stress and Protein S-Glutathionylation in Aortic Valve Sclerosis Patients with Overt Atherosclerosis. J. Clin. Med. 2019, 8, 552. https://doi.org/10.3390/jcm8040552
Valerio V, Myasoedova VA, Moschetta D, Porro B, Perrucci GL, Cavalca V, Cavallotti L, Songia P, Poggio P. Impact of Oxidative Stress and Protein S-Glutathionylation in Aortic Valve Sclerosis Patients with Overt Atherosclerosis. Journal of Clinical Medicine. 2019; 8(4):552. https://doi.org/10.3390/jcm8040552
Chicago/Turabian StyleValerio, Vincenza, Veronika A. Myasoedova, Donato Moschetta, Benedetta Porro, Gianluca L. Perrucci, Viviana Cavalca, Laura Cavallotti, Paola Songia, and Paolo Poggio. 2019. "Impact of Oxidative Stress and Protein S-Glutathionylation in Aortic Valve Sclerosis Patients with Overt Atherosclerosis" Journal of Clinical Medicine 8, no. 4: 552. https://doi.org/10.3390/jcm8040552
APA StyleValerio, V., Myasoedova, V. A., Moschetta, D., Porro, B., Perrucci, G. L., Cavalca, V., Cavallotti, L., Songia, P., & Poggio, P. (2019). Impact of Oxidative Stress and Protein S-Glutathionylation in Aortic Valve Sclerosis Patients with Overt Atherosclerosis. Journal of Clinical Medicine, 8(4), 552. https://doi.org/10.3390/jcm8040552





