A High Visceral-To-Subcutaneous Fat Ratio is an Independent Predictor of Surgical Site Infection after Gastrectomy
Abstract
1. Introduction
2. Materials and Methods
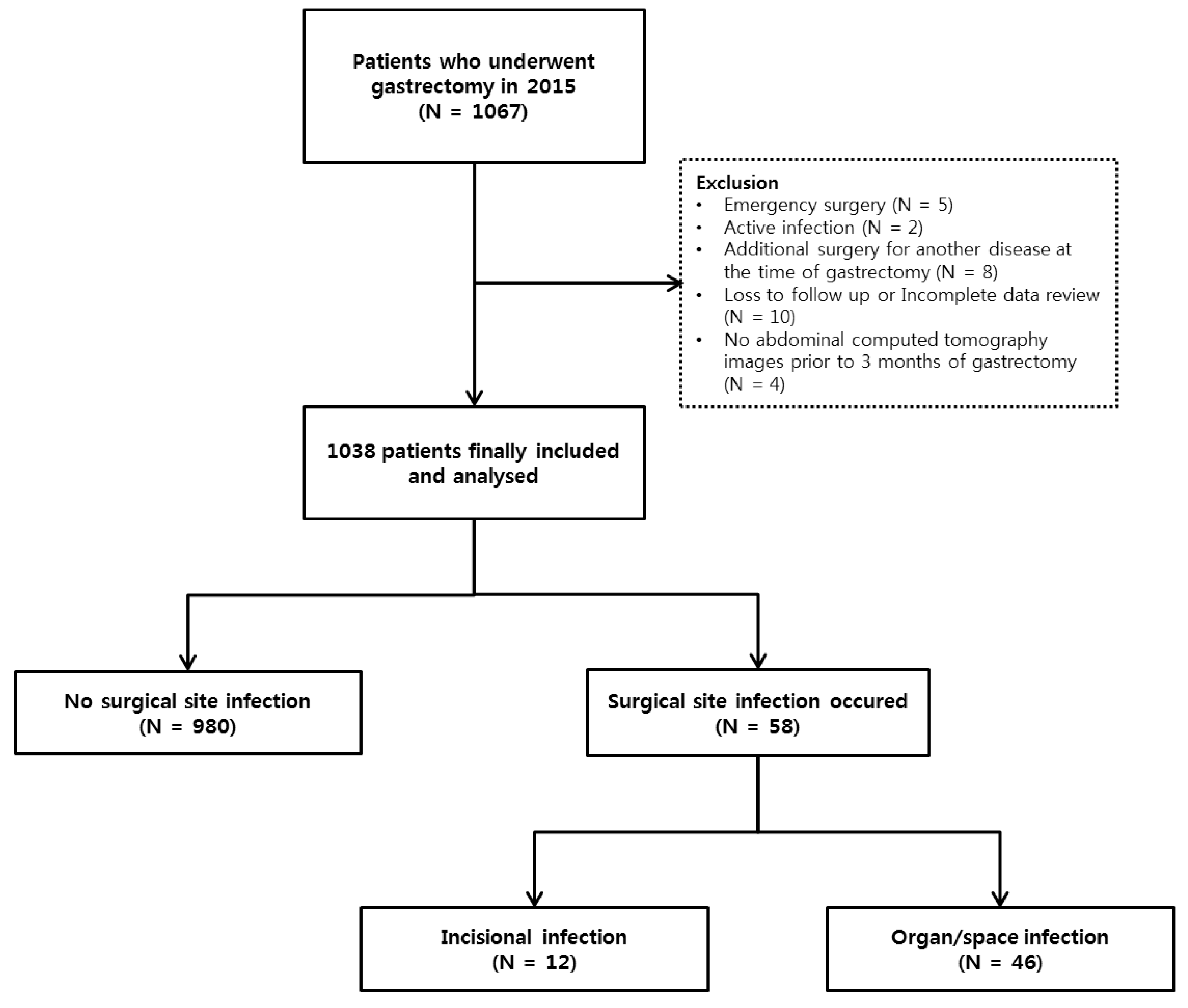
2.1. Study Design and Population
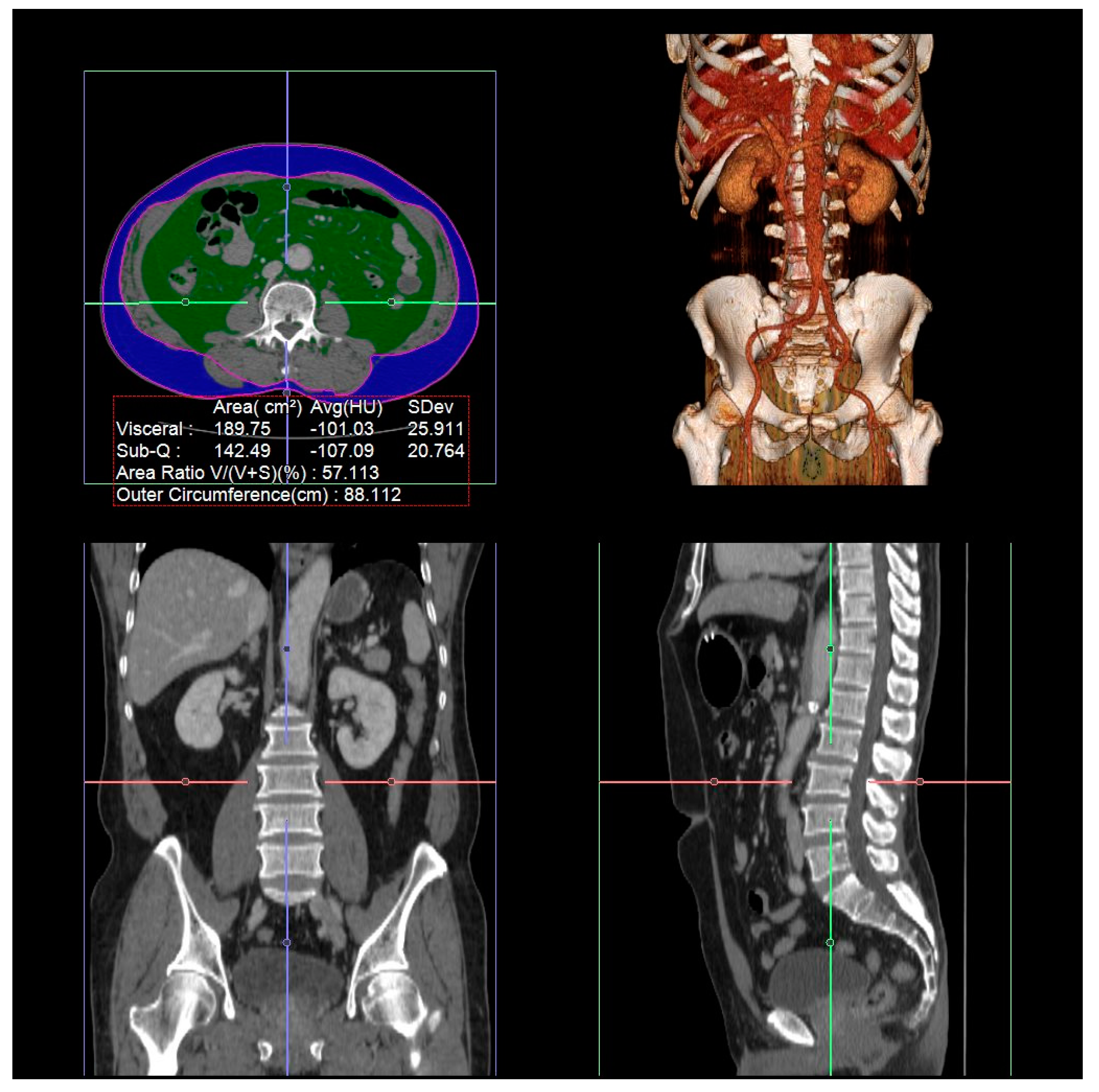
2.2. Measurement of Visceral Fat Area, Subcutaneous Fat Area, and Total Abdominal Muscle Area
2.3. Definition of SSI
2.4. Surgical Procedures and Perioperative Management
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients with SSI after Gastrectomy
3.2. Risk Factors for SSI after Gastrectomy
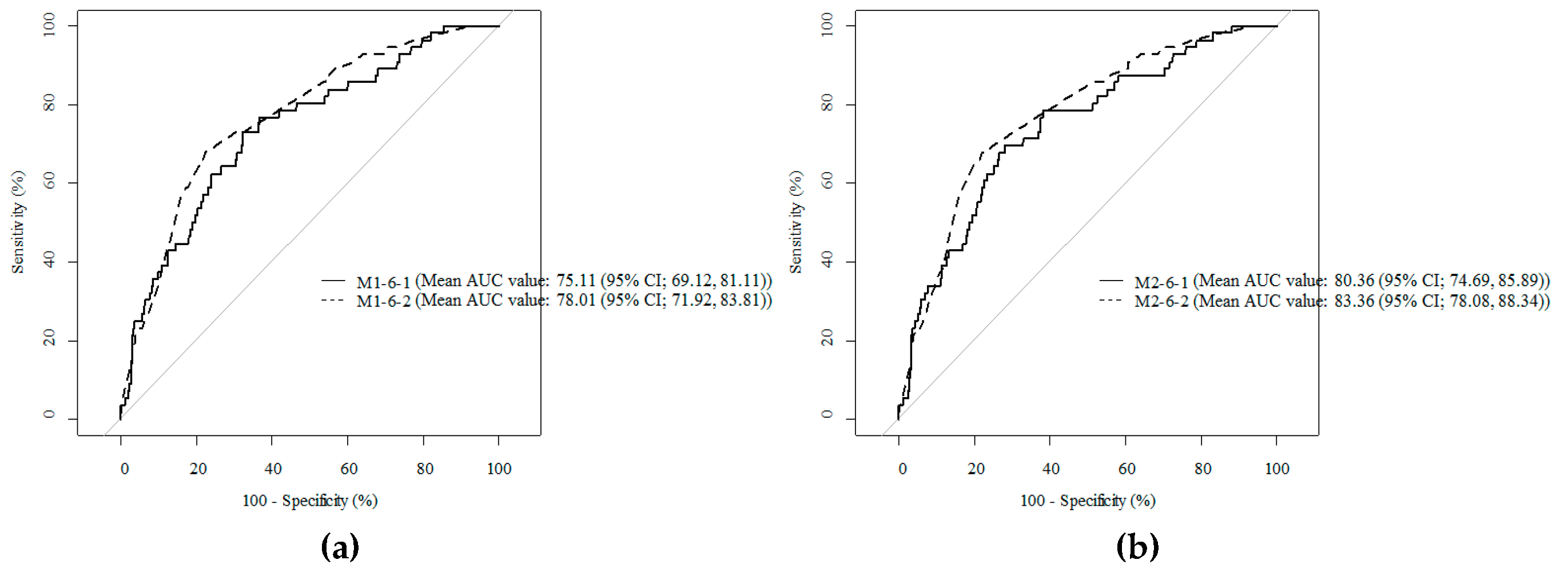
3.3. Comparison of Cody Composition Variables to Predict SSI after Gastrectomy
3.4. The Threshold Values of the VFA-To-SFA Ratios are related to SSI after Gastrectomy
3.5. Comparison of the Treatment Outcomes after Gastrectomy according to Abdominal Fat Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2015. Cancer Res. Treat. 2018, 50, 303–316. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Noh, S.H.; Cheong, J.H. Evolution of gastric cancer treatment: From the golden age of surgery to an era of precision medicine. Yonsei Med. J. 2015, 56, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Sasako, M.; Yamamoto, S.; Nashimoto, A.; Kurita, A.; Hiratsuka, M.; Tsujinaka, T.; Kinoshita, T.; Arai, K.; Yamamura, Y.; et al. Gastric cancer surgery: Morbidity and mortality results from a prospective randomized controlled trial comparing d2 and extended para-aortic lymphadenectomy—Japan clinical oncology group study 9501. J. Clin. Oncol. 2004, 22, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Bohl, J.K.; McElearney, S.T.; Friel, C.M.; Barclay, M.M.; Sawyer, R.G.; Foley, E.F. Wound infection after elective colorectal resection. Ann. Surg. 2004, 239, 599–605; discussion 597–605. [Google Scholar] [CrossRef]
- Yang, T.; Wei, M.; He, Y.; Deng, X.; Wang, Z. Impact of visceral obesity on outcomes of laparoscopic colorectal surgery: A meta-analysis. ANZ J. Surg. 2015, 85, 507–513. [Google Scholar] [PubMed]
