Ganoderma lucidum Ameliorates Non-Alcoholic Steatosis by Upregulating Energy Metabolizing Enzymes in the Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GL Extract
2.2. Animals and Treatments
2.3. Measurement of Blood Glucose Level
2.4. Glucose Tolerance Test and Insulin Tolerance Test
2.5. Biochemical Assay
2.6. H&E and Oil Red O Stain in Liver Tissue
2.7. Western Blot Analysis
2.8. Cell Culture and GL Treatment
2.9. Cytotoxicity of GL
2.10. Statistical Analysis
3. Results
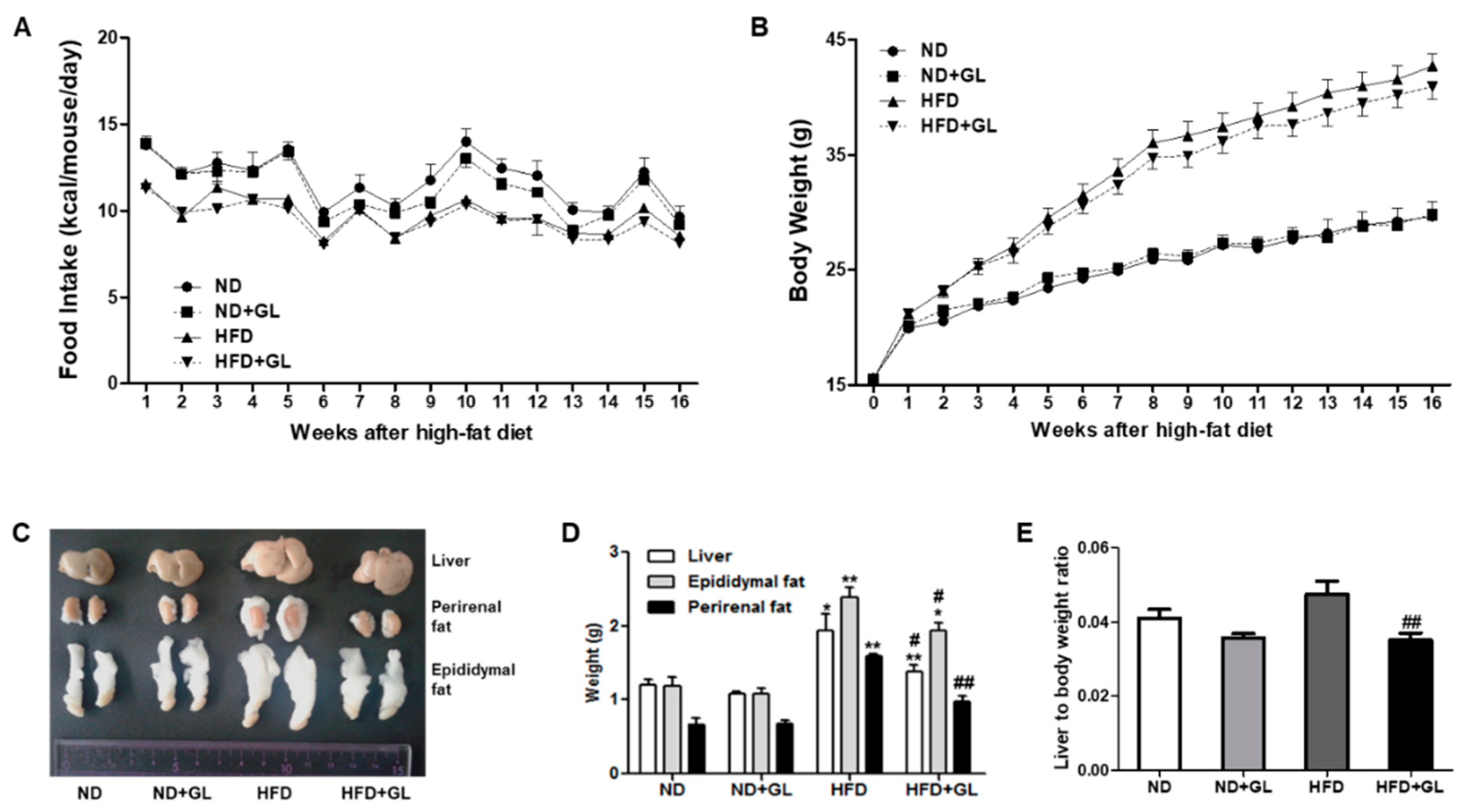
3.1. GL Attenuated the Increases in Liver and Adipose Tissue Weights in HFD-Fed Mice
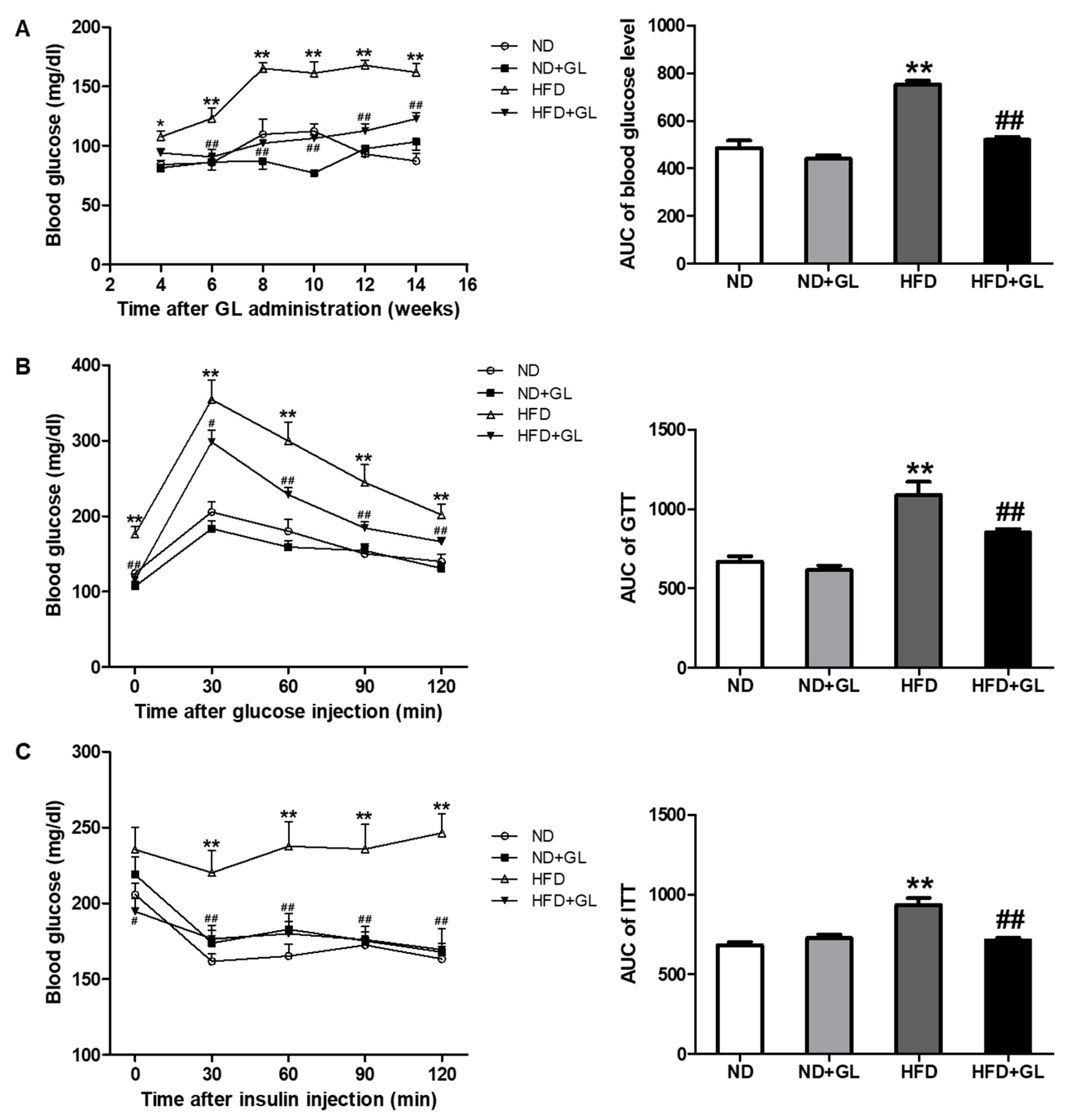
3.2. GL Reduced Insulin Resistance in HFD-Fed Mice
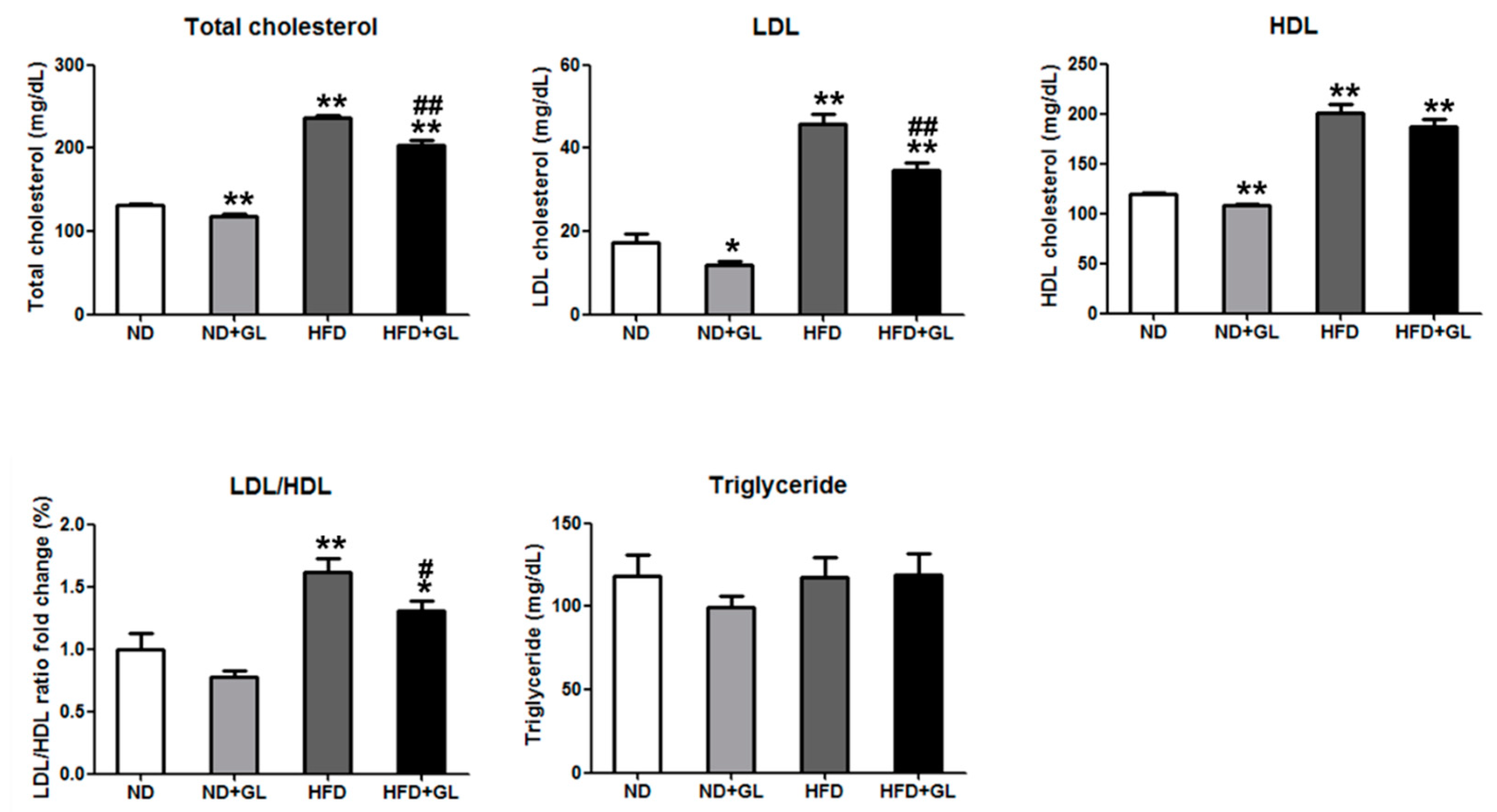
3.3. GL Reduced Serum Total Cholesterol and Low-Density Lipoprotein in HFD-fed Mice
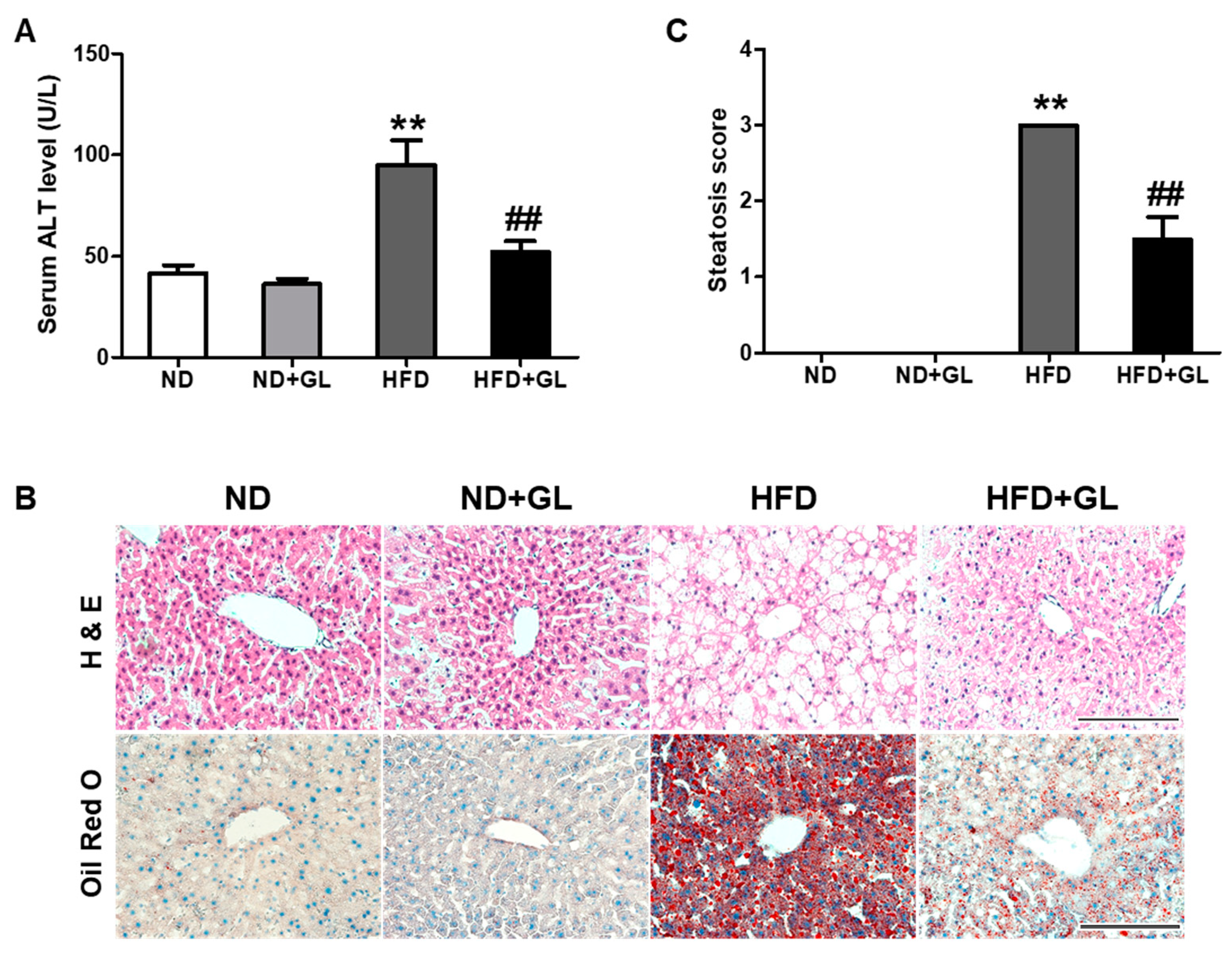
