Metastatic Tumors of the Sinonasal Cavity: A 15-Year Review of 17 Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical analysis
3. Results
3.1. Patients
3.2. Treatment Modality
3.3. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frazell, E.L.; Lewis, J.S. Cancer of the nasal cavity and accessory sinuses. A report of the management of 416 patients. Cancer 1963, 16, 1293–1301. [Google Scholar] [CrossRef]
- Carrau, R.L.; Myers, E.N. Neoplasms of the nose and paranasal sinuses. In Head and Neck Surgery-Otolaryngology, 3rd ed.; Bailey, B.J., Calhoun, K.H., Healy, G.B., Johnson, J.T., Jackler, R.K., Pillsbury, H.C., III, Tardy, M.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 1247–1264. [Google Scholar]
- Austin, J.R.; Kershiznek, M.M.; McGill, D.; Austin, S.G. Breast carcinoma metastatic to paranasal sinuses. Head Neck 1995, 17, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, E.; Grant, R.N. Cancer statistics, 1970. CA Cancer J. Clin. 1970, 20, 11–23. [Google Scholar] [CrossRef]
- Bernstein, J.M.; Montgomery, W.W.; Balogh, K., Jr. Metastatic tumors to the maxilla, nose, and paranasal sinuses. Laryngoscope 1966, 76, 621–650. [Google Scholar] [CrossRef]
- Azarpira, N.; Ashraf, M.J.; Khademi, B.; Asadi, N. Distant metastases to nasal cavities and paranasal sinuses case series. Indian J. Otolaryngol. Head Neck Surg. 2011, 63, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.E.; Majumdar, B. Metastatic tumours in the maxillary sinus. A report of two cases and a review of the literature. J. Laryngol. Otol. 1985, 99, 459–462. [Google Scholar] [CrossRef]
- Barnes, L. Metastases to the head and neck: An overview. Head Neck Pathol. 2009, 3, 217–224. [Google Scholar] [CrossRef]
- Lopez, F.; Devaney, K.O.; Hanna, E.Y.; Rinaldo, A.; Ferlito, A. Metastases to nasal cavity and paranasal sinuses. Head Neck 2016, 38, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, R.; Helmus, C. Hypernephroma metastatic to the head and neck. Laryngoscope 1973, 83, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Frigy, A.F. Metastatic hepatocellular carcinoma of the nasal cavity. Arch. Otolaryngol. 1984, 110, 624–627. [Google Scholar]
