10-Year Renal Function Trajectories in Community-Dwelling Older Adults: Exploring the Risk Factors for Different Patterns
Abstract
:1. Introduction
2. Methods
2.1. Study Procedure
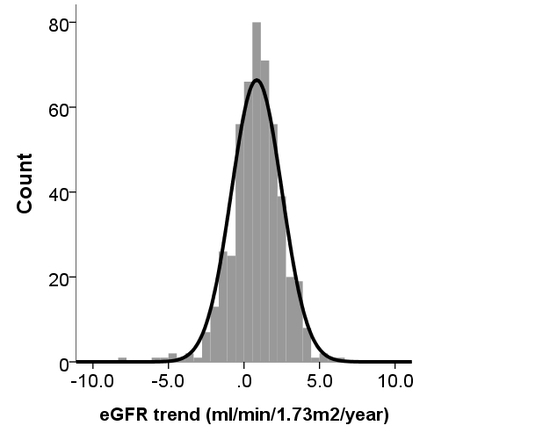
2.2. Analyzing the Trend of eGFR Changes among Participants Divided into Different Groups
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chapter 1: Definition and classification of CKD. Kidney. Int. Suppl. 2013, 3, 19–62. [CrossRef] [PubMed]
- Dousdampanis, P.; Trigka, K.; Fourtounas, C. Diagnosis and Management of Chronic Kidney Disease in the Elderly: A Field of Ongoing Debate. Aging. Dis. 2012, 3, 360–372. [Google Scholar] [PubMed]
- Maw, T.T.; Fried, L. Chronic Kidney Disease in the Elderly. Clin. Geriatr. Med. 2013, 29, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Wu, V.C.; Lai, C.F.; Shiao, C.C.; Huang, T.M.; Wu, P.C.; Tsai, I.J.; Hou, C.C.; Wang, W.J.; Tsai, H.B.; et al. Advanced age affects the outcome-predictive power of RIFLE classification in geriatric patients with acute kidney injury. Kidney. Int. 2012, 82, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.I.; Matsushita, K.; Sang, Y.; Mahmoodi, B.K.; Black, C.; Ishani, A.; Kleefstra, N.; Naimark, D.; Roderick, P.; Tonelli, M.; et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012, 308, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Wang, J.; Wu, H.Y.; Huang, J.W.; Chien, K.L. Age modifies the risk factor profiles for acute kidney injury among recently diagnosed type 2 diabetic patients: A population-based study. Geroscience. 2018, 40, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Turin, T.C.; Coresh, J.; Tonelli, M.; Stevens, P.E.; de Jong, P.E.; Farmer, C.K.; Matsushita, K.; Hemmelgarn, B.R. Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney. Int. 2013, 83, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Turin, T.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Coresh, J.; Ballew, S.H.; Woodward, M.; Levin, A.; Naimark, D.M.; Nally, J.; Rothenbacher, D.; Stengel, B.; Iseki, K.; et al. Past Decline Versus Current eGFR and Subsequent ESRD Risk. J. Am. Soc. Nephrol. 2016, 27, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.E.; Chubb, S.A.P.; Davis, W.A. The relationship between estimated glomerular filtration rate trajectory and all-cause mortality in type 2 diabetes: the Fremantle Diabetes Study. Eur. J. Endocrinol. 2016, 175, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelli, S.; Leonardis, D.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Esposito, C.; Mallamaci, F.; Zoccali, C.; Conte, G. Epidemiology of CKD Regression in Patients under Nephrology Care. PLoS ONE 2015, 10, e0140138. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Riella, M.C. Chronic kidney disease and the ageing population. Nat. Rev. Nephrol. 2014, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Hwang, A.-C.; Tosato, M.; Peng, L.N.; Calvani, R.; Picca, A.; Chen, L.K.; Landi, F. Age-related changes of skeletal muscle mass and strength among Italian and Taiwanese older people: Results from the Milan EXPO 2015 survey and the I-Lan Longitudinal Aging Study. Exp. Gerontol. 