Median Arcuate Ligament Compression in Orthotopic Liver Transplantation: Results from a Single-Center Analysis and a European Survey Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Ethics
2.2. Data Collection and Follow-Up
2.3. Surgical- and Perioperative Approach
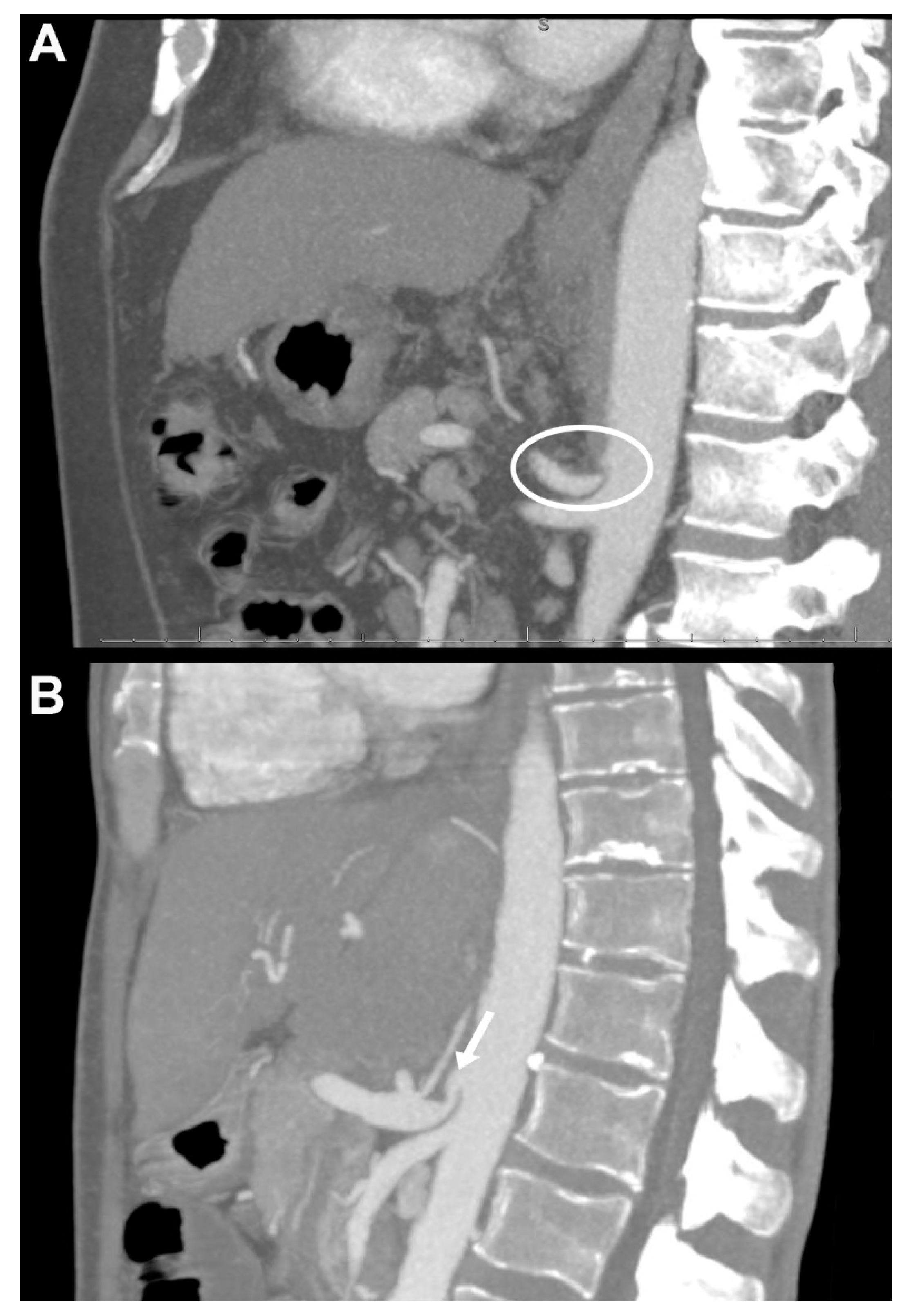
2.4. Contrast-Enhanced CT Detection of Coeliac Axis Stenosis and Other Vascular Alterations
2.5. European Survey
2.6. Statistical Analysis
3. Results
3.1. Imaging Based Preoperative Vascular Evaluation
3.2. Patient Characteristics and Perioperative Outcome
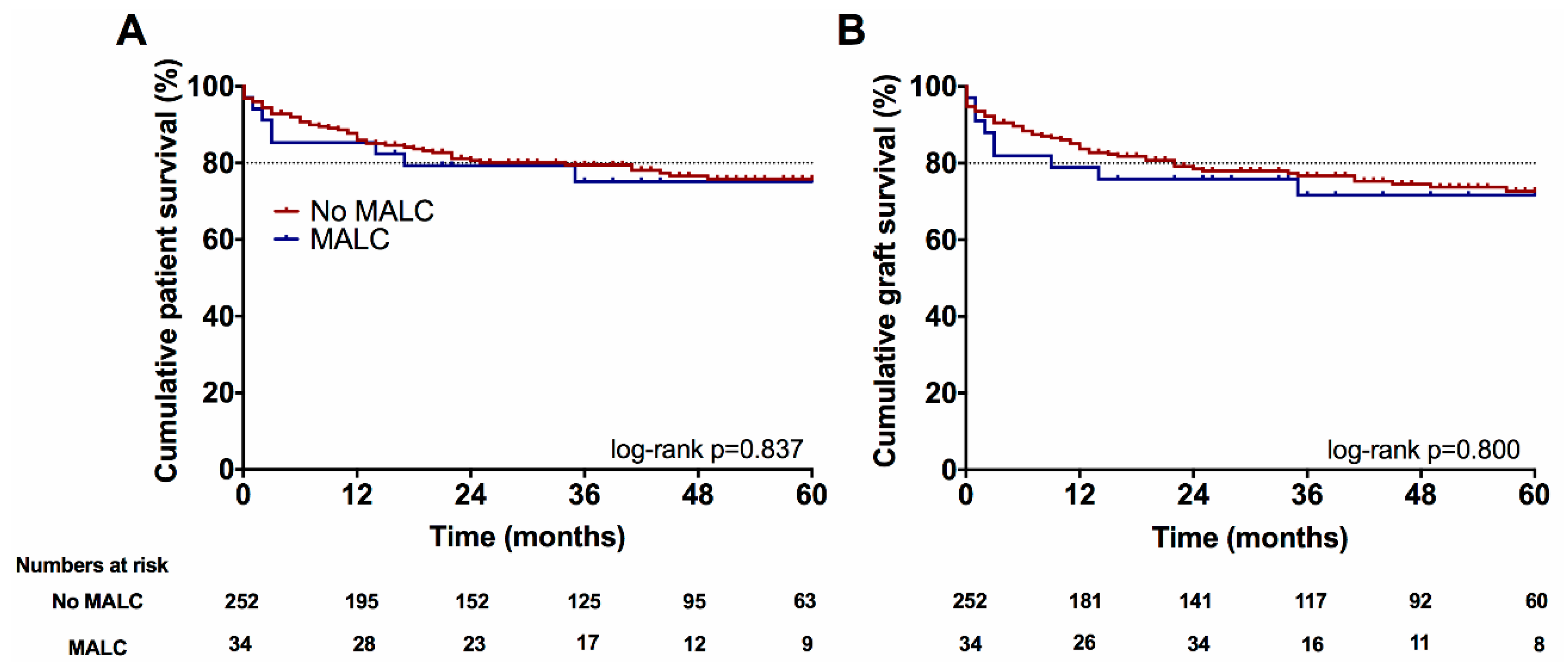
3.3. Long-Term Follow Up
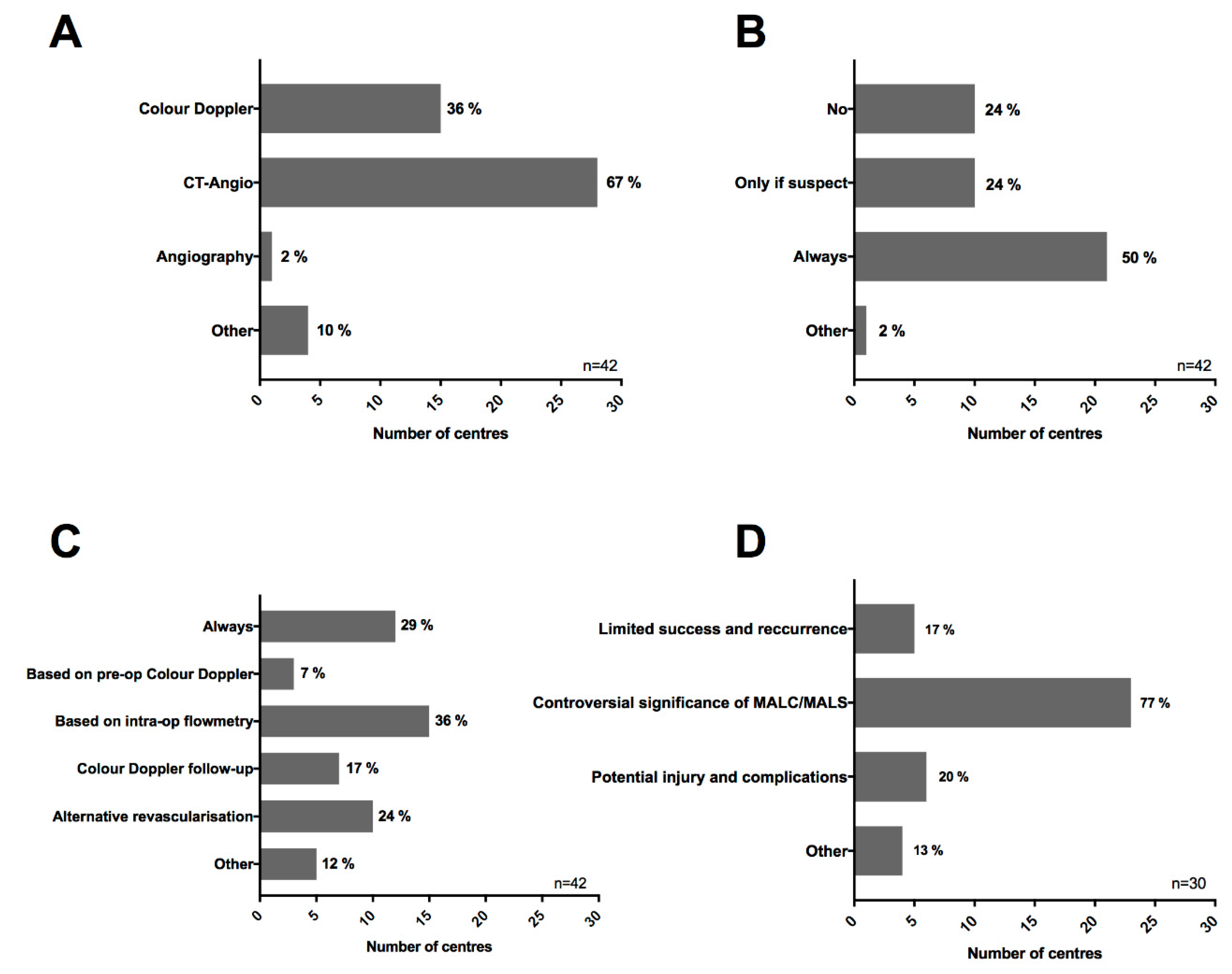
3.4. European Survey
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Starzl, T.E.; Fung, J.J. Themes of liver transplantation. Hepatology 2010, 51, 1869–1884. [Google Scholar] [CrossRef] [PubMed]
- Oberkofler, C.E.; Reese, T.; Raptis, D.A.; Kummerli, C.; de Rougemont, O.; De Oliveira, M.L.; Schlegel, A.; Dutkowski, P.; Clavien, P.A.; Petrowsky, H. Hepatic artery occlusion in liver transplantation—What counts more: Type of reconstruction or severity of recipient’s disease? Liver Transpl. 2018, 24, 790–802. [Google Scholar] [CrossRef]
- Harrison, J.; Harrison, M.; Doria, C. Hepatic Artery Pseudoaneurysm Following Orthotopic Liver Transplantation: Increasing Clinical Suspicion for a Rare but Lethal Pathology. Ann. Transpl. 2017, 22, 417–424. [Google Scholar] [CrossRef]
- Feltracco, P.; Barbieri, S.; Cillo, U.; Zanus, G.; Senzolo, M.; Ori, C. Perioperative thrombotic complications in liver transplantation. World J. Gastroenterol. 2015, 21, 8004–8013. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.R.; Vilchez, A.R.; Dominguez, M.B.; Garcia, A.N.; San Miguel, C.M.; Gonzalez, A.R.; Fundora, Y.S. Influence of Body Mass Index on Venous Thrombotic Complications of Liver Transplants. Transpl. Proc. 2016, 48, 3017–3020. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M.; Hashimoto, K.; Palaios, E.; Quintini, C.; Aucejo, F.N.; Uso, T.D.; Eghtesad, B.; Miller, C.M. Probability, management, and long-term outcomes of biliary complications after hepatic artery thrombosis in liver transplant recipients. Surgery 2017, 162, 1101–1111. [Google Scholar] [CrossRef]
