Could Cardiovascular Health Metrics Account for Age and Sex Disparities in Self-Reported Ischemic Heart Disease Prevalence?
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. CVH Metrics
2.3. Outcome Measurement
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
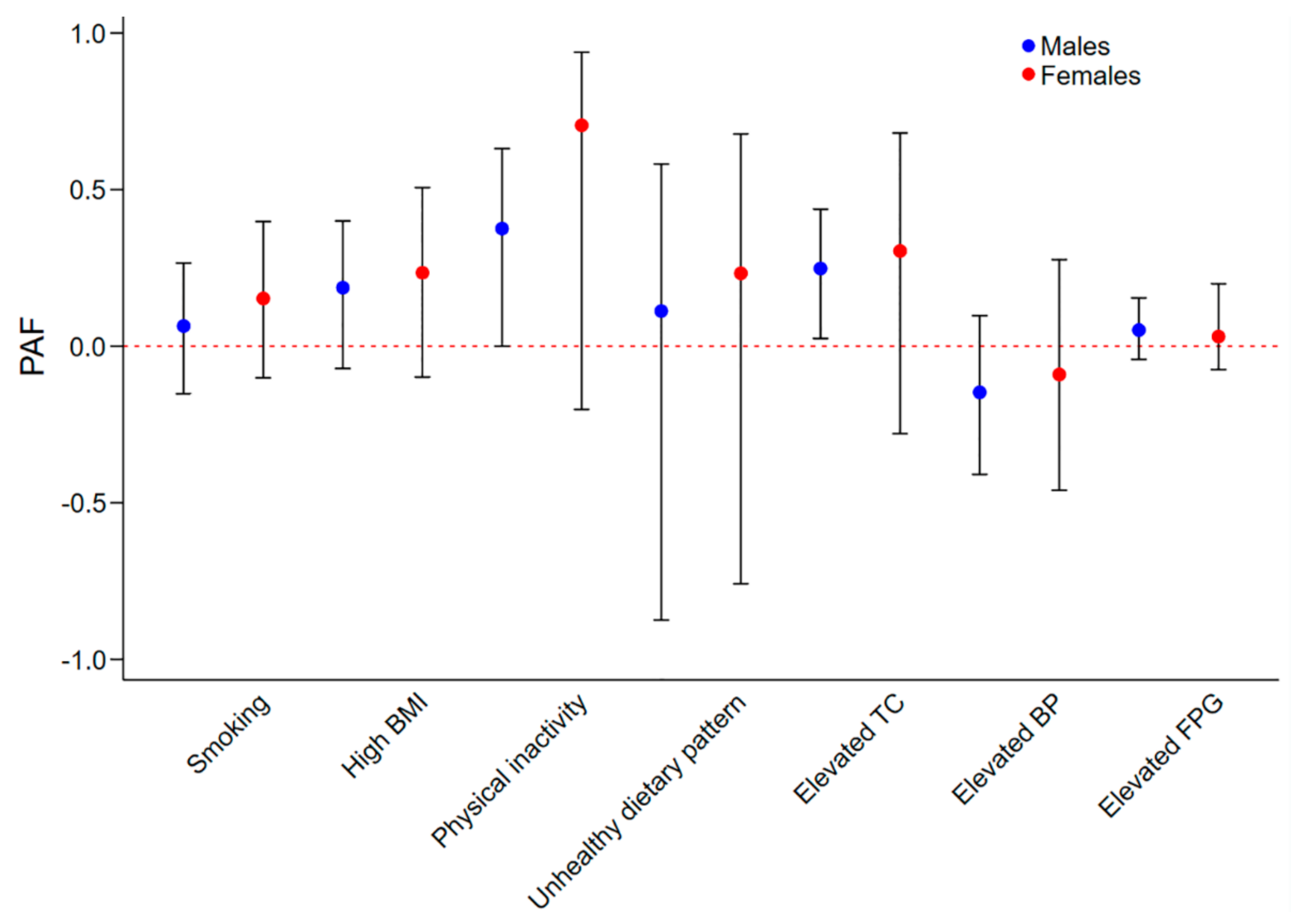
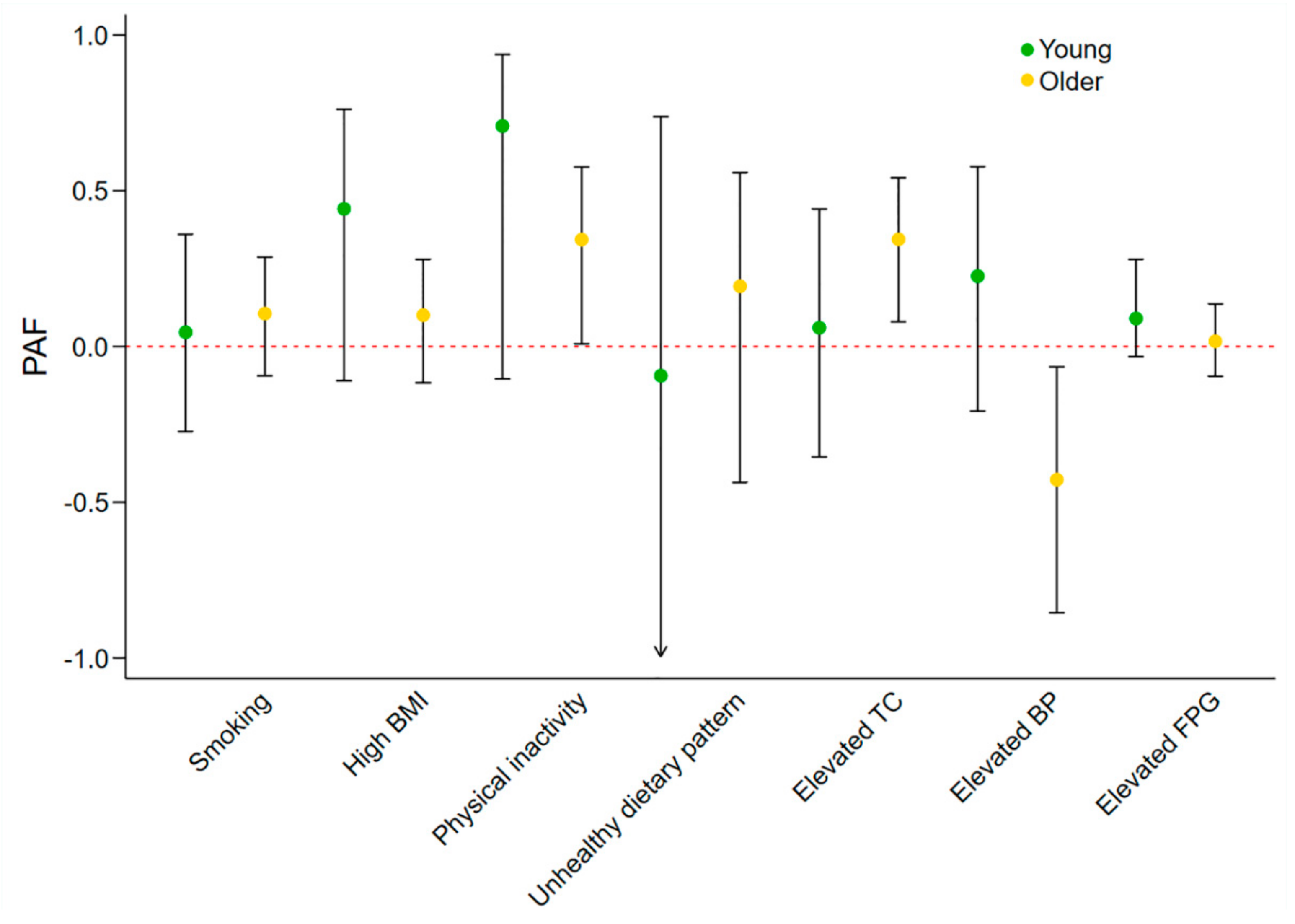
3.2. Sex and Age-Specific Effects of Individual Metrics on IHD Prevalence
3.3. Sex and Age-Specific Effects of Number of Ideal CVH Metrics on IHD Prevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Tang, X.; Zhang, W.; Qian, Y.; Huang, Y.; et al. Adherence to Healthy Lifestyle and Cardiovascular Diseases in the Chinese Population. J. Am. Coll. Cardiol. 2017, 69, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Chomistek, A.K.; Chiuve, S.E.; Eliassen, A.H.; Mukamal, K.J.; Willett, W.C.; Rimm, E.B. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J. Am. Coll. Cardiol. 2015, 65, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Z.; Dong, B.; Cao, S.; Hu, J.; Adegbija, O. Life’s Simple 7 and ischemic heart disease in the general Australian population. PLoS ONE 2017, 12, e0187020. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cogswell, M.E.; Dana Flanders, W.; Hong, Y.; Zhang, Z.; Loustalot, F.; Gillespie, C.; Merritt, R.; Hu, F.B. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among us adults. JAMA 2012, 307, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.; Morris, J.K.; Boniface, S.; Tang, J.L.; Milenkovic, D. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018, 360, j5855. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Hirakawa, Y.; Hussain, M.A.; Aekplakorn, W.; Wang, X.; Peters, S.A.; Mamun, A.; Woodward, M. Age- and Sex-Specific Burden of Cardiovascular Disease Attributable to 5 Major and Modifiable Risk Factors in 10 Asian Countries of the Western Pacific Region. Circ. J. 2015, 79, 1662–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaye, B.; Canonico, M.; Perier, M.C.; Samieri, C.; Berr, C.; Dartigues, J.F.; Tzourio, C.; Elbaz, A.; Empana, J.P. Ideal Cardiovascular Health, Mortality, and Vascular Events in Elderly Subjects: The Three-City Study. J. Am. Coll. Cardiol. 