3.4. GL Attenuated Serum ALT Level and Hepatic Lipid Accumulation in HFD-fed Mice
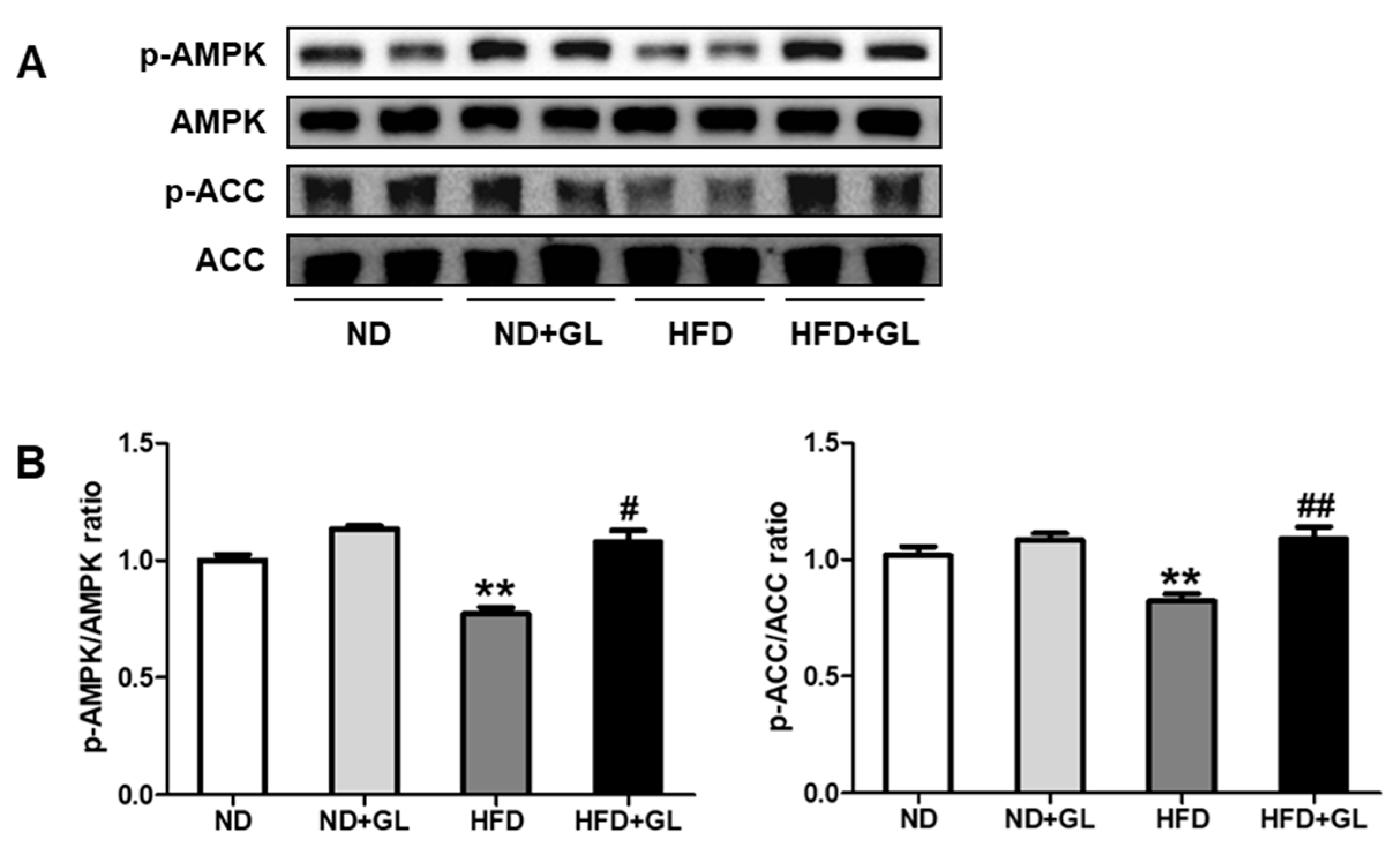
3.5. GL Increased Phosphorylation of AMPK and ACC in HFD-Fed Mice
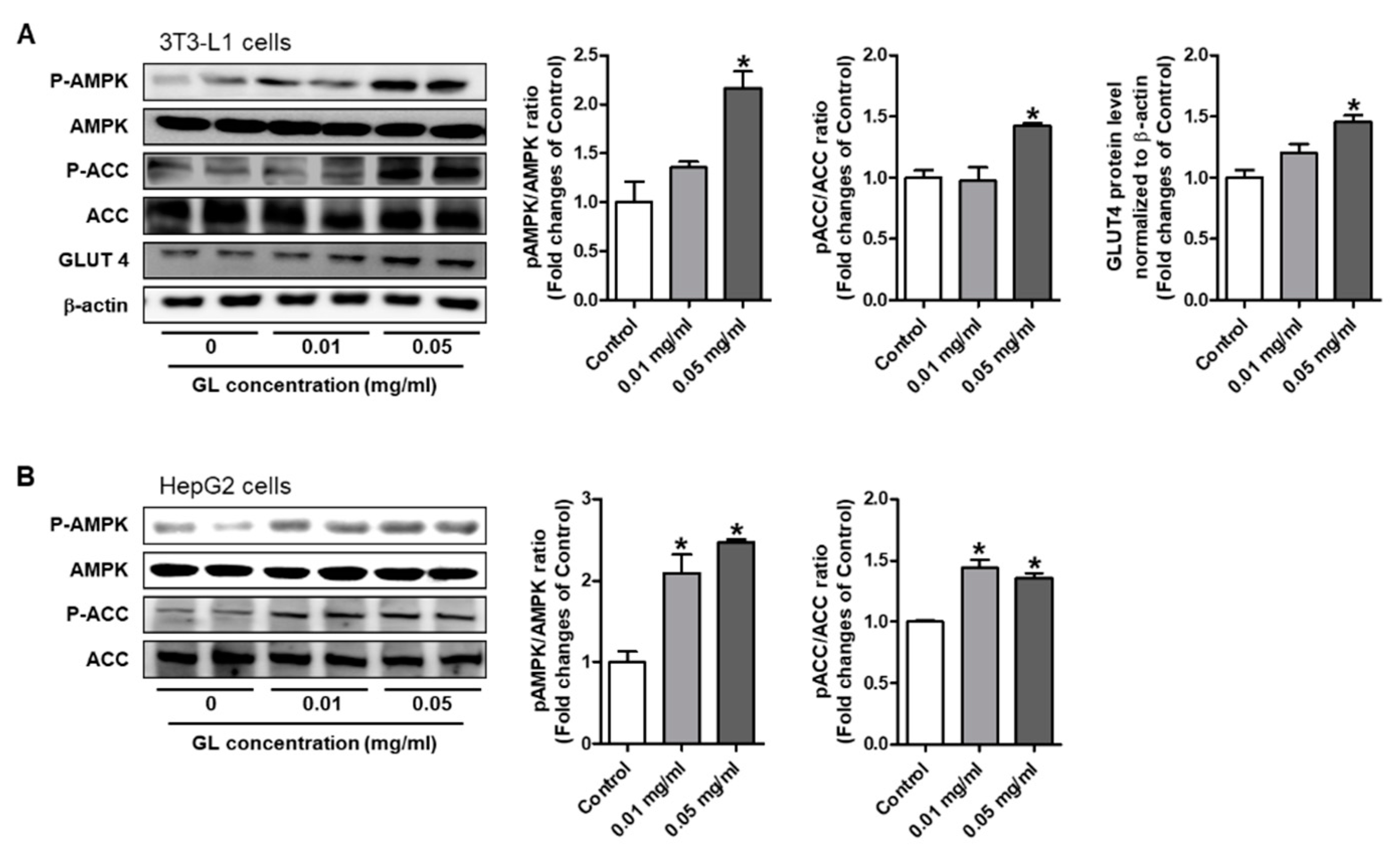
3.6. GL Increased Phosphorylation of AMPK and ACC and GLUT4 Expression in HepG2 and 3T3-L1 Cells
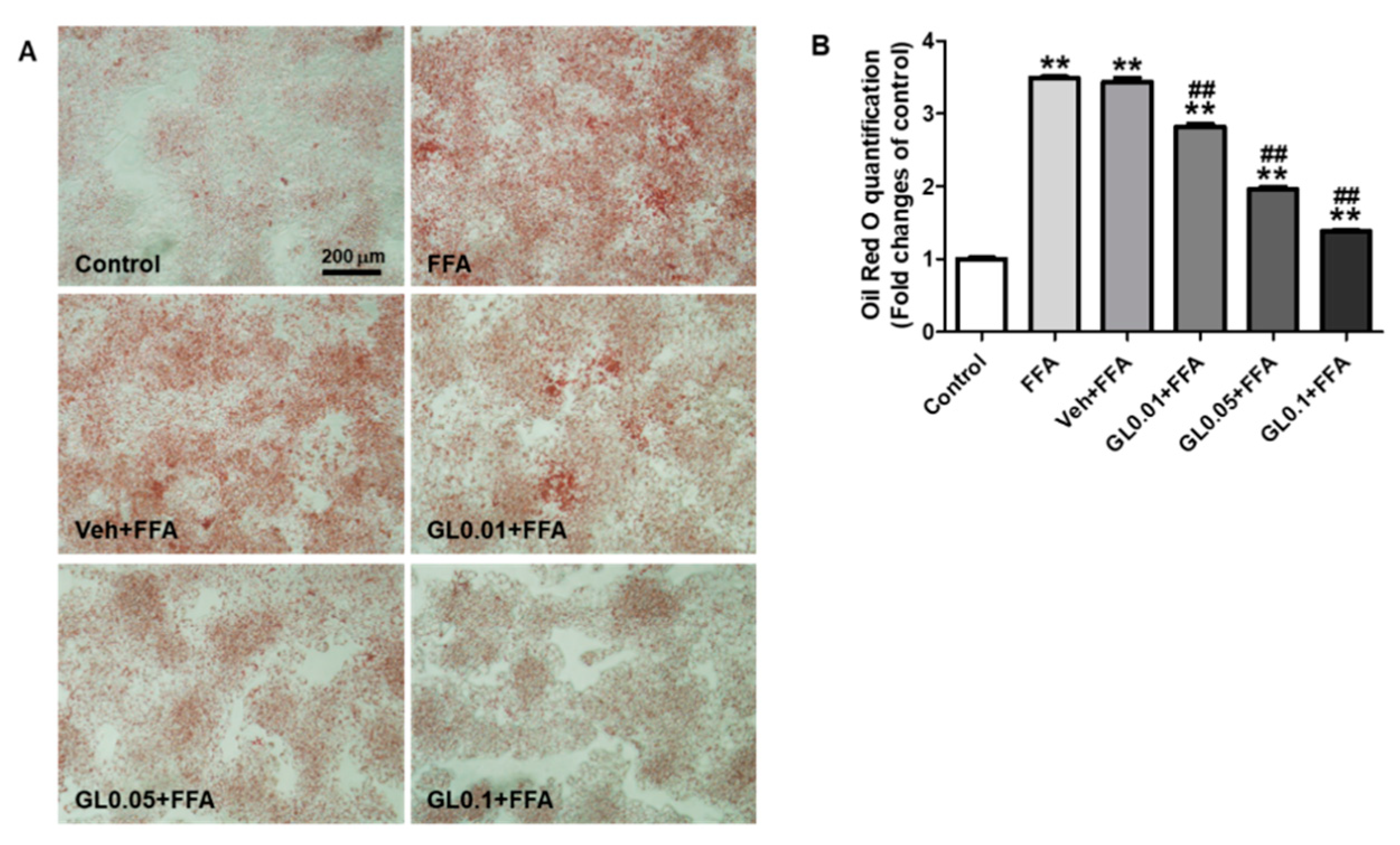
3.7. GL Attenuated Lipid Accumulation Induced by FFA in HepG2 Cells
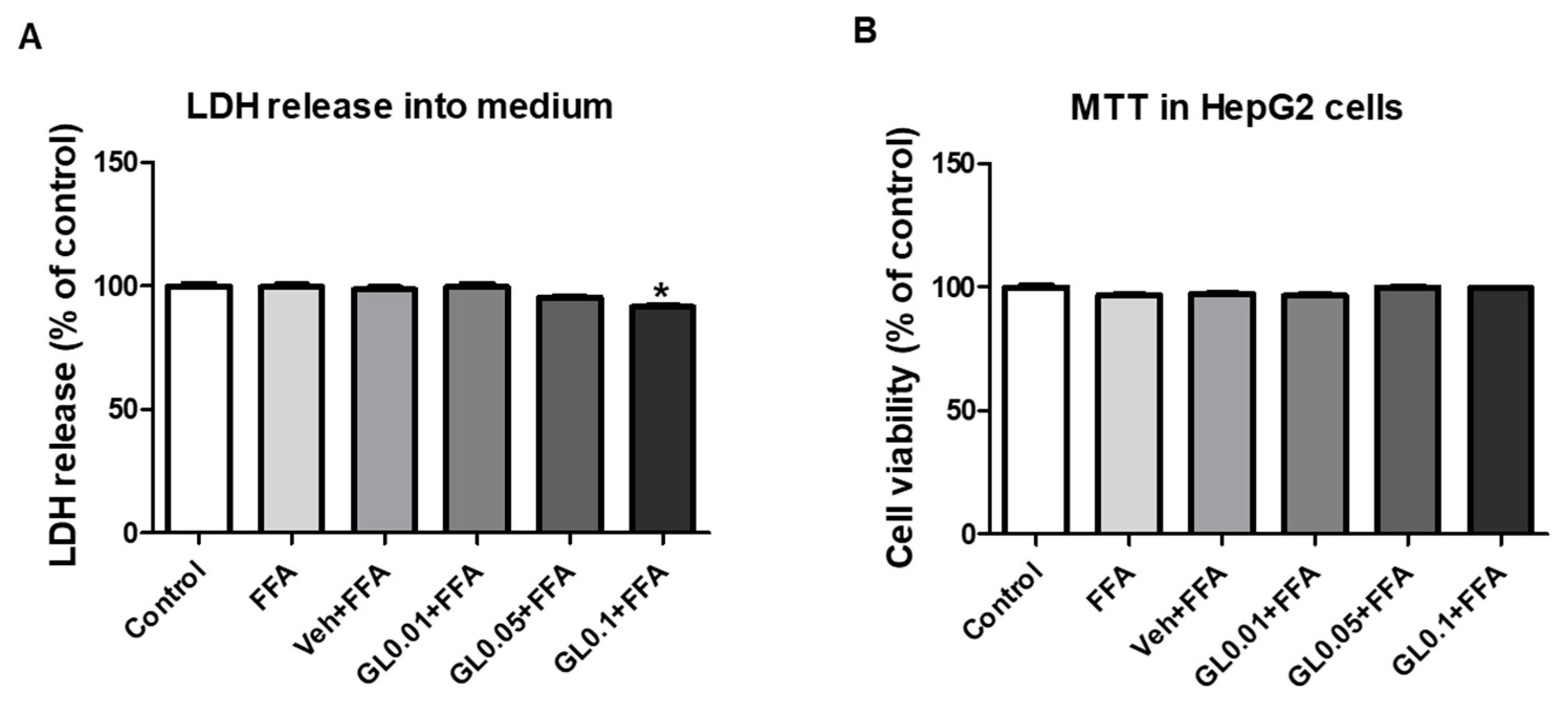
3.8. Cytotoxicity of GL Extract on HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, L.C.; Hellerstein, M.K.; Seidman, C.E.; Neese, R.A.; Tremaroli, J.D.; Hirsch, J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J. Lipid Res. 2000, 41, 595–604. [Google Scholar] [PubMed]
- Ma, H.T.; Hsieh, J.F.; Chen, S.T. Anti-diabetic effects of ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P.; Kozak, L. Phenolic and flavonoid content in hericium erinaceus, ganoderma lucidum, and agrocybe aegerita under selenium addition. Acta Aliment. 2016, 45, 300–308. [Google Scholar] [CrossRef]