- Mickel, R.A.; Zimmerman, M.C. The sphenoid sinus—A site for metastasis. Otolaryngol. Head Neck Surg. 1990, 102, 709–716. [Google Scholar] [CrossRef]
- Huang, H.H.; Chang, P.H.; Fang, T.J. Sinonasal metastatic hepatocellular carcinoma. Am. J. Otolaryngol. 2007, 28, 238–241. [Google Scholar] [CrossRef]
- Pourseirafi, S.; Shishehgar, M.; Ashraf, M.J.; Faramarzi, M. Papillary Carcinoma of Thyroid with Nasal Cavity Metastases: A Case Report. Iran. J. Med. Sci. 2018, 43, 90–93. [Google Scholar]
- Pittoni, P.; Di Lascio, S.; Conti-Beltraminelli, M.; Valli, M.C.; Espeli, V.; Bongiovanni, M.; Richetti, A.; Pagani, O. Paranasal sinus metastasis of breast cancer. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef]
- Terada, T. Renal cell carcinoma metastatic to the nasal cavity. Int. J. Clin. Exp. Pathol. 2012, 5, 588–591. [Google Scholar]
- Liu, C.Y.; Chang, L.C.; Yang, S.W. Metastatic hepatocellular carcinoma to the nasal cavity: A case report and review of the literature. J. Cancer Sci. Ther. 2011, 3, 81–83. [Google Scholar] [CrossRef]
- Barbosa, E.B.; Ferreira, E.; Mariano, F.V.; Altemani, A.; Sakuma, E.T.I.; Sakano, E. Metastasis to Paranasal Sinuses from Carcinoma of Prostate: Report of a Case and Review of the Literature. Case Rep. Otolaryngol. 2018, 2018, 5428975. [Google Scholar] [CrossRef]
- Bin Sabir Husin Athar, P.P.; bte Ahmad Norhan, N.; bin Saim, L.; bin Md Rose, I.; bte Ramli, R. Metastasis to the sinonasal tract from sigmoid colon adenocarcinoma. Ann. Acad. Med. Sing. 2008, 37, 788–790. [Google Scholar]
- Friedmann, I.; Osborn, D.A. Metastatic tumours in the ear, nose and throat region. J. Laryngol. Otol. 1965, 79, 576–591. [Google Scholar] [CrossRef]
- Choong, C.V.; Tang, T.; Chay, W.Y.; Goh, C.; Tay, M.H.; Zam, N.A.; Tan, P.H.; Tan, M.H. Nasal metastases from renal cell carcinoma are associated with Memorial Sloan-Kettering Cancer Center poor-prognosis classification. Chin. J. Cancer 2011, 30, 144–148. [Google Scholar] [CrossRef]
- Simo, R.; Sykes, A.J.; Hargreaves, S.P.; Axon, P.R.; Birzgalis, A.R.; Slevin, N.J.; Farrington, W.T. Metastatic renal cell carcinoma to the nose and paranasal sinuses. Head Neck 2000, 22, 722–727. [Google Scholar] [CrossRef]