2017, 69, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Barzi, F.; Woodward, M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ 2006, 332, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Woodward, M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet 2011, 378, 1297–1305. [Google Scholar] [CrossRef]
- Peters, S.A.; Singhateh, Y.; Mackay, D.; Huxley, R.R.; Woodward, M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 123–131. [Google Scholar] [CrossRef]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef] [PubMed]
- Lacey, B.; Lewington, S.; Clarke, R.; Kong, X.L.; Chen, Y.; Guo, Y.; Yang, L.; Bennett, D.; Bragg, F.; Bian, Z.; et al. Age-specific association between blood pressure and vascular and non-vascular chronic diseases in 0.5 million adults in China: A prospective cohort study. Lancet Glob. Health 2018, 6, e641–e649. [Google Scholar] [CrossRef]
- Rao Kondapally Seshasai, S.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar] [PubMed]
- Manson, J.E.; Greenland, P.; LaCroix, A.Z.; Stefanick, M.L.; Mouton, C.P.; Oberman, A.; Perri, M.G.; Sheps, D.S.; Pettinger, M.B.; Siscovick, D.S. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N. Engl. J. Med. 2002, 347, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, T.; Vaucher, J.; Okbay, A.; Pikhart, H.; Peasey, A.; Kubinova, R.; Pajak, A.; Tamosiunas, A.; Malyutina, S.; Hartwig, F.P.; et al. Education and coronary heart disease: Mendelian randomisation study. BMJ 2017, 358, j3542. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, H.; Kilpi, F.; Moustgaard, H.; Martikainen, P. Socioeconomic Position and Antidepressant Use as Predictors of Coronary Heart Disease Mortality: A Population-Based Registry Study of 362,271 Finns. Psychosom. Med. 2016, 78, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, H.J.; Wu, V.X.; He, H.G.; Jiang, Y.; Wang, W. Awareness, knowledge, healthy