- Lainas, P.; Fuks, D.; Gaujoux, S.; Machroub, Z.; Fregeville, A.; Perniceni, T.; Mal, F.; Dousset, B.; Gayet, B. Preoperative imaging and prediction of oesophageal conduit necrosis after oesophagectomy for cancer. Br. J. Surg. 2017, 104, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lamb, K.; Relles, D.; Moudgill, N.; DiMuzio, P.J.; Eisenberg, J.A. Median Arcuate Ligament Syndrome-Review of This Rare Disease. JAMA Surg. 2016, 151, 471–477. [Google Scholar] [CrossRef]
- Horton, K.M.; Talamini, M.A.; Fishman, E.K. Median arcuate ligament syndrome: Evaluation with CT angiography. Radiographics 2005, 25, 1177–1182. [Google Scholar] [CrossRef]
- Lipshutz, B. A composite study of the coeliac axis artery. Ann. Surg. 1917, 65, 159–169. [Google Scholar] [CrossRef]
- Harjola, P.T. A rare obstruction of the coeliac artery. report of a case. Ann. Chir. Gynaecol. Fenn. 1963, 52, 547–550. [Google Scholar]
- Dunbar, J.D.; Molnar, W.; Beman, F.F.; Marable, S.A. Compression of the celiac trunk and abdominal angina. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1965, 95, 731–744. [Google Scholar] [CrossRef]
- Sugae, T.; Fujii, T.; Kodera, Y.; Kanzaki, A.; Yamamura, K.; Yamada, S.; Sugimoto, H.; Nomoto, S.; Takeda, S.; Nakao, A. Classification of the celiac axis stenosis owing to median arcuate ligament compression, based on severity of the stenosis with subsequent proposals for management during pancreatoduodenectomy. Surgery 2012, 151, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, J.; Scatton, O.; Randone, B.; Molinier, N.; Massault, P.P.; Legmann, P.; Soubrane, O.; Dousset, B. Median arcuate ligament in orthotopic liver transplantation: Relevance to arterial reconstruction. Transpl. Proc. 2008, 40, 3532–3535. [Google Scholar] [CrossRef] [PubMed]
- Agnes, S.; Avolio, A.W.; Magalini, S.C.; Frongillo, F.; Castagneto, M. Celiac axis compression syndrome in liver transplantation. Transpl. Proc. 2001, 33, 1438–1439. [Google Scholar] [CrossRef]