- Tanaka, K.; Miyashiro, I.; Yano, M.; Kishi, K.; Motoori, M.; Seki, Y.; Noura, S.; Ohue, M.; Yamada, T.; Ohigashi, H.; et al. Accumulation of excess visceral fat is a risk factor for pancreatic fistula formation after total gastrectomy. Ann. Surg. Oncol. 2009, 16, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Ishii, K.; Seki, H.; Yasui, N.; Sakata, M.; Shimada, A.; Matsumoto, H. Excessive visceral fat area as a risk factor for early postoperative complications of total gastrectomy for gastric cancer: A retrospective cohort study. BMC Surg. 2016, 16, 54. [Google Scholar] [CrossRef]
- Peng, P.; Hyder, O.; Firoozmand, A.; Kneuertz, P.; Schulick, R.D.; Huang, D.; Makary, M.; Hirose, K.; Edil, B.; Choti, M.A.; et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, K.W.; van Vugt, J.L.; Tegels, J.J.; Snijders, C.; Hulsewe, K.W.; Hoofwijk, A.G.; Stoot, J.H.; Von Meyenfeldt, M.F.; Beets, G.L.; Derikx, J.P.; et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann. Surg. 2015, 261, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Watanabe, M.; Yoshida, N.; Baba, Y.; Umezaki, N.; Harada, K.; Karashima, R.; Imamura, Y.; Iwagami, S.; Baba, H. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann. Surg. Oncol. 2015, 22, 4432–4437. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Kvist, H.; Sjostrom, L.; Tylen, U. Adipose tissue volume determinations in women by computed tomography: Technical considerations. Int. J. Obes. 1986, 10, 53–67. [Google Scholar]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. (1985) 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Endo, S.; Tsujinaka, T.; Fujitani, K.; Fujita, J.; Tamura, S.; Yamasaki, M.; Kobayashi, S.; Akamaru, Y.; Mizushima, T.; Shimizu, J.; et al. Risk factors for superficial incisional surgical site infection after gastrectomy: Analysis of patients enrolled in a prospective randomized trial comparing skin closure methods. Gastric Cancer 2016, 19, 639–644. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for prevention of surgical site infection, 1999. Hospital infection control practices advisory committee. Infect. Control Hosp. Epidemiol. 1999, 20, 250–278. [Google Scholar]