- Boh, B. Ganoderma lucidum: A potential for biotechnological production of anti-cancer and immunomodulatory drugs. Recent Pat. Anticancer Drug Discov. 2013, 8, 255–287. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.B.; Li, C.H.; Lee, S.S.; Kan, L.S. Triterpene-enriched extracts from ganoderma lucidum inhibit growth of hepatoma cells via suppressing protein kinase C, activating mitogen-activated protein kinases and G2-phase cell cycle arrest. Life Sci. 2003, 72, 2381–2390. [Google Scholar] [CrossRef]
- Seto, S.W.; Lam, T.Y.; Tam, H.L.; Au, A.L.; Chan, S.W.; Wu, J.H.; Yu, P.H.; Leung, G.P.; Ngai, S.M.; Yeung, J.H.; et al. Novel hypoglycemic effects of ganoderma lucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine 2009, 16, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective effects of ganoderma lucidum peptides against d-galactosamine-induced liver injury in mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Joo, O.S.; Hwang, C.E.; Hong, S.Y.; Sin, E.C.; Nam, S.H.; Cho, K.M. Antioxidative and digestion enzyme inhibitory activity of ganoderma lucidum depends on the extraction solvent. Korean J. Food Preserv. 2018, 25, 124–135. [Google Scholar]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.K.; Sheng, L.; Nagar, N.; Wu, C.; Barile, D.; Mills, D.A.; Wan, Y.Y. Synbiotics bifidobacterium infantis and milk oligosaccharides are effective in reversing cancer-prone nonalcoholic steatohepatitis using western diet-fed fxr knockout mouse models. J. Nutr. Biochem. 2018, 57, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kim, J.H.; Kim, H.J.; Chang, K.C.; Park, S.W. Honokiol activates the LKB1-AMPK signaling pathway and attenuates the lipid accumulation in hepatocytes. Toxicol. Appl. Pharmacol. 