Prognostic Laboratory Parameters in Placental Abruption: A Retrospective Case-Control Study
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Population and Study Design
2.2. Parameters Analyzed
2.3. Statistical Analysis
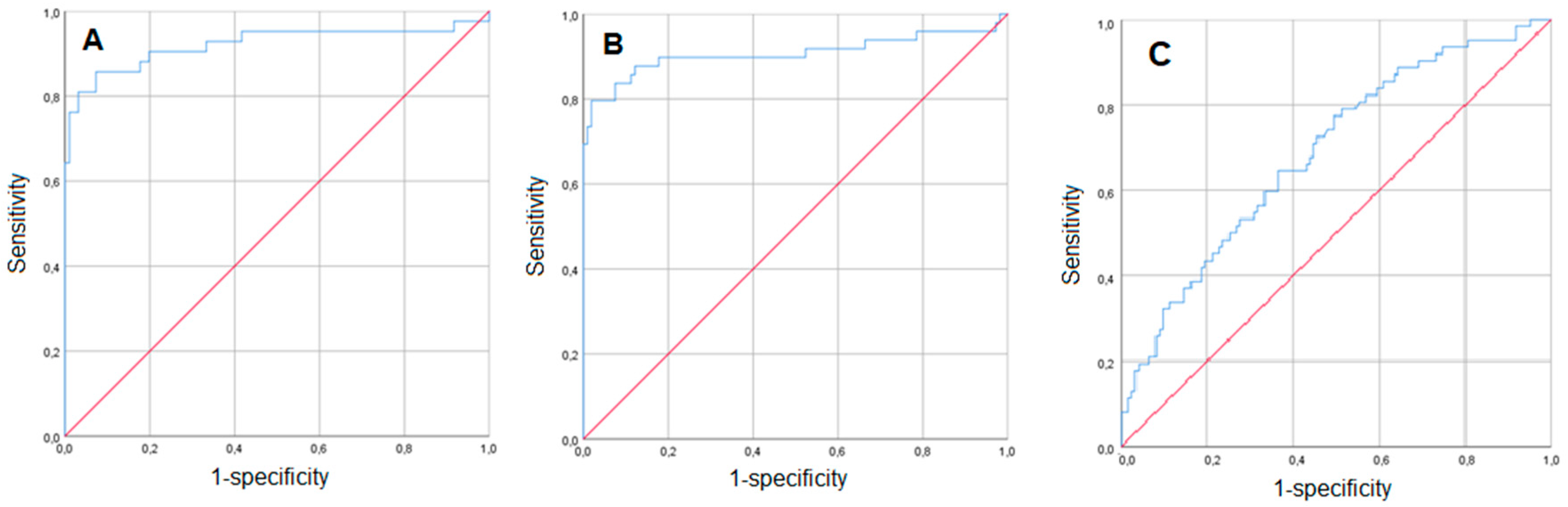
3. Results
3.1. Basic Comparison between Women with and without Placental Abruption
3.2. Prediction of Placental Abruption Using Routine Laboratory Parameters in Women with Vaginal Bleeding
3.3. Routine Laboratory Parameters’ Predictive Value for Placental Abruption in Women without Vaginal Bleeding
3.4. Women with Placental Abruption: Comparison between Bleeding and Non-Bleeding Patients
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Oyelese, Y.; Ananth, C.V. Placental abruption. Obstet. Gynecol. 2006, 108, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Lavery, J.A.; Vintzileos, A.M.; Skupski, D.W.; Varner, M.; Saade, G.; Biggio, J.; Williams, M.A.; Wapner, R.J.; Wright, J.D. Severe placental abruption: Clinical definition and associations with maternal complications. Am. J. Obstet. Gynecol. 2016, 214, 272.e1–272.e9. [Google Scholar] [CrossRef]
- Parker, S.E.; Werler, M.M. Epidemiology of ischemic placental disease: A focus on preterm gestations. Semin. Perinatol. 2014, 38, 133–138. [Google Scholar] [CrossRef]
- Ananth, C.V. Ischemic placental disease: A unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin. Perinatol. 2014, 38, 131–132. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hayashi, K.; Shiozaki, A.; Kawamichi, Y.; Satoh, S.; Saito, S. Comparison of risk factors for placental abruption and placenta previa: Case-cohort study. J. Obstet. Gynaecol. Res. 2011, 37, 538–5346. [Google Scholar] [CrossRef] [PubMed]
- Pariente, G.; Wiznitzer, A.; Sergienko, R.; Mazor, M.; Holcberg, G.; Sheiner, E. Placental abruption: Critical analysis of risk factors and perinatal outcomes. J. Matern Fetal Neonatal Med. 2011, 24, 698–702. [Google Scholar] [CrossRef]
- Gelaye, B.; Sumner, S.J.; McRitchie, S.; Carlson, J.E.; Ananth, C.V.; Enquobahrie, D.A.; Qiu, C.; Sorensen, T.K.; Williams, M.A. Maternal Early Pregnancy Serum Metabolomics Profile and Abnormal Vaginal Bleeding as Predictors of Placental Abruption: A Prospective Study. PLoS ONE 2016, 11, e0156755. [Google Scholar] [CrossRef]
- Arlier, S.; Adiguzel, C.; Yilmaz, E.S.; Seyfettinoglu, S.; Helvacioglu, C.; Ekin, G.U.; Nazik, H.; Yucel, O. The role of mean platelet volume and platelet distribution width in the prediction of placental abruption. J. Obstet. Gynaecol. 2016, 36, 950–953. [Google Scholar] [CrossRef]