- Uchida, H.; Sakamoto, S.; Matsunami, M.; Sasaki, K.; Shigeta, T.; Kanazawa, H.; Fukuda, A.; Nakazawa, A.; Miyazaki, O.; Nosaka, S.; et al. Hepatic artery reconstruction preserving the pancreaticoduodenal arcade in pediatric liver transplantation with celiac axis compression syndrome: Report of a case. Pediatric Transpl. 2014, 18, E232–E235. [Google Scholar] [CrossRef] [PubMed]
- Jurim, O.; Shaked, A.; Kiai, K.; Millis, J.M.; Colquhoun, S.D.; Busuttil, R.W. Celiac compression syndrome and liver transplantation. Ann. Surg. 1993, 218, 10–12. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Liang, T.B.; Feng, X.N.; Wang, W.L.; Shen, Y.; Zhang, M.; Wu, J.; Xu, X.; Zheng, S.S. Arcuate ligament syndrome inducing hepatic artery thrombosis after liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 433–436. [Google Scholar]
- Ali, M.; Patel, J. Dunbar syndrome following liver transplantation. BMJ Case Rep. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, K.; Schwartz, M.E.; Katz, E.; Mor, E.; Emre, S.; Acarli, K.; Miller, C.M. The arcuate ligament syndrome in liver transplantation. Transplantation 1993, 56, 223–224. [Google Scholar] [CrossRef]
- Vilatoba, M.; Zamora-Valdes, D.; Guerrero-Hernandez, M.; Romero-Talamas, H.; Leal-Villalpando, R.P.; Mercado, M.A. Arcuate ligament compression as a cause of early-onset thrombosis of the hepatic artery after liver transplantation. Ann. Hepatol. 2011, 10, 88–92. [Google Scholar] [PubMed]
- Schlegel, A.; Linecker, M.; Kron, P.; Gyori, G.; De Oliveira, M.L.; Mullhaupt, B.; Clavien, P.A.; Dutkowski, P. Risk Assessment in High- and Low-MELD Liver Transplantation. Am. J. Transpl. 2017, 17, 1050–1063. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.A. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef]
- Richtlinien für die Wartelistenführung und Organvermittlung zur Lebertransplantation gem. § 16 Abs. 1 S. 1 Nrn. 2 u. 5 TPG. Available online: https://bundesaerztekammer.de (accessed on 10 April 2019).