2015, 284, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan-Sahu, A.; Lane, B.; Sliva, D. Reishimax, mushroom based dietary supplement, inhibits adipocyte differentiation, stimulates glucose uptake and activates AMPK. BMC Complement. Altern. Med. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Abate, N.; Garg, A.; Peshock, R.M.; Stray-Gundersen, J.; Grundy, S.M. Relationships of generalized and regional adiposity to insulin sensitivity in men. J. Clin. Investig. 1995, 96, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Capeau, J. Insulin resistance and steatosis in humans. Diabetes Metab. 2008, 34, 649–657. [Google Scholar] [CrossRef]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; DeFronzo, R.A.; Shulman, R.G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 1990, 322, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q.; Keating, A.F. Functional properties and genomics of glucose transporters. Curr. Genomics 2007, 8, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, J.; Kim, H.; Heo, R.W.; Yi, C.O.; Lee, J.E.; Jeon, B.T.; Kim, W.H.; Hahm, J.R.; Roh, G.S. Exendin-4 improves nonalcoholic fatty liver disease by regulating glucose transporter 4 expression in ob/ob mice. Korean J. Physiol. Pharmacol. 2014, 18, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Cullen, P.; Pacy, P.; Halliday, D.; Scott, J. Stable isotopes show a direct relation between vldl apob overproduction and serum triglyceride levels and indicate a metabolically and biochemically coherent basis for familial combined hyperlipidemia. Arterioscler. Thromb. 1993, 13, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.H.; Watts, G.F.; Umpleby, A.M.; Hennessy, T.R.; Naoumova, R.; Slavin, B.M.; Thompson, G.R.; Sonksen, P.H. Increased