- Tikkanen, M. Etiology, clinical manifestations, and prediction of placental abruption. Acta. Obstet. Gynecol. Scand. 2010, 89, 732–740. [Google Scholar] [CrossRef]
- Ananth, C.V.; Vintzileos, A.M. Ischemic placental disease: Epidemiology and risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Oyelese, Y.; Prasad, V.; Getahun, D.; Smulian, J.C. Evidence of placental abruption as a chronic process: Associations with vaginal bleeding early in pregnancy and placental lesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 15–21. [Google Scholar] [CrossRef]
- Nielsen, H.S.; Mogensen, M.; Steffensen, R.; Kruse, C.; Christiansen, O.B. Indications of anti-HY immunity in recurrent placental abruption. J. Reprod. Immunol. 2007, 75, 63–69. [Google Scholar] [CrossRef]
- Tikkanen, M.; Surcel, H.M.; Bloigu, A.; Nuutila, M.; Hiilesmaa, V.; Ylikorkala, O.; Paavonen, J. Prediction of placental abruption by testing for C-reactive protein and chlamydial antibody levels in early pregnancy. BJOG 2008, 115, 486–491. [Google Scholar] [CrossRef]
- Alexander, K.S.; Madden, T.E.; Farrell, D.H. Association between γ’ fibrinogen levels and inflammation. Thromb. Haemost. 2011, 105, 605–609. [Google Scholar] [CrossRef]
- Wang, L.; Matsunaga, S.; Mikami, Y.; Takai, Y.; Terui, K.; Seki, H. Pre-delivery fibrinogen predicts adverse maternal or neonatal outcomes in patients with placental abruption. J. Obstet. Gynaecol. Res. 2016, 42, 796–802. [Google Scholar] [CrossRef]
- Dayan, N.; Lanes, A.; Walker, M.C.; Spitzer, K.A.; Laskin, C.A. Effect of chronic hypertension on assisted pregnancy outcomes: A population-based study in Ontario, Canada. Fertil. Steril. 2016, 105, 1003–1009. [Google Scholar] [CrossRef]
- Baumann, P.; Blackwell, S.C.; Schild, C.; Berry, S.M.; Friedrich, H.J. Mathematic modeling to predict abruptio placentae. Am. J. Obstet. Gynecol. 2000, 183, 815–822. [Google Scholar] [CrossRef]
- Christian, L.M.; Porter, K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine 2014, 70, 134–140. [Google Scholar] [CrossRef]
- Höllig, A.; Stoffel-Wagner, B.; Clusmann, H.; Veldeman, M.; Schubert, G.A.; Coburn, M. Time Courses of Inflammatory Markers after Aneurysmal Subarachnoid Hemorrhage and Their Possible Relevance for Future Studies. Front. Neurol. 2017, 8, 694. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef]
- Hale, S.A.; Sobel, B.; Benvenuto, A.; Schonberg, A.; Badger, G.J.; Bernstein, I.M. Coagulation and Fibrinolytic System Protein Profiles in Women with Normal Pregnancies and Pregnancies Complicated by Hypertension. Pregnancy Hypertens. 2012, 2, 152–157. [Google Scholar] [CrossRef][Green Version]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. The biology and medical implications of interleukin-6. Cancer Immunol. Res. 2014, 2, 288–294. [Google Scholar] [CrossRef]