- Czigany, Z.; Schoning, W.; Ulmer, T.F.; Bednarsch, J.; Amygdalos, I.; Cramer, T.; Rogiers, X.; Popescu, I.; Botea, F.; Fronek, J.; et al. Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): A prospective multicenters randomised controlled trial (HOPE ECD-DBD). BMJ Open 2017, 7, e017558. [Google Scholar] [CrossRef]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010, 16, 943–949. [Google Scholar] [CrossRef]
- Andert, A.; Becker, N.; Ulmer, F.; Schoning, W.; Hein, M.; Rimek, A.; Neumann, U.; Schmeding, M. Liver Transplantation and Donor Body Mass Index >30: Use or Refuse? Ann. Transpl. 2016, 21, 185–193. [Google Scholar] [CrossRef]
- Kienlein, S.; Schoening, W.; Andert, A.; Kroy, D.; Neumann, U.P.; Schmeding, M. Biliary complications in liver transplantation: Impact of anastomotic technique and ischemic time on short- and long-term outcome. World J. Transpl. 2015, 5, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Scherer, M.N.; Pratschke, J.; Guba, M.; Nadalin, S.; Mehrabi, A.; Berlakovich, G.; Rogiers, X.; Pirenne, J.; Lerut, J.; et al. Technical aspects of orthotopic liver transplantation—a survey-based study within the Eurotransplant, Swisstransplant, Scandiatransplant and British Transplantation Society networks. J. Gastrointest. Surg. 2018. [Google Scholar] [CrossRef]
- Czigany, Z.; Lurje, I.; Tolba, R.; Neumann, U.P.; Tacke, F.; Lurje, G. Machine perfusion for liver transplantation in the era of marginal organs—new kids on the block. Liver Int. 2019, 39, 228–249. [Google Scholar] [CrossRef]
- Warner, P.; Fusai, G.; Glantzounis, G.K.; Sabin, C.A.; Rolando, N.; Patch, D.; Sharma, D.; Davidson, B.R.; Rolles, K.; Burroughs, A.K. Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation—univariable and multivariable analysis. Transpl. Int. 2011, 24, 401–408. [Google Scholar] [CrossRef]