2018, 102, 76–80. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.M.; Walker, R.; Haneuse, S.; Crane, P.K.; McCormick, W.C.; Bowen, J.D.; Larson, E.B. Relatiosnip between longitudinal measures of renal function and onset of dementia in a community cohort of older adults. J. Am. Geriatr. Soc. 2012, 60, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Golper, T.A.; Hartle, P.M.; Bian, A. Arteriovenous fistula creation may slow estimated glomerular filtration rate trajectory. Nephrol. Dial. Transplant. 2015, 30, 2014–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Bowe, B.; Xian, H.; Balasubramanian, S.; Al-Aly, Z. Estimated GFR trajectories of people entering CKD stage 4 and subsequent kidney disease outcomes and mortality. Am. J. Kidney Dis. 2016, 68, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.E.; Davis, W.A.; Ito, J.; Winfield, K.; Stoll, T.; Bringans, S.D.; Lipscombe, R.J.; Davis, T.M.E. Identification of novel circulating biomarkers predicting rapid decline in renal function in type 2 diabetes: the Fermantle Diabetes Study Phase II. Diabetes Care 2017, 40, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Weldegiorgis, M.; de Zeeuw, D.; Li, L.; Parving, H.H.; Hou, F.F.; Remuzzi, G.; Greene, T.; Heerspink, H.J.L. Longitudinal estimated GFR trajectories in patients with and without type 2 diabetes and nephropathy. Am. J. Kidney Dis. 2018, 71, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Yu, T.Y.; Li, C.I.; Liu, C.S.; Lin, W.Y.; Lin, C.H.; Yang, S.Y.; Chiang, J.H.; Lin, C.C. Three-year renal function trajectory and its association with adverse renal event in patients with type 2 diabetes. J. Diabetes Complicat. 2018, 32, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Liu, S.; Gurung, R.L.; Ching, J.; Kovalik, J.P.; Tan, T.Y.; Lim, S.C. Urine tricarboxylic acid (TCA) cycle metabolites predict progressive chronic kidney disease in type 2 diabetes. J. Clin. Endocrinol. Metab. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Huang, H.C.; Chiang, H.Y.; Chung, C.W.; Chiu, H.T.; Liang, C.C.; Yu, T.; Kuo, C.C. First-year estimated glomerular filtration rate variability after pre-end-stage renal disease program enrollment and adverse outcomes of chronic kidney disease. Nephrol. Dial. Transplant. 2018. [Google Scholar] [CrossRef]
- Matsumura, K.; Sugii, K.; Awazu, M. Trajectory of estimated glomerular filtration rate predicts renal injury in children with multicystic dysplastic kidney. Nephron 2018, 140, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zee, J.; Mansfield, S.; Mariani, L.H.; Gillespie, B.W. Using all longitudinal data to define time to specified percentages of estimated GFR decline: A simulation study. Am. J. Kidney Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Huang, J.W.; Chiang, C.K. Functional assessment of chronic illness therapy—The fatigue scale exhibits stronger associations with clinical parameters in chronic dialysis patients compared to other fatigue-assessing instruments. PeerJ 2016, 4, e1818. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Wang, J.; Chien, K.L.; COGENT study group. Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2018, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Hsu, Y.H.; Chang, P.Y.; He, Y.T.; Ueng, R.S.; Lai, C.F.; Chiang, C.K.; Huang, J.W.; Huang, S.J. Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology 2015, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Ting, I.W.; Yeh, H.C.; Kuo, C.C. Longitudinal change in estimated GFR among CKD patients: A 10-year follow-up study of an integrated kidney disease care program in Taiwan. PLoS ONE 2017, 12, e0173843. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Shardell, M.D.; Seliger, S.L.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Inflammation and trajectory of renal function in community-dwelling older adults. J. Am. Geriatr. Soc. 2018, 66, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A.; Katz, R.; Boulware, L.E.; Kestenbaum, B.; de Boer, I.H.; Wang, W.; Fulop, T.; Bansal, N.; Robinson-Cohen, C.; Griswold, M.; et al. Risk factor for rapid kidney function decline among African Americans: The Jackson Heart Study (JHS). Am. J. Kidney Dis. 2016, 68, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Pickering, J.W.; Ralib, A.M.; Endre, Z.H. Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit. Care 2013, 17, R7. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Astor, B.C.; Lewis, J.; Hu, B.; Appel, L.J.; Lipkowitz, M.S.; Toto, R.D.; Wang, X.; Wright, J.T., Jr.; Greene, T.H. Longitudinal Progression Trajectory of GFR Among Patients With CKD. Am. J. Kidney Dis. 2012, 59, 504–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hare, A.M.; Batten, A.; Burrows, N.R.; Pavkov, M.E.; Taylor, L.; Gupta, I.; Todd-Stenberg, J.; Maynard, C.; Rodriguez, R.A.; Murtagh, F.E.; et al. Trajectories of Kidney Function Decline in the 2 Years Before Initiation of Long-term Dialysis. Am. J. Kidney Dis. 2012, 59, 513–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turin, T.C.; Coresh, J.; Tonelli, M.; Stevens, P.E.; de Jong, P.E.; Farmer, C.K.; Matsushita, K.; Hemmelgarn, B.R. Short-term change in kidney function and risk of end-stage renal disease. Nephrol. Dial. Transplant. 2012, 27, 3835–3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, Y.Y.; Lee, K.B.; Rhee, E.J.; Park, C.Y.; Chang, Y.; Ryu, S. Chronic kidney disease and high eGFR according to body composition phenotype in adults with normal BMI. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-M.; Chen, Y.-T.; Hung, S.-C.; Shih, C.-J.; Lin, C.-H.; Chiang, C.-K.; Tarng, D.-C. Association of estimated glomerular filtration rate with all-cause and cardiovascular mortality: The role of malnutrition–inflammation–cachexia syndrome. J. Cachexia Sarcopenia Muscle 2016, 7, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.H.; Bolina, A.F.; Luiz, R.B.; de Oliveira, K.F.; Virtuoso, J.S., Jr.; Rodrigues, R.A.; Silva, L.C.; da Cunha, D.F.; De Mattia, A.L.; Barichello, E. Body mass index as discriminator of the lean mass deficit and excess body fat in institutionalized elderly people. Geriatr. Nurs. 2015, 36, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kwon, H.S.; Hwang, H.