- Song, J.; Oh, S.J.; Kang, W.H.; Hyung, W.J.; Choi, S.H.; Noh, S.H. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: Lessons learned from an initial 100 consecutive procedures. Ann. Surg. 2009, 249, 927–932. [Google Scholar] [CrossRef]
- Song, J.; Kang, W.H.; Oh, S.J.; Hyung, W.J.; Choi, S.H.; Noh, S.H. Role of robotic gastrectomy using da vinci system compared with laparoscopic gastrectomy: Initial experience of 20 consecutive cases. Surg. Endosc. 2009, 23, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Hyung, W.J.; Lim, J.S.; Song, J.; Choi, S.H.; Noh, S.H. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J. Am. Coll. Surg. 2008, 207, e6–e11. [Google Scholar] [CrossRef] [PubMed]
- Hyung, W.J.; Song, C.; Cheong, J.H.; Choi, S.H.; Noh, S.H. Factors influencing operation time of laparoscopy-assisted distal subtotal gastrectomy: Analysis of consecutive 100 initial cases. Eur. J. Surg. Oncol. 2007, 33, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [CrossRef] [PubMed]
- Kim, H.I.; Han, S.U.; Yang, H.K.; Kim, Y.W.; Lee, H.J.; Ryu, K.W.; Park, J.M.; An, J.Y.; Kim, M.C.; Park, S.; et al. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann. Surg. 2016, 263, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Takayama, T.; Matsumoto, S.; Wakatsuki, K.; Enomoto, K.; Tanaka, T.; Ito, M.; Nakajima, Y. Risk factors for surgical site infections after elective gastrectomy. J. Gastrointest. Surg. 2012, 16, 1107–1115. [Google Scholar] [CrossRef]
- Robertson, C.S.; Chung, S.C.; Woods, S.D.; Griffin, S.M.; Raimes, S.A.; Lau, J.T.; Li, A.K. A prospective randomized trial comparing r1 subtotal gastrectomy with r3 total gastrectomy for antral cancer. Ann. Surg. 1994, 220, 176–182. [Google Scholar] [CrossRef]