- Puliti Reigada, C.H.; de Ataide, E.C.; de Almeida Prado Mattosinho, T.; Boin, I. Hepatic Artery Thrombosis After Liver Transplantation: Five-Year Experience at the State University of Campinas. Transpl. Proc. 2017, 49, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.; Souche, R.; Joly, E.; Boisset, G.; Habibeh, H.; Bouyabrine, H.; Panaro, F.; Ursic-Bedoya, J.; Jaber, S.; Guiu, B.; et al. Early Hepatic Artery Thrombosis After Liver Transplantation: What is the Impact of the Arterial Reconstruction Type? World J. Surg. 2017, 41, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Jambulingam, P.S.; Gunson, B.K.; Mayer, D.; Buckels, J.A.; Mirza, D.F.; Bramhall, S.R. Hepatic artery thrombosis following orthotopic liver transplantation: A 10-year experience from a single centers in the United Kingdom. Liver Transpl. 2006, 12, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Gilbo, N.; Van Praet, L.; Jochmans, I.; Sainz-Barriga, M.; Verslype, C.; Maleux, G.; Laleman, W.; van der Merwe, S.; Cassiman, D.; Nevens, F.; et al. Pre-operative trans-catheter arterial chemo-embolization increases hepatic artery thrombosis after liver transplantation—a retrospective study. Transpl. Int. 2018, 31, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Giorgakis, E.; Tedeschi, M.; Bonaccorsi-Riani, E.; Khorsandi, S.E.; Menon, K.; Vilca-Melendez, H.; Jassem, W.; Srinivasan, P.; Prachalias, A.; Heaton, N. The Effect of Recipient Body Mass Index and Its Extremes on Survival and Graft Vascular and Biliary Complications After Liver Transplantation: A Single Center Retrospective Study. Ann. Transpl. 2017, 22, 611–621. [Google Scholar] [CrossRef]
- Park, C.M.; Chung, J.W.; Kim, H.B.; Shin, S.J.; Park, J.H. Celiac axis stenosis: Incidence and etiologies in asymptomatic individuals. Korean J. Radiol. 2001, 2, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Sharafuddin, M.J.; Olson, C.H.; Sun, S.; Kresowik, T.F.; Corson, J.D. Endovascular treatment of celiac and mesenteric arteries stenoses: Applications and results. J. Vasc. Surg. 2003, 38, 692–698. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ceken, K.; Gurkan, A.; Erdogan, O.; Demirbas, A.; Kabaalioglu, A.; Sindel, T.; Luleci, E. Endovascular treatment of a recipient celiac trunk stenosis after orthotopic liver transplantation. J. Endovasc. Ther. 2003, 10, 376–380. [Google Scholar] [CrossRef]