lifestyle behaviors, and their correlates to coronary heart disease among working women in Singapore. Heart Lung 2016, 45, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Yasaitis, L.C.; Berkman, L.F.; Chandra, A. Comparison of self-reported and Medicare claims-identified acute myocardial infarction. Circulation 2015, 131, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Muggah, E.; Graves, E.; Bennett, C.; Manuel, D.G. Ascertainment of chronic diseases using population health data: A comparison of health administrative data and patient self-report. BMC Public Health 2013, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Okura, Y.; Urban, L.H.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J. Clin. Epidemiol. 2004, 57, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Barber, A.; Doughty, R.N.; Grey, A.; Gamble, G.; Reid, I.R. Differences between self-reported and verified adverse cardiovascular events in a randomised clinical trial. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed]
| Metrics | Status | Males, n (%) | Females, n (%) | p | Young, n (%) | Older, n (%) | p |
|---|---|---|---|---|---|---|---|
| IHD | Yes | 214 (4.2) | 143 (2.5) | <0.01 | 72 (1.1) | 285 (10.2) | <0.01 |
| No | 3115 (95.8) | 4027 (97.5) | 4751 (98.9) | 2391 (89.8) | |||
| Smoking | Ideal | 1420 (49.8) | 2342 (61.1) | <0.01 | 2521 (57.7) | 1241 (49.0) | <0.01 |
| Non-ideal | 1909 (50.2) | 1828 (38.9) | 2302 (42.3) | 1435 (51.0) | |||
| BMI | Ideal | 833 (32.3) | 1562 (46.2) | <0.01 | 1750 (43.1) | 645 (27.2) | <0.01 |
| Non-ideal | 2388 (67.7) | 2344 (53.8) | 2843 (56.9) | 1889 (72.8) | |||
| Physical activity | Ideal | 945 (32.7) | 829 (20.9) | <0.01 | 1320 (30.0) | 454 (16.6) | <0.01 |
| Non-ideal | 2382 (67.3) | 3339 (79.1) | 3500 (70.0) | 2221 (83.4) | |||
| Dietary pattern | Ideal | 93 (2.1) | 341 (7.4) | <0.01 | 223 (4.1) | 211 (6.9) | <0.01 |
| Non-ideal | 3236 (97.9) | 3829 (92.6) | 4600 (95.9) | 2465 (93.1) | |||
| TC | Ideal | 1259 (45.1) | 1568 (45.8) | 0.70 | 2246 (52.5) | 581 (23.9) | <0.01 |