- Smith, J.K.; McPhee, J.T.; Hill, J.S.; Whalen, G.F.; Sullivan, M.E.; Litwin, D.E.; Anderson, F.A.; Tseng, J.F. National outcomes after gastric resection for neoplasm. Arch. Surg. (Chicago, Ill.: 1960) 2007, 142, 387–393. [Google Scholar] [CrossRef]
- Lawson, E.H.; Hall, B.L.; Ko, C.Y. Risk factors for superficial vs deep/organ-space surgical site infections: Implications for quality improvement initiatives. JAMA Surg. 2013, 148, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol. Metab. Syndr. 2011, 3, 12. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018, 17, 218. [Google Scholar] [CrossRef]
- Mitsui, Y.; Sadahira, T.; Araki, M.; Maruyama, Y.; Wada, K.; Tanimoto, R.; Kobayashi, Y.; Watanabe, M.; Watanabe, T.; Nasu, Y. Clinical impact of abdominal fat distribution measured by 3-D computed tomography volumetry on post-transplant renal function in recipients after living kidney transplantation: A retrospective study. Clin. Exp. Nephrol. 2018. [Google Scholar] [CrossRef]
- Kaess, B.M.; Pedley, A.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012, 55, 2622–2630. [Google Scholar] [CrossRef]
- Nishigori, T.; Tsunoda, S.; Okabe, H.; Tanaka, E.; Hisamori, S.; Hosogi, H.; Shinohara, H.; Sakai, Y. Impact of sarcopenic obesity on surgical site infection after laparoscopic total gastrectomy. Ann. Surg. Oncol. 2016, 23, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, N.; Carrara, G.; De Cobelli, F.; Cristel, G.; Damascelli, A.; Balzano, G.; Beretta, L.; Braga, M. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br. J. Surg. 2016, 103, 434–442. [Google Scholar] [CrossRef]
- Pecorelli, N.; Capretti, G.; Sandini, M.; Damascelli, A.; Cristel, G.; De Cobelli, F.; Gianotti, L.; Zerbi, A.; Braga, M. Impact of sarcopenic obesity on failure to rescue from major complications following pancreaticoduodenectomy for cancer: Results from a multicenter study. Ann. Surg. Oncol. 2018, 25, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Miyata, H.; Gotoh, M.