| Placental Abruption (n = 118) | No Placental Abruption (n = 253) | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Age (years) * | 31.9 (26.3; 36.4) | 33.65 (28.9; 36.8) | 0.952 (0.917; 0.989) | 0.011 | |
| Body mass index (kg/m2) * | 23.4 (21.2; 26.2) | 22.7 (20.2; 25.7) | 1.046 (0.987; 1.110) | 0.130 | |
| Pregnancy after IVF treatment # | 9 (7.6) | 13 (5.1) | 1.545 (0.633-3.673) | 0.345 | |
| Parity # | 0 | 58 (48.7) | 121 (48.0) | Reference | - |
| 1 | 28 (23.5) | 75 (29.8) | 0.779 (0.456–1.330) | 0.360 | |
| ≥2 | 33 (27.7) | 56 (22.2) | 1.171 (0.686–1.998) | 0.562 | |
| Pregnancy-induced/preexisting hyper-tension # | 21 (17.8) | 8 (3.2) | 6.630 (2.841–15.475) | <0.001 | |
| Smoking # | 25 (21.4) | 39 (15.4) | 1.491 (0.853–2.606) | 0.159 | |
| Gestational diabetes mellitus # | 9 (7.6) | 30 (11.9) | 0.614 (0.282–1.338) | 0.216 | |
| Placenta previa # | 14 (11.9) | 53 (20.9) | 0.508 (0.269; 0.958) | 0.034 | |
| Neonatal weight (g) * | 1839 (1076; 2500) | 3044 (2400; 3430) | 0.999 (0.999; 0.999) | <0.001 | |
| Gestational age at delivery (completed weeks) * | 33.43 (28.86; 36.00) | 38.14 (35.29; 38.86) | 0.974 (0.966; 0.981) | <0.001 | |
| Leukocytes (g/L) *+ | 11.99 (9.82; 14.10) | 10.07 (8.36; 12.05) | 1.172 (1.091; 1.258) | <0.001 | |
| Thrombocytes (g/L) *+ | 218.5 (172; 267) | 226 (184.5; 264.5) | 0.998 (0.995; 1.001) | 0.195 | |
| C-reactive protein (mg/dL) *+ | 0.58 (0.37; 1.14) | 0.46 (0.24; 0.79) | 1.093 (0.936; 1.276) | 0.260 | |
| Fibrinogen (mg/dL) *+ | 437 (337; 519) | 488 (431; 554) | 0.994 (0.936; 1.276) | <0.001 | |
| Hemoglobin (g/dL) *+ | 11.20 (10.1–12.1) | 11.70 (10.80–12.60) | 1.009 (0.987–1.032) | 0.423 | |
| Placental Abruption (n = 64) | No Placental Abruption (n = 123) | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Age (years) * | 32.9 (28.5; 36.9) | 33.6 (28.7; 36.5) | 0.985 (0.932; 1.040) | 0.581 | - | - | |
| Body mass index (kg/m2) * | 23.15 (21.20; 29.05) | 22.90 (20.20; 25.40) | 1.067 (0.986; 1.155) | 0.109 | - | - | |
| Pregnancy after IVF treatment # | 8 (12.5) | 4 (3.3) | 4.250 (1.228; 14.709) | 0.014 | 5.594 (1.365; 22.915) | 0.017 | |
| Parity # | 0 | 32 (50.0) | 48 (39.0) | Reference | 0.301 | - | - |
| 1 | 15 (23.4) | 40 (32.5) | 1.373 (0.660; 2.854) | 0.396 | - | - | |
| ≥2 | 17 (26.6) | 35 (28.5) | 0.772 (0.337; 1.769) | 0.541 | - | - | |
| Pregnancy-induced/preexisting hypertension # | 8 (12.5) | 1 (0.4) | 17.429 (2.128; 142.72) | 0.008 | 25.477 (2.347; 276:585) | 0.008 | |