hepatic secretion of very-low-density lipoprotein apolipoprotein b-100 in niddm. Diabetologia 1995, 38, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Bjorntorp, P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990, 10, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Hong, S.W.; Yeon, S.H.; Kim, Y.M.; Um, K.A.; Kim, J.H.; Kim, H.J.; Chang, K.C.; Park, S.W. Magnolia officinalis attenuates free fatty acid-induced lipogenesis via AMPK phosphorylation in hepatocytes. J. Ethnopharmacol. 2014, 157, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Minireview: The amp-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of amp-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Son, H.; Hwang, C.E.; Cho, K.M.; Park, S.W.; Kim, H.J. Ganoderma lucidum Ameliorates Non-Alcoholic Steatosis by Upregulating Energy Metabolizing Enzymes in the Liver. J. Clin. Med. 2018, 7, 152. https://doi.org/10.3390/jcm7060152
Jung S, Son H, Hwang CE, Cho KM, Park SW, Kim HJ. Ganoderma lucidum Ameliorates Non-Alcoholic Steatosis by Upregulating Energy Metabolizing Enzymes in the Liver. Journal of Clinical Medicine. 2018; 7(6):152. https://doi.org/10.3390/jcm7060152
Chicago/Turabian StyleJung, Soonwoong, Hyeonwi Son, Chung Eun Hwang, Kye Man Cho, Sang Won Park, and Hyun Joon Kim. 2018. "Ganoderma lucidum Ameliorates Non-Alcoholic Steatosis by Upregulating Energy Metabolizing Enzymes in the Liver" Journal of Clinical Medicine 7, no. 6: 152. https://doi.org/10.3390/jcm7060152
APA StyleJung, S., Son, H., Hwang, C. E., Cho, K. M., Park, S. W., & Kim, H. J. (2018). Ganoderma lucidum Ameliorates Non-Alcoholic Steatosis by Upregulating Energy Metabolizing Enzymes in the Liver. Journal of Clinical Medicine, 7(6), 152. https://doi.org/10.3390/jcm7060152