- Kaminski, B.; Kobiorska-Nowak, J.; Bien, S. [Distant metastases to nasal cavities and paranasal sinuses, from the organs outside the head and neck]. Otolaryngol. Polska = Pol. Otolaryngol. 2008, 62, 422–425. [Google Scholar] [CrossRef]
- Parida, P.K. Renal cell carcinoma metastatic to the sinonasal region: Three case reports with a review of the literature. Ear Nose Throat J. 2012, 91, E11–E16. [Google Scholar] [CrossRef] [PubMed]
- Ravnik, J.; Smigoc, T.; Bunc, G.; Lanisnik, B.; Ksela, U.; Ravnik, M.; Velnar, T. Hypophyseal metastases: A report of three cases and literature review. Neurologia i Neurochirurgia Polska 2016, 50, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Fang, T.J.; Chang, P.H.; Lee, T.J. Sinonasal metastatic tumors in Taiwan. Chang Gung Med. J. 2008, 31, 457–462. [Google Scholar] [PubMed]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Chiang, C.J.; Lo, W.C.; Yang, Y.W.; You, S.L.; Chen, C.J.; Lai, M.S. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J. Formos. Med. Assoc. = Taiwan Yi Zhi 2016, 115, 1076–1088. [Google Scholar] [CrossRef]
- Ringelhan, M.; McKeating, J.A.; Protzer, U. Viral hepatitis and liver cancer. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Trepo, C.; Chan, H.L.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef]
- Parkin, D.M.; Pisani, P.; Ferlay, J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int. J. Cancer 1999, 80, 827–841. [Google Scholar] [CrossRef]
- Chen, D.S. Hepatocellular carcinoma in Taiwan. Hepatol. Res. 2007, 37 (Suppl. 2), S101–S105. [Google Scholar] [CrossRef] [PubMed]
- Prescher, A.; Brors, D. Metastases to the paranasal sinuses: Case report and review of the literature. Laryngo Rhino Otologie 2001, 80, 583–594. [Google Scholar] [CrossRef]
- Dogan, S.; Can, I.H.; Sayn, M.; Ozer, E.; Bayz, U.; Yazici, G.; Samim, E.E. The nasal septum: An unusual presentation of metastatic renal cell carcinoma. J. Craniofac. Surg. 2009, 20, 1204–1206. [Google Scholar] [CrossRef]
- Sountoulides, P.; Metaxa, L.; Cindolo, L. Atypical presentations and rare metastatic sites of renal cell carcinoma: A review of case reports. J. Med. Case Rep. 2011, 5, 429. [Google Scholar] [CrossRef]