| Smoking # | 13 (20.6) | 19 (15.4) | 1.423 (0.651; 3.111) | 0.375 | - | - | |
| Gestational diabetes mellitus # | 3 (4.7) | 13 (10.6) | 0.416 (0.114; 1.517) | 0.172 | - | - | |
| Placenta previa # | 11 (17.2) | 50 (40.7) | 0.303 (0.144; 0.637) | 0.001 | 0.219 (0.089; 0.538) | 0.001 | |
| Neonatal weight (g) * | 1665 (907; 2475) | 2400 (1830; 2940) | 0.999 (0.999; 1.000) | <0.001 | - | - | |
| Gestational age at bleeding onset (weeks) * | 32.57 (26.43; 35.00) | 29.14 (26.29; 32.86) | 1.011 (1.002; 1.021) | 0.020 | 1.108 (1.030; 1.192) | 0.006 | |
| Gestational age at delivery (weeks) * | 32.57 (26.71; 35.14) | 35 (31.71; 37.57) | 0.982 (0.973; 0.992) | <0.001 | - | - | |
| Hemoglobin (g/dL) *+ | 11.60 (10.60; 12.10) | 11.30 (10.60; 12.00) | 0.969 (0.776; 1.210) | 0.782 | - | - | |
| Leukocytes (g/L) *+ | 10.91 (8.99; 13.50) | 10.59 (8.92; 12.11) | 1.080 (0.977; 1.217) | 0.124 | - | - | |
| Thrombocytes (g/L) *+ | 217 (180; 276) | 235 (187; 283) | 0.996 (0.992; 1.001) | 0.101 | - | - | |
| C-reactive protein (mg/dL) *+ | 0.56 (0.28; 1.24) | 0.51 (0.28; 0.84) | 1.469 (1.050; 2.005) | 0.025 | 1.506 (1.071; 2.117) | 0.019 | |
| Fibrinogen (mg/dL) *+ | 478.0 (435.5; 532.5) | 457.0 (428.0; 528.0) | 0.998 (0.994; 1.001) | 0.200 | - | - | |
| Placental Abruption (n = 54) | No Placental Abruption (n = 130) | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Age (years) * | 29.75 (25.90; 35.50) | 33.65 (28.90; 37.50) | 0.923 (0.875; 0.974) | 0.004 | 0.897 (0.807; 0.997) | 0.043 | |
| Body mass index (kg/m2) * | 23.50 (21.00; 25.90) | 22.40 (20.20; 25.90) | 1.016 (0.928; 1.112) | 0.734 | - | - | |
| Pregnancy after IVF treatment # | 1 (1.9) | 9 (6.9) | 0.254 (0.031; 2.053) | 0.199 | - | - | |
| Parity # | 0 | 26 (48.1) | 73 (56.2) | Reference | 0.525 | - | - |
| 1 | 13 (24.1) | 35 (26.9) | 0.522 (0.236; 1.156) | 0.109 | - | - | |
| ≥2 | 15 (27.8) | 22 (16.9) | 0.545 (0.218; 1.359) | 0.545 | - | - | |
| Pregnancy-induced/preexisting hypertension # | 13 (24.1) | 8 (6.2) | 4.835 (1.872; 12.492) | <0.001 | 1.204 (0.123; 11.748) | 0.873 | |
| Smoking # | 12 (22.2) | 20 (15.4) | 1.571 (0.707; 3.494) | 0.265 | - | - | |
| Gestational diabetes mellitus # | 6 (11.1) | 17 (13.1) | 0.831 (0.309; 2.236) | 0.711 | - | - | |
| Placenta previa # | 3 (5.6) | 3 (2.3) | 2.490 (0.486; 12.747) | 0.259 | - | - | |
| Neonatal weight (g) * | 1918 (1440; 2680) | 3340 (3075; 3620) | 0.997 (0.996; 0.998) | <0.001 | - | - | |
| Gestational age at delivery (weeks) * | 34.00 (30.29–37.43) | 38.57 (38.29; 39.00) | 0.873 (0.830–0.918) | <0.001 | 0.869 (0.814; 0.926) | <0.001 | |