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Kitagawa, Y.; Sugihara, K.; et al. Total gastrectomy risk model: Data from 20,011 Japanese patients in a nationwide internet-based database. Ann. Surg. 2014, 260, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Kurita, N.; Miyata, H.; Gotoh, M.; Shimada, M.; Imura, S.; Kimura, W.; Tomita, N.; Baba, H.; Kitagawa, Y.; Sugihara, K.; et al. Risk model for distal gastrectomy when treating gastric cancer on the basis of data from 33,917 Japanese patients collected using a nationwide web-based data entry system. Ann. Surg. 2015, 262, 295–303. [Google Scholar] [CrossRef]
| Total (n = 1038) | SSI (−) (n = 980) | SSI (+) (n = 58) | p-Value | |
|---|---|---|---|---|
| Age | 59 (50–67) | 59 (50–67) | 62.5 (53–68) | 0.206 |
| Sex | 0.037 | |||
| Male | 654 (63) | 610 (62.2) | 44 (75.9) | |
| Female | 384 (37) | 370 (37.8) | 14 (24.1) | |
| BMI >25 | 330 (31.8) | 308 (31.4) | 22 (37.9) | 0.301 |
| Smoking | 561 (54) | 520 (53.1) | 41 (70.7) | 0.009 |
| ASA score | 0.142 | |||
| >3 | 794 (76.5) | 756 (77.1) | 38 (65.5) | |
| ≤3 | 244 (23.5) | 141 (22.9) | 11 (34.5) | |
| Hypertension | 338 (32.6) | 313 (31.9) | 25 (43.1) | 0.078 |
| Diabetes | 150 (14.5) | 140 (14.3) | 10 (17.2) | 0.534 |
| Cerebrovascular accident | 32 (3.1) | 31 (3.2) | 1 (1.7) | 1.000 |
| Arrhythmia | 19 (1.8) | 19 (1.9) | 0 (0) | 0.621 |
| Congestive heart failure | 16 (1.5) | 16 (1.6) | 0 (0) | 1.000 |
| Coronary artery disease | 43 (4.1) | 41 (4.2) | 2 (3.4) | 1.000 |
| Chronic pulmonary disease | 14 (1.3) | 14 (1.4) | 0 (0) | 1.000 |
| Old tuberculosis | 79 (7.6) | 72 (7.3) | 7 (12.1) | 0.188 |
| Chronic kidney disease | 48 (4.6) | 44 (4.5) | 4 (6.9) | 0.337 |
| Neoadjuvant chemotherapy | 53 (5.1) | 46 (4.7) | 7 (12.1) | 0.013 |
| Pathological stage | <0.001 | |||
| Stage I and II | 800 (79.3) | 768 (80.6) | 32 (57.1) | |
| Stage III and IV | 209 (20.7) | 185 (19.4) | 24 (42.9) | |
| Extent of surgery | <0.001 | |||