- Weber, A.L.; Stanton, A.C. Malignant tumors of the paranasal sinuses: Radiologic, clinical, and histopathologic evaluation of 200 cases. Head Neck Surg. 1984, 6, 761–776. [Google Scholar] [CrossRef]
- Gottlieb, M.D.; Roland, J.T., Jr. Paradoxical spread of renal cell carcinoma to the head and neck. Laryngoscope 1998, 108, 1301–1305. [Google Scholar] [CrossRef]
- Fukuda, M.; Miyata, M.; Okabe, K.; Sakashita, H. A case series of 9 tumors metastatic to the oral and maxillofacial region. J. Oral Maxillofac. Surg. 2002, 60, 942–944. [Google Scholar] [CrossRef]
- Aziz, S.A.; Sznol, J.; Adeniran, A.; Colberg, J.W.; Camp, R.L.; Kluger, H.M. Vascularity of primary and metastatic renal cell carcinoma specimens. J. Transl. Med. 2013, 11, 15. [Google Scholar] [CrossRef]
- Evgeniou, E.; Menon, K.R.; Jones, G.L.; Whittet, H.; Williams, W. Renal cell carcinoma metastasis to the paranasal sinuses and orbit. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef][Green Version]
- Nahum, A.M.; Bailey, B.J. malignant tumors metastatic to the paranasal sinuses: case report and review of the literature. Laryngoscope 1963, 73, 942–953. [Google Scholar] [CrossRef]
- Sim, R.S.; Tan, H.K. A case of metastatic hepatocellular carcinoma of the sphenoid sinus. J. Laryngol. Otol. 1994, 108, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.D.; Cheng, K.S.; Tsai, C.H.; Chen, C.L.; Tsai, M.H. Metastatic hepatocellular carcinoma in the nasal septum: Report of a case. J. Formos. Med. Assoc. = Taiwan Yi Zhi 2002, 101, 715–718. [Google Scholar] [PubMed]
- Matsuda, H.; Tanigaki, Y.; Yoshida, T.; Matsuda, R.; Tsukuda, M. A case of metastatic hepatocellular carcinoma in the nasal cavity. Eur. Arch. Oto-Rhino-Laryngol. 2006, 263, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Baldwin, M.E.; Achen, M.G. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002, 16, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Batson, O.V. The function of the vertebral veins and their role in the spread of metastases. Ann. Surg. 1940, 112, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Wiltse, L.L.; Fonseca, A.S.; Amster, J.; Dimartino, P.; Ravessoud, F.A. Relationship of the