| All Patients (n = 286) | κ | |
|---|---|---|
| Normal vascular anatomy | 149 (52%) | 0.916 |
| Intrinsic celiac axis stenosis/arteriosclerosis | 16 (6%) | 0.930 |
| Extrinsic celiac axis stenosis/MALC | 34 (12%) | 0.892 |
| Portal vein thrombosis | 20 (7%) | 0.848 |
| Other vascular abnormalities | 93 (33%) | 0.777 |
| All Patients (n = 286) | No MAL Compression (n = 252) | MAL Compression (n = 34) | P Value | |
|---|---|---|---|---|
| Donor age (years) | 56 ± 15 | 56 ± 15 | 52 ± 17 | 0.167 |
| Donor BMI | 30 ± 7 | 30 ± 8 | 28 ± 6 | 0.146 |
| Donor sex ratio (F:M) | 135 (47%):151 (53%) | 117 (46%):135 (54%) | 18 (53%):16 (47%) | 0.475 |
| Pre-transplant Child-Pugh Score | 7.4 ± 2.0 | 7.4 ± 2.0 | 7.6 ± 2.2 | 0.683 |
| Donor cause of death | CVA 181 (63%) | CVA 161 (64%) | CVA 20 (59%) | 0.565 |
| Anoxia 60 (21%) | Anoxia 52 (21%) | Anoxia 8 (24%) | 0.697 | |
| Trauma 34 (12%) | Trauma 30 (12%) | Trauma 4 (12%) | 0.981 | |
| Other 11 (4%) | Other 9 (4%) | Other 2 (6%) | 0.626 | |
| Extended Criteria Donors 1 | 181 (63%) | 162 (64%) | 19 (56%) | 0.325 |
| Recipient age (years) | 54 ± 10 | 55 ± 11 | 52 ± 10 | 0.174 |
| Recipient BMI | 27 ± 5 | 27 ± 5 | 25 ± 5 | 0.040 |
| Recipient sex ratio (F:M) | 91 (32%):195 (68%) | 81 (32%):171 (68%) | 10 (29%):24 (71%) | 0.748 |
| Etiology of liver disease | ALF 33 (12%) | ALF 26 (10%) | ALF 7 (20%) | 0.088 |
| HCC 70 (24%) | HCC 61 (24%) | HCC 9 (26%) | 0.773 | |
| Alcoholic cirrhosis 75 (26%) | Alcoholic cirrhosis 70 (28%) | Alcoholic cirrhosis 5 (15%) | 0.104 | |
| Viral 22 (8%) | Viral 18 (7%) | Viral 4 (12%) | 0.312 | |
| PSC/PBC 28 (10%) | PSC/PBC 25 (10%) | PSC/PBC 3 (9%) | 0.840 | |
| AIH 9 (3%) | AIH 7 (3%) | AIH 2 (6%) | 0.291 | |
| Other 49 (17%) | Other 45 (18%) | Other 4 (12%) | 0.404 | |
| Pre-transplant labMELD | 19 ± 10 | 19 ± 10 | 20 ± 11 | 0.697 |
| BAR Score 2 | 8.51 ± 5.45 | 8.45 ± 5.31 | 8.76 ± 6.30 | 0.863 |
| Recipient pre-transplant mechanical/ventilation support 3 | 26 (9%) | 22 (9%) | 4 (12%) | 0.528 |
| Recipient pre-transplant ICU | 65 (23%) | 56 (22%) | 9 (27%) | 0.579 |