| Total gastrectomy | 228 (22) | 203 (20.7) | 25 (43.1) | |
| Partial gastrectomy | 810 (78) | 777 (79.3) | 33 (56.9) | |
| Surgical approach | 0.005 | |||
| Open | 441 (42.5) | 406 (41.4) | 35 (60.3) | |
| Minimally invasive | 597 (57.5) | 574 (58.6) | 23 (39.7) | |
| Combined resection | 105 (10.1) | 97 (9.9) | 8 (13.8) | 0.339 |
| Lymph node dissection | 0.012 | |||
| D1 and D1+ | 451 (43.4) | 435 (44.4) | 16 (27.6) | |
| D2 or more | 587 (56.6) | 545 (55.6) | 42 (72.4) | |
| Operation time (min) | 177 (145–211) | 175 (144–210) | 203 (171.5–242) | <0.001 |
| OR (95% CI) | p-Value | |
|---|---|---|
| Gender | ||
| Female | Ref | |
| Male | 3.71 (0.79, 17.42) | 0.097 |
| Smoking | ||
| No | Ref | |
| Yes | 1.79 (0.74, 4.34) | 0.196 |
| Surgical method | ||
| Minimal invasive | Ref | |
| Open surgery | 1.12 (0.58, 2.16) | 0.737 |
| Extent of surgery | ||
| Partial resection | Ref | |
| Total resection | 2.13 (1.15, 3.94) | 0.017 |
| Staging | ||
| Stage I or II | Ref | |
| Stage I or IV | 2.66 (1.39, 5.08) | 0.003 |
| VFA-to-SFA ratio | 8.09 (2.91, 22.54) | <0.001 |
| Model | Added Variable | Mean AUC (95% CI) | p-Value * |
|---|---|---|---|
| M1-1 | Control | 71.38(64.34, 78.57) | 0.447 |
| M1-2 | VFA | 73.84(66.84, 80.38) | 0.700 |
| M1-3 | TAMA | 71.81(64.76, 78.86) | 0.466 |
| M1-4 | Muscle index | 71.65(64.80, 78.88) | 0.591 |
| M1-5 | Visceral fat index | 73.41(66.48, 79.95) | 0.679 |
| M1-6 | VFA-to-SFA | 75.11(69.12, 81.11) | 0.381 |
| M1-7 | VFA-to-TAMA | 73.47(66.37, 79.90) | 0.786 |
| M1-8 | VFA-to-Muscle index | 73.97(66.91, 80.30) | 0.812 |
| For Model (Using the Binary Variable) | |
|---|---|
| VFA-to-SFA cut-off value | 0.94 |
| Sensitivity (95% CI) % | 67.86 (55.63, 80.09) |
| Specificity (95% CI) % | 77.65 (75.00, 80.29) |
| PPV (95% CI) % | 15.14 (10.71, 19.57) |
| NPV (95% CI) % | 97.63 (96.54, 98.71) |
| Accuracy (95% CI) % | 77.11 (74.51, 79.70) |
| AUC (95% CI) % | 72.75 (66.44, 79.06) |