dura, Hofmann’s ligaments, Batson’s plexus, and a fibrovascular membrane lying on the posterior surface of the vertebral bodies and attaching to the deep layer of the posterior longitudinal ligament. An anatomical, radiologic, and clinical study. Spine 1993, 18, 1030–1043. [Google Scholar] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.J. Metastatic tumours of the paranasal sinuses simulating primary growths. J. Fac. Radiol. 1959, 10, 151–155. [Google Scholar] [CrossRef]
- Som, P.M.; Norton, K.I.; Shugar, J.M.; Reede, D.L.; Norton, L.; Biller, H.F.; Som, M.L. Metastatic hypernephroma to the head and neck. AJNR Am. J. Neuroradiol. 1987, 8, 1103–1106. [Google Scholar] [PubMed]
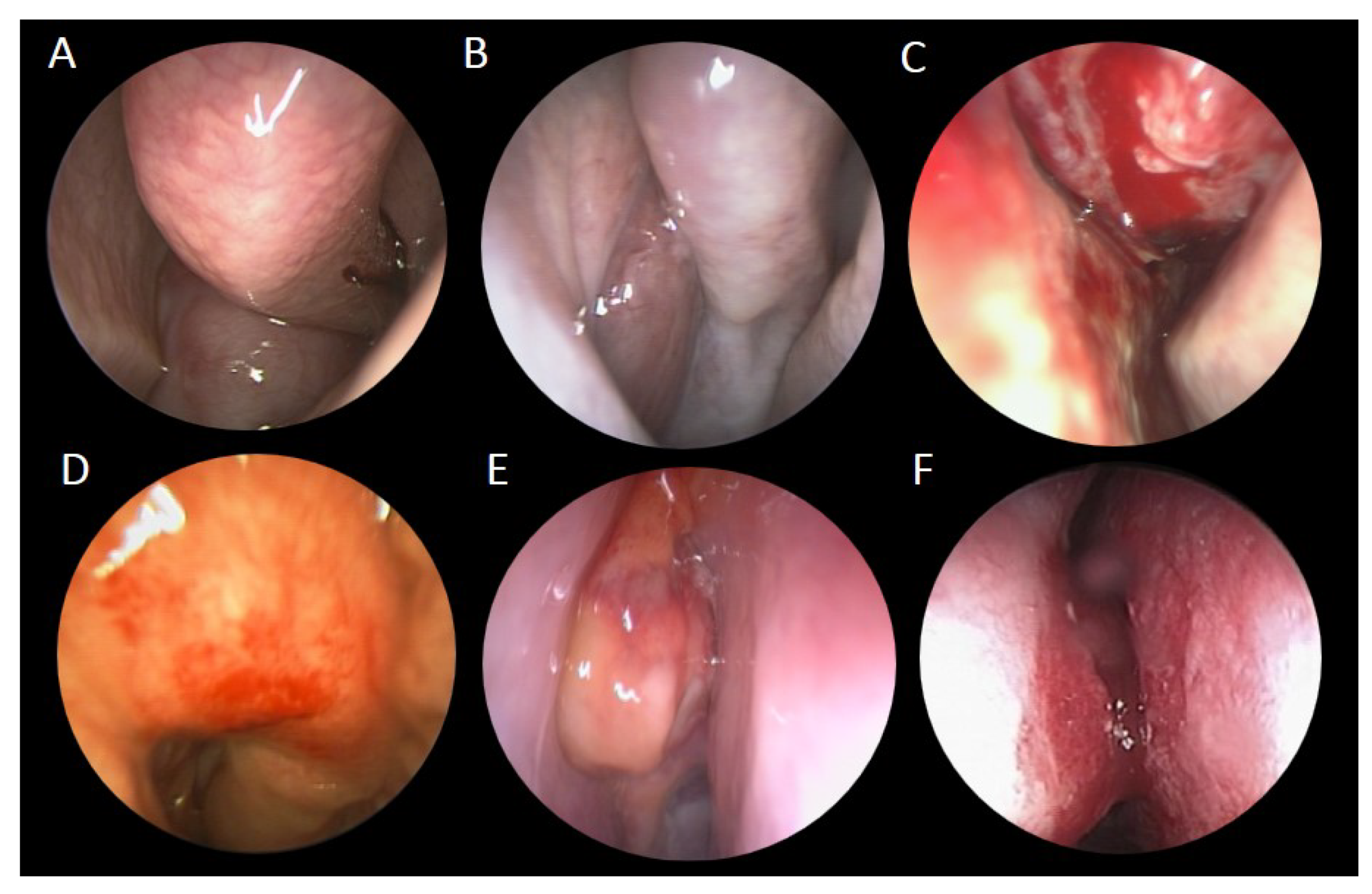
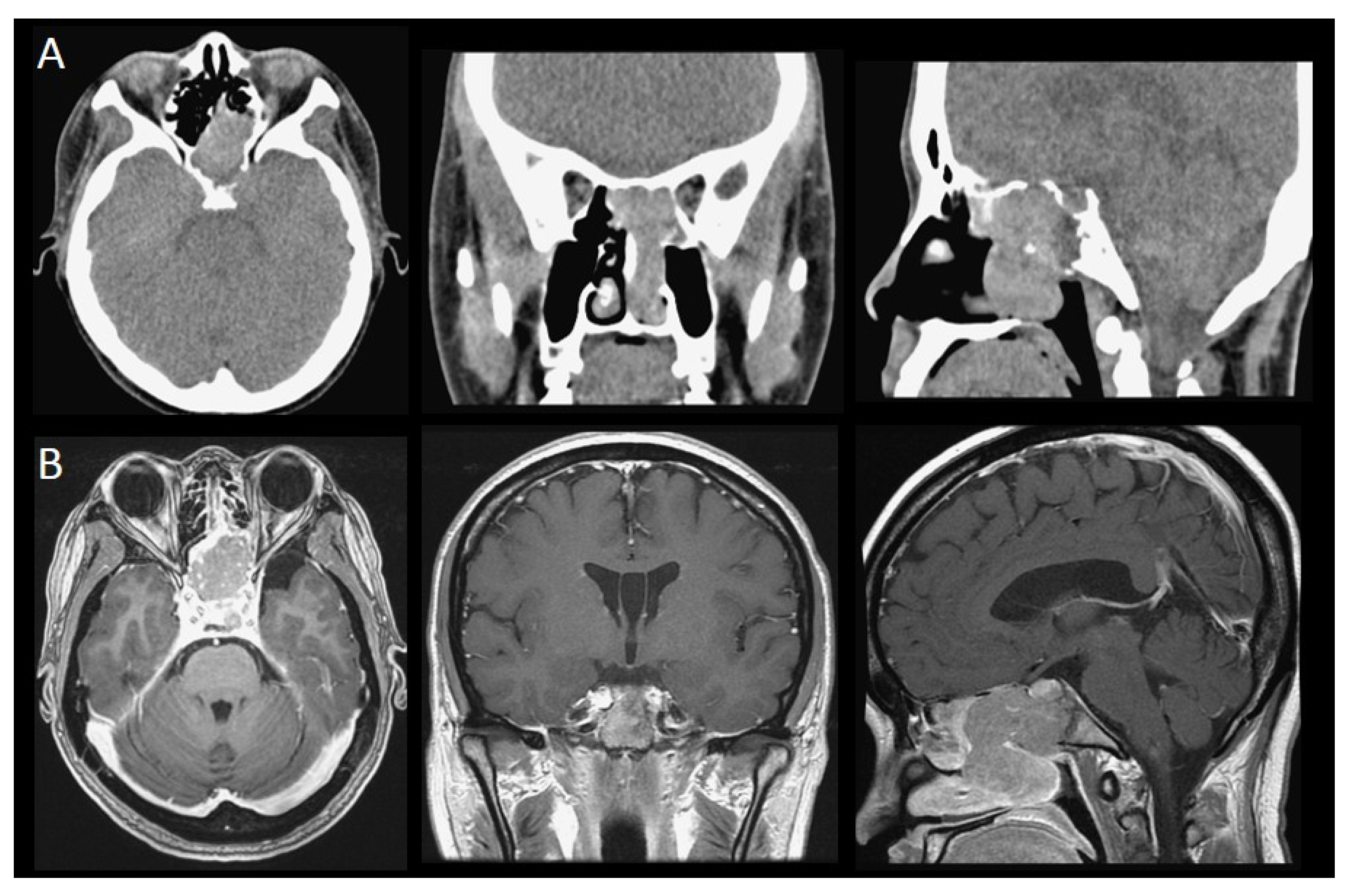
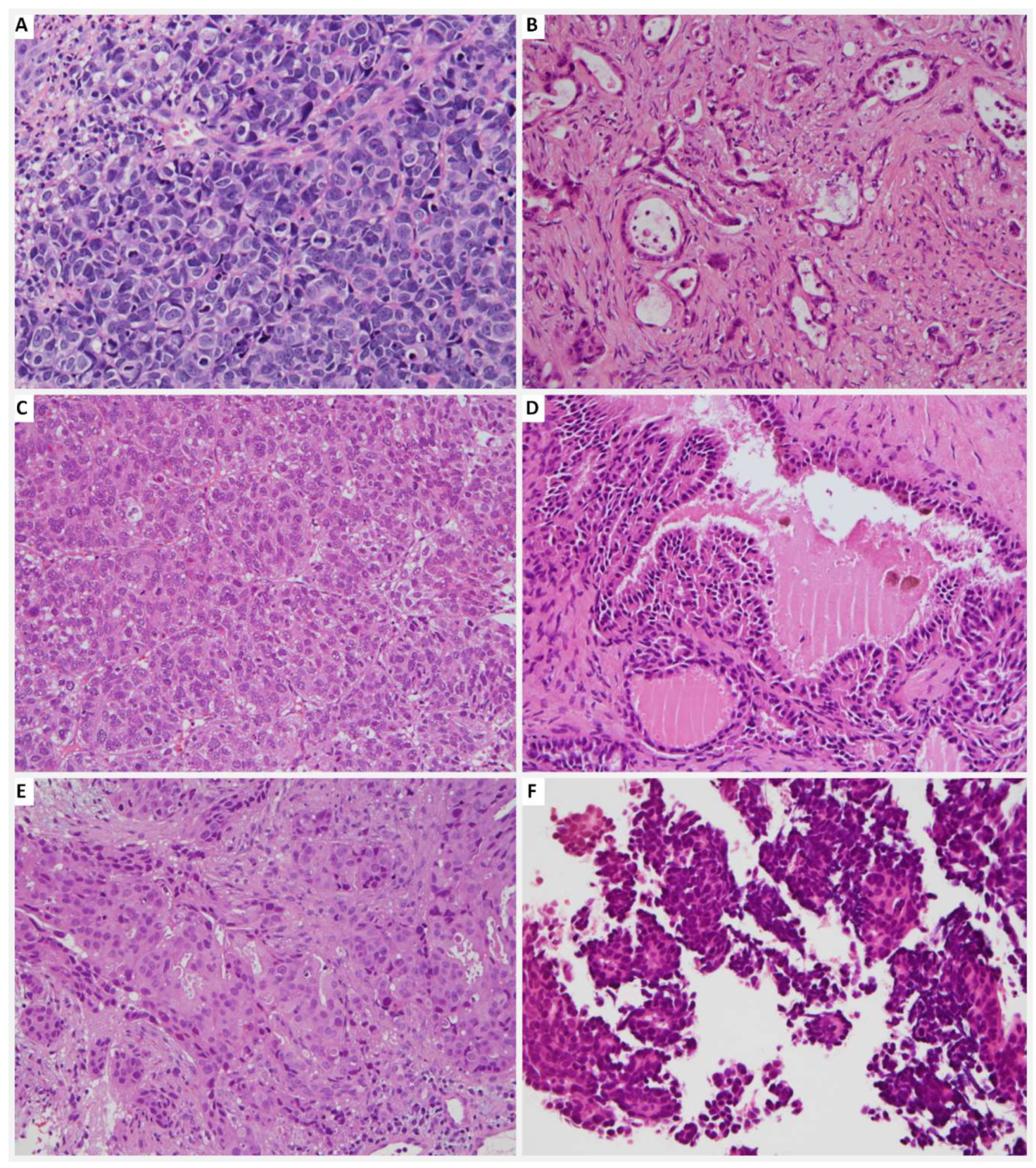
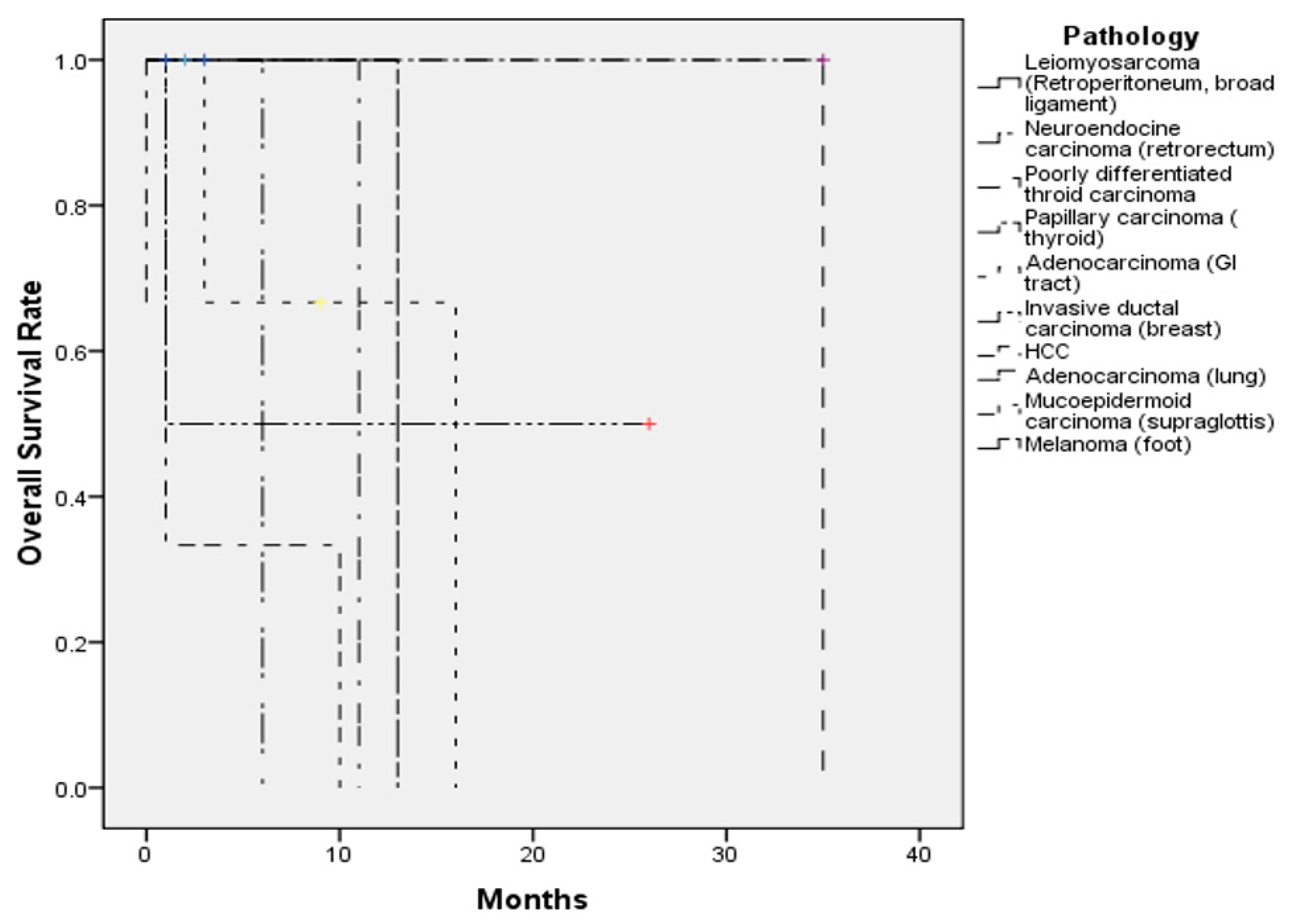
| Case Number (n = 17) | |
|---|---|
| Age | 27–80 (mean 56.8 ± 14.2) |
| Sex | M:F = 9:8 |
| Metastatic time | 2 months–18 years and 7 months |
| Symptoms | |
| Epistaxis | 7 |
| Headache | 3 |
| Nasal obstruction | 2 |
| Diplopia | 1 |
| Extraocular movement limitation | 1 |
| Facial swelling | 1 |
| Side | |
| Unilateral (L/R) | 8 (3/5) |
| Bilateral | 7 |
| Sinonasal metastases | |
| Single metastases | 9 |
| Multifocal metastases | 7 |
| Widespread metastasis | 11 |
| Sinonasal metastatic sites | |
| Nasal cavity | 8 |
| Skull base | 6 |
| Nasal septum | 5 |
| Maxillary sinus | 4 |
| Ethmoid sinus | 4 |
| Sphenoid sinus | 4 |
| Frontal sinus | 1 |
| Number (n = 17) | |
|---|---|
| Primary tumor site | |
| -GI tract | 4 |
| -Liver | 3 |
| -Breast | 2 |
| -Thyroid | 2 |
| -Retroperitoneum | 1 |
| -Broad ligament | 1 |
| -Supraglottis | 1 |
| -Lung | 1 |
| -Tibia | 1 |
| -Foot | 1 |
| Pathology | |
| -Adenocarcinoma (GI tract) | 3 |
| -Hepatocellular carcinoma (HCC) | 3 |
| -Invasive ductal carcinoma (breast) | 2 |
| -Leiomyosarcoma (retroperitoneum, broad ligament) | 2 |
| -Poorly differentiated carcinoma (thyroid) | 1 |
| -Papillary carcinoma (thyroid) | 1 |
| -Adenocarcinoma (lung) | 1 |
| -Neuroendocrine carcinoma (retrorectum) | 1 |
| -Mucoepidermoid carcinoma (supraglottis) | 1 |
| -Osteosarcoma (tibia) | 1 |