| Hemoglobin (g/dL) *+ | 11.30 (10.25; 12.00) | 12.10 (11.30; 13.00) | 1.013 (0.986; 1.041) | 0.343 | - | - | |
| Leukocytes (g/L) *+ | 12.01 (9.41; 14.10) | 9.54 (8.13; 10.97) | 1.392 (1.214–1.595) | <0.001 | 1.124 (0.863; 1.464) | 0.385 | |
| Thrombocytes (g/L) *+ | 215 (169.5; 259) | 219 (184; 260) | 0.998 (0.993–1.003) | 0.398 | - | - | |
| C-reactive protein (mg/dL) *+ | 0.64 (0.48; 1.08) | 0.32 (0.18; 0.61) | 6.099 (2.381–15.624) | <0.001 | 7.454 (1.538; 36.121) | 0.013 | |
| Fibrinogen (mg/dL) *+ | 418 (334; 534) | 485 (442; 535) | 0.994 (0.990–0.998) | 0.002 | 1.003 (0.995; 1.012) | 0.234 | |
| Vaginal Bleeding (n = 64) | No Vaginal Bleeding (n = 54) | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Age (years) * | 32.9 (28.5; 36.9) | 29.8 (25.9; 35.5) | 1.066 (1.001; 1.136) | 0.046 | 0.989 (0.933; 1.049) | 0.720 | |
| Body mass index (kg/m2) * | 23.2 (21.2; 29.05) | 23.5 (21.0; 25.9) | 1.056 (0.955; 1.169) | 0.288 | - | - | |
| Pregnancy after IVF treatment # | 8 (12.5) | 1 (1.9) | 7.571 (0.916; 62.608) | 0.030 | 4.076 (1.132; 14.679) | 0.032 | |
| Parity# | 0 | 32 (50.0) | 26 (48.1) | Reference | 0.980 | - | - |
| 1 | 15 (23.4) | 13 (24.1) | 1.086 (0.457; 2.582) | 0.852 | - | - | |
| ≥2 | 17 (26.6) | 15 (27.8) | 1.018 (03.68; 2.841) | 0.972 | - | - | |
| Pregnancy-induced/preexisting hypertension # | 8 (12.5) | 13 (24.1) | 0.451 (0.171; 1.187) | 0.101 | - | - | |
| Smoking # | 13 (20.6) | 12 (22.2) | 0.910 (0.375; 2.206) | 0.835 | - | - | |
| Gestational diabetes mellitus # | 3 (4.7) | 6 (11.1) | 0.393 (0.094; 1.655) | 0.190 | - | - | |
| Placenta previa # | 11 (17.2) | 3 (5.6) | 3.528 (0.930; 13.384) | 0.052 | - | - | |
| Neonatal weight (g) * | 1665 (907; 2475) | 1918 (1440; 2680) | 1.000 (0.999; 1.000) | 0.082 | - | - | |
| Gestational age at delivery (weeks) * | 32.6 (26.7; 34.0) | 34.0 (30.3; 37.1) | 0.985 (0.974; 0.997) | 0.011 | 0.983 (0.973; 0.992) | <0.001 | |
| Hemoglobin (g/dL) *+ | 11.2 (9.7; 12.1) | 11.3 (10.25; 12.0) | 0.879 (0.701; 1.103) | 0.265 | - | - | |
| Leukocytes (g/L) *+ | 11.89 (9.89; 14.1) | 12.00 (9.41; 14.10) | 0.987 (0.889; 1.094) | 0.799 | - | - | |
| Thrombocytes (g/L) *+ | 218.5 (175.0; 277.0) | 215.0 (169.5; 259.0) | 1.000 (0.995; 1.005) | 0.900 | - | - | |
| C-reactive protein (mg/dL) *+ | 0.56 (0.28; 1.24) | 0.64 (0.48; 1.08) | 1.002 (0.773; 1.299) | 0.985 | - | - | |
| Fibrinogen (mg/dL) * + | 440.5 (344.0; 494.5) | 418 (334.0; 534.0) | 1.000 (0.997; 1.003) | 0.965 | - | - | |
| Placental Abruption (n = 54) | No Placental Abruption (n = 46) | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Age (years) * | 29.75 (25.90; 35.50) | 30.55 (26,21; 33.70) | 1.020 (0.951; 1.093) | 0.588 | |||