-J. White blood cell counts, insulin resistance, vitamin D levels and sarcopenia in Korean elderly men. Scand. J. Clin. Lab. Investig. 2017, 77, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, P.F.; Fujii, N.; Roy, J.; Chen, H.Y.; Lee Hamm, L.; Sondheimer, J.H.; He, J.; Fischer, M.J.; Rincon-Choles, H.; Krishnan, G.; et al. Hematuria as a risk factor for progression of chronic kidney disease and death: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. BMC Nephrol. 2018, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Tsai, H.B.; Chiang, C.K.; Huang, J.W.; Hung, K.Y. Dipstick proteinuria level is significantly associated with pre-morbid and in-hospital functional status among hospitalized older adults: A preliminary study. Sci. Rep. 2017, 7, 42030. [Google Scholar] [CrossRef] [PubMed]

| Clinical Features | Total (n = 500) | Annual Change < 0 (n = 136) | Reference Group (n = 126) | Annual Change ≥ 1 (n = 238) | p-Value |
|---|---|---|---|---|---|
| Demographic profile | |||||
| Age (years) | 71.3 ± 4.2 | 72.4 ± 4.6 | 71.7 ± 3.9 | 70.4 ± 4 | <0.01 |
| Gender (male%) | 259 (52) | 84 (62) | 70 (56) | 105 (44) | <0.01 |
| Smoking (%) | 31 (6) | 17 (13) | 1 (1) | 13 (5) | 0.02 |
| Alcohol (%) | 167 (33) | 52 (38) | 51 (40) | 64 (27) | 0.03 |
| Anthropometric parameters | |||||
| Body height (cm) | 160.5 ± 8.2 | 161.2 ± 7.9 | 160.7 ± 8.1 | 159.9 ± 8.4 | 0.31 |
| Body weight (kg) | 61.4 ± 9.7 | 62.5 ± 9.4 | 61.4 ± 9.3 | 60.7 ± 10.1 | 0.23 |
| BMI (kg/m2) | 23.8 ± 3.1 | 24 ± 3.1 | 23.7 ± 3.1 | 23.7 ± 3.1 | 0.56 |
| Waist circumference (cm) | 84.8 ± 9.7 | 86 ± 10.2 | 83.8 ± 10.1 | 84.6 ± 9 | 0.15 |
| Other comorbidities by history | |||||
| Hypertension (%) | 229 (46) | 69 (51) | 59 (47) | 101 (42) | 0.29 |
| Diabetes mellitus (%) | 41 (8) | 21 (15) | 6 (5) | 14 (6) | <0.01 |
| Cardiovascular disease (%) | 107 (21) | 38 (28) | 25 (20) | 44 (18) | 0.09 |
| Hyperlipidemia (%) | 155 (31) | 45 (33) | 43 (34) | 67 (28) | 0.42 |
| Gout/hyperuricemia (%) | 53 (11) | 19 (14) | 17 (13) | 17 (7) | 0.06 |
| Chronic kidney disease (%) | 1 (0) | 1 (1) | 0 (0) | 0 (0) | 0.26 |
| Chronic liver disease (%) | 49 (10) | 11 (8) | 14 (11) | 24 (10) | 0.7 |
| Chronic lung disease (%) | 37 (7) | 11 (8) | 10 (8) | 16 (7) | 0.86 |
| Dementia (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| Physical parameters | |||||
| Systolic BP (mmHg) | 133.2 ± 15.9 | 136.9 ± 18.6 | 132.2 ± 15.3 | 131.6 ± 14 | <0.01 |
| Diastolic BP (mmHg) | 78 ± 10.7 | 79 ± 11.5 | 77.4 ± 10.3 | 77.7 ± 10.3 | 0.42 |
| Heart rate (/minute) | 70.3 ± 9.7 | 69.5 ± 9.5 | 69.6 ± 10.1 | 71.1 ± 9.6 | 0.2 |
| Blood test results | |||||
| Hemoglobin (g/dL) | 13.7 ± 1.2 | 13.7 ± 1.2 | 13.8 ± 1.2 | 13.7 ± 1.2 | 0.58 |
| Platelet (×103/µL) | 207.8 ± 47.2 | 205.2 ± 49.5 | 202.4 ± 47.7 | 212.2 ± 45.4 | 0.13 |
| Leuokocyte (×103/µL) | 5.6 ± 1.3 | 5.6 ± 1.2 | 5.4 ± 1.4 | 5.7 ± 1.3 | 0.11 |
| Total protein (mg/dL) | 7.3 ± 0.4 | 7.3 ± 0.4 | 7.3 ± 0.4 | 7.3 ± 0.4 | 0.34 |