| -Melanoma (foot) | 1 |
| No. | Age/Sex | Symptoms | Primary Tumor | Clinical Stage before Metastasis to Sinonasal Region | Sinonasal Metastatic Site | Time Before Metastasis | Extrasinonasal Metastasis (Initial) | Extrasinonasal Metastasis (Before Sinonasal Metastasis) | Treatment Modality | Follow-up Duration | Disease Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27M | Headache Ptosis | Tibiaosteosarcoma | Stage III (M1) | NA | 4y5m | N | Lung | CT (Ifosfamide + Etoposide) | NA | NA |
| 2 | 37F | Epistaxis | Retroperitoneum leiomyosarcoma | Stage IV (M1) | Sphenoid, ethmoid sinuses, clivus | 3y4m | N | Liver | Tumor resection + RT | 1m | Alive, no obvious residual tumor |
| 3 | 45F | Headache | Retrorectalneuroendocrine carcinoma | Stage IIIa (T2N1M0) | Sphenoid sinus, pituitary gland | 4m | N | N | Debulking surgery + CT (cisplatin+ etoposide) | 6m | Dead |
| 4 | 47M | Epistaxis | Thyroidpoorly differentiated carcinoma | Stage IVb (pT3N1bM1) | Maxillary, ethmoid, sphenoid sinuses, nasal cavity, orbit, brain | 6m | Left iliac | Left iliac | Tumor biopsy + RT + iodine ablation therapy | 3y11m | Dead |
| 5 | 48F | Nasal mass | Thyroid papillary carcinoma | Stage IVc (M1) | Nasal septum | Synchronous | N | N | Tumor excision | 2y11m | Alive, no obvious residual tumor |
| 6 | 49M | Diplopia | Gastricadenocarcinoma | Stage Ib (T1N1M0) | Sphenoid sinus, cavernous sinus | 6y3m | N | N | Tumor excision + CT (Capecitabine+ oxaliplatin+ Paclitaxel)+ Target therapy (Cetuximab+ Uracil-Tegafur) | 1y4m | Dead |
| 7 | 51F | EOM limitation, hearing impairment | Breast invasive ductal carcinoma | Stage IV (pT3N3M1) | Ethmoid sinus, nasal cavity, nasal septum | 11y | Bone, pleural, lung | Bone, pleural, lung | Tumor biopsy + CT | 1m22d | Dead |
| 8 | 53F | Facial pain, headache, ptosis | Rectal adenocarcinoma | Stage IVb (M1) | Nasal cavity, skull base, | 1y1m | Lung, liver, adrenal, bone | Lung, liver, adrenal, bone | Tumor biopsy + CT (Irinotecan + Fluorouracil + Leucovorin) + Target therapy (Bevacizumab + Cetuximab) | 9m | Alive with residual tumor |