| Cold ischemic time (min) | 503 ± 126 | 503 ± 123 | 511 ± 147 | 0.716 |
| Warm ischemic time (min) | 45 ± 8 | 45 ± 8 | 45 ± 7 | 0.996 |
| Intra-operative red blood cell transfusions (units) | 9 ± 9 | 10 ± 10 | 8 ± 6 | 0.181 |
| Post-operative red blood cell transfusions (units) 4 | 4 ± 7 | 4 ± 7 | 4 ± 6 | 0.717 |
| Early allograft dysfunction 5 | 80 (28%) | 69 (27%) | 11 (32%) | 0.563 |
| Major complications (≥CD3b) 6 | 145 (51%) | 127 (50%) | 18 (53%) | 0.814 |
| 90-days CCI 7 | 52 ± 32 | 52 ± 32 | 56 ± 30 | 0.544 |
| 90-days morbidity | CD3 96 (34%) | CD3 82 (33%) | CD3 14 (41%) | 0.317 |
| CD4 78 (27%) | CD4 70 (28%) | CD4 8 (24%) | 0.602 | |
| CD5 14 (5%) | CD5 12 (5%) | CD5 2 (6%) | 0.676 | |
| 90-days graft loss | 25 (9%) | 22 (9%) | 3 (9%) | 0.542 |
| Alternative arterial revascularization 8 | 21 (7%) | 17 (7%) | 4 (12%) | 0.274 |
| ICU stay (days) 9 | 12 ± 20 | 12 ± 20 | 12 ± 16 | 0.379 |
| Hospital stay (days) 10 | 38 ± 35 | 39 ± 36 | 36 ± 23 | 0.707 |
| First Author | Type of Study | Patients n (%) * | Management n (%) | Structured Summary |
|---|---|---|---|---|
| Czigany et al. (present study) | Retrospective cohort | 34/286 (12%) | MAL division 26/34 (77), Alternative reconstruction 4/34 (12) | Treatment of MALC with MAL division was feasible and safe and may be considered in patients with relevant celiac axis stenosis as assessed by preoperative ceCT imaging. |
| Fukuzawa et al., 1993 [20] | Retrospective cohort | 5/307 (1.6%) | Aorto-hepatic reconstruction 2/5 (40), MAL division 3/5 (60) | Celiac axis compression resulted in decreased hepatic artery flow to the allograft. In three cases, celiac decompression by division of the arcuate ligament resulted in adequate flow improvement. |
| Jurim et al., 1993 [17] | Retrospective cohort | 17/164 (10%) | MAL division 17/17 (100), Aorto-hepatic reconstruction after unsuccessful MAL division 2/17 (12) | Identification and removal of the obstruction of the celiac axis by MAL division is crucial to prevent severe complications and potential graft loss. |