| Total (n = 1038) | VFA-to-SFA ≥0.94 (n = 616) | VFA-to-SFA <0.94 (n = 422) | p-Value | |
|---|---|---|---|---|
| SSI occurrence | 58 (5.6) | 52 (8.4) | 6 (1.4) | <0.001 |
| Type of SSI | ||||
| Incisional | 12 (1.2) | 8 (1.3) | 4 (0.9) | 0.771 |
| Organ/space | 46 (4.4) | 44 (7.1) | 2 (0.5) | <0.001 |
| Clavien-Dindo score of IIIa or higher postoperative complication | 78 (7.5) | 59 (9.6) | 19 (4.5) | 0.002 |
| Postoperative hospital stay (days) | 7.64 ± 5.72 | 8.1 ± 6.96 | 6.95 ± 3.01 | <0.001 |
| Re-admission within 30 days | 42 (4) | 33 (5.4) | 9 (2.1) | 0.011 |
| Mortality | 3 (0.3) | 2 (0.3) | 1 (0.2) | 1.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, J.; Lee, W.J.; Seong, H.; Choi, H.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Son, T.; Kim, H.-I.; et al. A High Visceral-To-Subcutaneous Fat Ratio is an Independent Predictor of Surgical Site Infection after Gastrectomy. J. Clin. Med. 2019, 8, 494. https://doi.org/10.3390/jcm8040494
Kim JH, Kim J, Lee WJ, Seong H, Choi H, Ahn JY, Jeong SJ, Ku NS, Son T, Kim H-I, et al. A High Visceral-To-Subcutaneous Fat Ratio is an Independent Predictor of Surgical Site Infection after Gastrectomy. Journal of Clinical Medicine. 2019; 8(4):494. https://doi.org/10.3390/jcm8040494
Chicago/Turabian StyleKim, Jung Ho, Jinnam Kim, Woon Ji Lee, Hye Seong, Heun Choi, Jin Young Ahn, Su Jin Jeong, Nam Su Ku, Taeil Son, Hyoung-Il Kim, and et al. 2019. "A High Visceral-To-Subcutaneous Fat Ratio is an Independent Predictor of Surgical Site Infection after Gastrectomy" Journal of Clinical Medicine 8, no. 4: 494. https://doi.org/10.3390/jcm8040494
APA StyleKim, J. H., Kim, J., Lee, W. J., Seong, H., Choi, H., Ahn, J. Y., Jeong, S. J., Ku, N. S., Son, T., Kim, H.-I., Han, S. H., Choi, J. Y., Yeom, J.-S., Hyung, W. J., Song, Y. G., & Noh, S. H. (2019). A High Visceral-To-Subcutaneous Fat Ratio is an Independent Predictor of Surgical Site Infection after Gastrectomy. Journal of Clinical Medicine, 8(4), 494. https://doi.org/10.3390/jcm8040494