| Albumin (mg/dL) | 4.5 ± 0.2 | 4.5 ± 0.2 | 4.5 ± 0.2 | 4.5 ± 0.2 | 0.24 |
| Globulin (mg/dL) | 2.8 ± 0.4 | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.9 ± 0.4 | 0.03 |
| Urea nitrogen (mg/dL) | 16.5 ± 3.9 | 17.4 ± 4.2 | 16.4 ± 3.6 | 16 ± 3.7 | <0.01 |
| Creatinine (mg/dL) | 0.98 ± 0.2 | 0.99 ± 0.3 | 1 ± 0.2 | 0.97 ± 0.2 | 0.43 |
| eGFR (mL/min/1.73 m2) | 72.7 ± 15 | 76.4 ± 20.1 | 71.2 ± 12.8 | 71.4 ± 12 | <0.01 |
| Fasting glucose (mg/dL) | 99.7 ± 16.8 | 105.3 ± 23.1 | 96.4 ± 13.3 | 98.3 ± 13.1 | <0.01 |
| Total cholesterol (g/dL) | 195 ± 32.8 | 190.4 ± 34.2 | 192.9 ± 33.4 | 198.7 ± 31.3 | 0.05 |
| Triglyceride (mg/dL) | 113.3 ± 58.6 | 119.8 ± 67.1 | 106.6 ± 51.6 | 113.2 ± 56.7 | 0.19 |
| Uric acid (mg/dL) | 6.1 ± 1.4 | 6.3 ± 1.4 | 6.2 ± 1.4 | 5.9 ± 1.4 | 0.01 |
| Urinalysis results | |||||
| Occult blood (0–4+) | 0.1 ± 0.36 | 0.19 ± 0.49 | 0.06 ± 0.29 | 0.07 ± 0.29 | <0.01 |
| Protein (0–4+) | 0.05 ± 0.29 | 0.11 ± 0.43 | 0.03 ± 0.2 | 0.03 ± 0.22 | 0.02 |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age (year) | 1.08 | 1.01–1.16 | 0.03 |
| Diastolic blood pressure (mmHg) | 1.03 | 0.998–1.05 | 0.07 |
| Urine protein (titer) | 9.86 | 1.42–68.4 | 0.02 |
| Urine OB (titer) | 3.39 | 1.3–8.8 | 0.01 |
| Hemoglobin (g/dL) | 0.74 | 0.56–0.97 | 0.03 |
| Glucose (mg/dL) | 1.04 | 1.02–1.06 | <0.01 |
| Creatinine (mg/dL) | 13.2 | 1.13–153.7 | 0.04 |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age (year) | 0.92 | 0.87–0.97 | <0.01 |
| Male gender | 0.5 | 0.3–0.82 | <0.01 |
| BMI (kg/m2) | 0.85 | 0.75–0.96 | <0.01 |
| Waist circumference (cm) | 1.06 | 1.02–1.11 | <0.01 |
| Leukocyte (K/µL) | 1.21 | 1.01–1.45 | 0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-T.; Chen, Y.-M.; Ho, F.-H.; Lin, K.-P.; Chen, J.-H.; Yen, C.-J.; COGENT Study Group (COhort of GEriatric Nephrology in NTUH). 10-Year Renal Function Trajectories in Community-Dwelling Older Adults: Exploring the Risk Factors for Different Patterns. J. Clin. Med. 2018, 7, 373. https://doi.org/10.3390/jcm7100373
Chao C-T, Chen Y-M, Ho F-H, Lin K-P, Chen J-H, Yen C-J, COGENT Study Group (COhort of GEriatric Nephrology in NTUH). 10-Year Renal Function Trajectories in Community-Dwelling Older Adults: Exploring the Risk Factors for Different Patterns. Journal of Clinical Medicine. 2018; 7(10):373. https://doi.org/10.3390/jcm7100373
Chicago/Turabian StyleChao, Chia-Ter, Yung-Ming Chen, Fu-Hui Ho, Kun-Pei Lin, Jen-Hau Chen, Chung-Jen Yen, and COGENT Study Group (COhort of GEriatric Nephrology in NTUH). 2018. "10-Year Renal Function Trajectories in Community-Dwelling Older Adults: Exploring the Risk Factors for Different Patterns" Journal of Clinical Medicine 7, no. 10: 373. https://doi.org/10.3390/jcm7100373
APA StyleChao, C.-T., Chen, Y.-M., Ho, F.-H., Lin, K.-P., Chen, J.-H., Yen, C.-J., & COGENT Study Group (COhort of GEriatric Nephrology in NTUH). (2018). 10-Year Renal Function Trajectories in Community-Dwelling Older Adults: Exploring the Risk Factors for Different Patterns. Journal of Clinical Medicine, 7(10), 373. https://doi.org/10.3390/jcm7100373