| 9 | 56M | Epistaxis, nasal obstruction | Hepatocellular carcinoma | StageIVb (M1) | Frontal, ethmoid sinuses, nasal septum, nasal cavity, orbit | 10y | N | Lung, brain | Tumor biopsy + RT+ Target therapy (Sorafenib) | 2m | Dead |
| 10 | 57F | Epistaxis | Lungadenocarcinoma | Stage IVa (cT3N2M1b) | Nasal cavity | Synchronous | Lung, bone | Lung, bone | Tumor biopsy + RT + CT (Alimta + Cisplatin + Docetaxel) + Target therapy (Erlotinib + Pembrolizumab) | 11m | Dead |
| 11 | 66F | Nasal mass Epistaxis | Breast invasive ductal carcinoma | Stage IV (pT4cN0M1) | Nasal vestibule | 4y3m | Lung | Lung | Tumor biopsy + Hormone therapy (Tamoxifen) | 2y2m | Alive with residual tumor |
| 12 | 66F | No | Broad ligamentleiomyosarcoma | Stage IV (M1) | ITF | 18y7m | N | Liver, abdomen, muscle, bone | Tumor biopsy + CT (Gemcitabine + Taxotere + Cisplatin + Everolimus + Adriamycin) + Target therapy (Pazopanib) | 3m | Alive with residual tumor |
| 13 | 67M | Epistaxis | Right plantar foot melanoma | Stage IIIb (T4aN2bM0) | Maxillary sinus | 6 m | N | N | Tumor biopsy + CT (Dacarbazine + Cisplatin + Vinblastine + Proleukin + Cyclophosphamide + paclitaxel) + interferon-A + immunotherapy (IL-2) | 1y1m | Dead |
| 14 | 69M | Nasal mass | Hepatocellular carcinoma | StageIVb (M1) | Nasal cavity | 2m | Lung | Lung | Tumor biopsy + CT (Etoposide + Doxorubicin + Cisplatin +5-FU +Leucovorin) | 3d | Dead |
| 15 | 70M | Epistaxis | Cecaladenocarcinoma | Stage IVc (M1) | Maxilla | Synchronous | N | N | Tumor resection + CT (fluorouracil + oxaliplatin + calcium folinate + irinotecan) + Target therapy (bevacizumab) | 3m | Dead |
| 16 | 77M | Nasal obstruction | Supraglottic mucoepidermoid carcinoma | Stage IVa (pT4N2cM0) | Maxillary sinus, nasal cavity, nasal septum | 6m | N | N | Tumor resection + RT | 2m | Alive, no obvious residual tumor |
| 17 | 80M | NA | Hepatocellular carcinoma | Stage IVb (M1) | nasal septum | 5y9m | N | Lung, muscle, bone | Tumor biopsy | 10m | Dead |
| Years of Publication | Authors | Total Patients | Age (mean, y/o) | Sex | Metastatic Site | Primary Tumor Sites | Follow-up (months) | Alive with Residual Tumor (grossly) | Death |
|---|---|---|---|---|---|---|---|---|---|
| 1959 | Garrett [49] | 6 | 58.8 | 5M1F | 5M, 1E | 1B, 1G, 1L, 1K, 2T | 0.3–16 | 1 | 4 |