| Body mass index (kg/m2) * | 23.50 (21.00; 25.90) | 22.40 (21.10; 26.70) | 0.971 (0.885; 1.066) | 0.540 | |||
| Pregnancy after IVF treatment # | 1 (1.9) | 1 (2.2) | 0.830 (0.050; 13.659) | 0.896 | |||
| Parity # | 0 | 26 (48.1) | 28 (60.9) | Reference | 0.200 | ||
| 1 | 13 (24.1) | 12 (26.1) | 1.167 (0.452; 3.014) | 0.750 | |||
| ≥2 | 15 (27.8) | 6 (13.0) | 2.692 (0.908; 7.983) | 0.074 | |||
| Pregnancy-induced/preexisting hyper-tension # | 13 (24.1) | 2 (4.3) | 6.817 (1.448; 32.085) | 0.015 | 4.565 (0.788; 26.433) | 0.090 | |
| Smoking # | 12 (22.2) | 2 (4.3) | 6.143 (1.296; 29.121) | 0.022 | 4.412 (0.778; 25.032) | 0.094 | |
| Gestational diabetes mellitus # | 6 (11.1) | 3 (6.5) | 1.792 (0.422; 7.605) | 1.792 | |||
| Placenta previa # | 3 (5.6) | 0 (0) | |||||
| Gestational age at blood retrieval (weeks) * | 34.00 (30.29–37.43) | 33.86 (32.29; 37.00) | 1.000 (0.984; 1.015) | 0.953 | |||
| Hemoglobin (g/dL) *+ | 11.30 (10.25; 12.00) | 11.55 (11.00; 12.10) | 1.016 (0.965; 1.069) | 0.542 | |||
| Leukocytes (g/L) *+ | 12.01 (9.41; 14.10) | 9.21 (7.95; 10.49) | 1.498 (1.229; 1.826) | <0.001 | 1.378 (1.095; 1.735) | 0.006 | |
| Thrombocytes (g/L) *+ | 215 (169.5; 259) | 217 (189; 261) | 0.998 (0.991; 1.005) | 0.600 | |||
| C-reactive protein (mg/dL) *+ | 0.64 (0.48; 1.08) | 0.33 (0.20; 0.50) | 20.849 (4.172; 104.182) | <0.001 | 7.942 (1.435; 43.958) | 0.018 | |
| Fibrinogen (mg/dL) *+ | 418 (334; 534) | 428 (387; 510) | 1.000 (0.997; 1.003) | 0.991 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pils, S.; Paternostro, C.; Bekos, C.; Hager, M.; Ristl, R.; Ott, J. Prognostic Laboratory Parameters in Placental Abruption: A Retrospective Case-Control Study. J. Clin. Med. 2019, 8, 482. https://doi.org/10.3390/jcm8040482
Pils S, Paternostro C, Bekos C, Hager M, Ristl R, Ott J. Prognostic Laboratory Parameters in Placental Abruption: A Retrospective Case-Control Study. Journal of Clinical Medicine. 2019; 8(4):482. https://doi.org/10.3390/jcm8040482
Chicago/Turabian StylePils, Sophie, Chiara Paternostro, Christine Bekos, Marlene Hager, Robin Ristl, and Johannes Ott. 2019. "Prognostic Laboratory Parameters in Placental Abruption: A Retrospective Case-Control Study" Journal of Clinical Medicine 8, no. 4: 482. https://doi.org/10.3390/jcm8040482
APA StylePils, S., Paternostro, C., Bekos, C., Hager, M., Ristl, R., & Ott, J. (2019). Prognostic Laboratory Parameters in Placental Abruption: A Retrospective Case-Control Study. Journal of Clinical Medicine, 8(4), 482. https://doi.org/10.3390/jcm8040482