| Agnes et al., 2001 [15] | Retrospective cohort | 5/140 (3.6%) | MAL division 5/5 (100) | Celiac compression must be identified and corrected in liver transplant recipients to avoid acute or chronic ischemic complications. Surgical therapy consists of arcuate ligament division, usually with excellent prognosis. |
| Lubrano et al., 2008 [14] | Retrospective cohort | 10/168 (6%) | Aorto-hepatic reconstruction 4/10 (40), MAL division 1/10 (10) | The presence of an arcuate ligament does not contraindicate a routine hepatic artery reconstruction and MAL division should be performed if required. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czigany, Z.; Boecker, J.; Morales Santana, D.A.; Bednarsch, J.; Meister, F.A.; Amygdalos, I.; Isfort, P.; Liebl, M.; Neumann, U.P.; Lurje, G. Median Arcuate Ligament Compression in Orthotopic Liver Transplantation: Results from a Single-Center Analysis and a European Survey Study. J. Clin. Med. 2019, 8, 550. https://doi.org/10.3390/jcm8040550
Czigany Z, Boecker J, Morales Santana DA, Bednarsch J, Meister FA, Amygdalos I, Isfort P, Liebl M, Neumann UP, Lurje G. Median Arcuate Ligament Compression in Orthotopic Liver Transplantation: Results from a Single-Center Analysis and a European Survey Study. Journal of Clinical Medicine. 2019; 8(4):550. https://doi.org/10.3390/jcm8040550
Chicago/Turabian StyleCzigany, Zoltan, Joerg Boecker, Daniel Antonio Morales Santana, Jan Bednarsch, Franziska Alexandra Meister, Iakovos Amygdalos, Peter Isfort, Martin Liebl, Ulf Peter Neumann, and Georg Lurje. 2019. "Median Arcuate Ligament Compression in Orthotopic Liver Transplantation: Results from a Single-Center Analysis and a European Survey Study" Journal of Clinical Medicine 8, no. 4: 550. https://doi.org/10.3390/jcm8040550
APA StyleCzigany, Z., Boecker, J., Morales Santana, D. A., Bednarsch, J., Meister, F. A., Amygdalos, I., Isfort, P., Liebl, M., Neumann, U. P., & Lurje, G. (2019). Median Arcuate Ligament Compression in Orthotopic Liver Transplantation: Results from a Single-Center Analysis and a European Survey Study. Journal of Clinical Medicine, 8(4), 550. https://doi.org/10.3390/jcm8040550