| 1966 | Bernstein et al. [5] | 10 | 54.2 | 3M7F | 5M, 1E, 2F, 2N | 1B, 2G, 5K, 1L, 1U | 1–48 | 4 | 5 |
| 1987 | Som et al. [50] | 6 | 54 | NA | 3M, 2S, 1 E,1F | 6K | NA | 0 | 3 |
| 1990 | Mickel et al. [12] | 7 | 49.4 | 5M2F | 7S | 3L, 1U, 2O, 1 humerus | 0.25–7 | NA | NA |
| 2000 | Simo et al. [22] | 6 | 67.8 | 3M3F | NA | 6K | 6–48 | 2 | 4 |
| 2008 | Huang et al. [26] | 17 | 50.8 | 9M8F | 7M, 7N, 3E, 1S, 1NP | 5G, 3H, 3K, 3B, 2T, 1L | NA | 0 | 7 |
| 2008 | Kaminski et al. [23] | 4 | 61 | 1M3F | 2M,1S,1N | 1B, 2G, 1K | 2–23 | 0 | 3 |
| 2011 | Azarpira et al. [6] | 3 | 58 | 2M1F | 1E, 1M, 1S | 1B, 1K, 1U | 6–11 | 0 | 3 |
| 2011 | Choong et al. [21] | 4 | 55.8 | 3M1F | 4N | 4K | 8–24 | 1 | 3 |
| 2012 | Parida et al. [24] | 3 | 54.3 | 1M2F | 2F, 1N | 3K | 4–6 | 1 | 0 |
| 2016 | Ravnik et al. [25] | 3 | 57 | 1M2F | 3P | 1B, 1K, 1 lymphoma | 8–48 | 2 | 0 |
| 2019 | Chang et al. (our study) | 17 | 56.8 | 9M8F | 8N, 6SB, 5 septum, 4M, 4E, 4S, 1F, | 2B, 4G, 3H, 1L, 2T, 1 supraglottis, 2 leg, 1 retroperitoneum, 1 broad ligament | 2–228 | 7 | 9 |
| Total | 86 | 55.5 | 42M38F | 27M, 11E, 16S, 4F, 23N, 1 NP, 6 SB, 5 septum | 10B, 14G, 6H, 7L, 31K, 6T, 3U, 1 supraglottis, 3 extremities, 1 retroperitoneum, 1 broad ligament | 0.3–228 | 18 | 41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.-H.; Kuo, Y.-J.; Ho, C.-Y.; Kuan, E.C.; Lan, M.-Y. Metastatic Tumors of the Sinonasal Cavity: A 15-Year Review of 17 Cases. J. Clin. Med. 2019, 8, 539. https://doi.org/10.3390/jcm8040539
Chang M-H, Kuo Y-J, Ho C-Y, Kuan EC, Lan M-Y. Metastatic Tumors of the Sinonasal Cavity: A 15-Year Review of 17 Cases. Journal of Clinical Medicine. 2019; 8(4):539. https://doi.org/10.3390/jcm8040539
Chicago/Turabian StyleChang, Miao-Hsu, Ying-Ju Kuo, Ching-Yin Ho, Edward C. Kuan, and Ming-Ying Lan. 2019. "Metastatic Tumors of the Sinonasal Cavity: A 15-Year Review of 17 Cases" Journal of Clinical Medicine 8, no. 4: 539. https://doi.org/10.3390/jcm8040539
APA StyleChang, M.-H., Kuo, Y.-J., Ho, C.-Y., Kuan, E. C., & Lan, M.-Y. (2019). Metastatic Tumors of the Sinonasal Cavity: A 15-Year Review of 17 Cases. Journal of Clinical Medicine, 8(4), 539. https://doi.org/10.3390/jcm8040539