| Non-ideal | 2070 (54.9) | 2602 (54.2) | 2577 (47.5) | 2095 (76.1) | |||
| BP | Ideal | 1047 (36.7) | 1781 (51.6) | <0.01 | 2234 (51.4) | 594 (21.9) | <0.01 |
| Non-ideal | 2189 (63.3) | 2204 (48.4) | 2427 (48.6) | 1966 (78.1) | |||
| FPG | Ideal | 2426 (79.4) | 3513 (87.7) | <0.01 | 4162 (88.6) | 1777 (68.4) | <0.01 |
| Non-ideal | 903 (20.6) | 657 (12.3) | 661 (11.4) | 899 (31.6) | |||
| Age | <60 years | 2078 (76.2) | 2745 (74.7) | 0.01 | 4823 (100.0) | 0 (0.0) | NA |
| ≥60 years | 1251 (23.8) | 1425 (25.3) | 0 (0.0) | 2676 (100.0) | |||
| Sex | Male | 3329 (100.0) | 0 (0.0) | NA | 2078 (49.8) | 1251 (47.8) | 0.01 |
| Female | 0 (0.0) | 4170 (100.0) | 2745 (50.2) | 1425 (52.2) | |||
| Education level | High | 1620 (57.6) | 2113 (58.1) | 0.76 | 2969 (67.5) | 764 (28.3) | <0.01 |
| Low | 1709 (42.4) | 2057 (41.9) | 1854 (32.5) | 1912 (71.7) | |||
| Income | High | 1642 (56.5) | 1758 (49.9) | <0.01 | 2771 (62.0) | 629 (26.6) | <0.01 |
| Low | 1441 (43.5) | 1976 (50.1) | 1628 (38.0) | 1789 (73.4) | |||
| Region | Major cities | 2024 (72.0) | 2538 (73.0) | 0.71 | 3012 (74.2) | 1550 (67.2) | <0.01 |
| Inner regional | 760 (20.0) | 949 (19.2) | 1025 (18.3) | 684 (23.7) | |||
| Other | 545 (8.0) | 683 (7.8) | 786 (7.5) | 442 (9.1) |
| Metrics | Population | Crude IRR (95% CI) | p | Adjusted IRR * (95% CI) | p | Pinteraction |
|---|---|---|---|---|---|---|
| Smoking | Males | 1.84 (1.21–2.79) | 0.01 | 1.12 (0.77–1.63) | 0.56 | 0.49 |
| Females | 1.30 (0.78–2.18) | 0.31 | 1.41 (0.79–2.51) | 0.24 | ||
| High BMI | Males | 1.93 (1.26–2.97) | <0.01 | 1.32 (0.91–1.90) | 0.14 | 0.14 |
| Females | 2.13 (1.27–3.58) | 0.01 | 1.51 (0.85–2.71) | 0.15 | ||
| Physical inactivity | Males | 3.57 (1.94–6.55) | <0.01 | 1.84 (1.00–3.39) | 0.048 | 0.38 |
| Females | 9.47 (1.88–47.73) | 0.01 | 3.99 (0.79–20.12) | 0.09 | ||
| Unhealthy dietary pattern | Males | 0.42 (0.17–1.06) | 0.06 | 1.13 (0.52–2.43) | 0.76 | 0.82 |
| Females | 1.24 (0.50–3.07) | 0.64 | 1.33 (0.53–3.29) | 0.54 | ||
| Elevated TC | Males | 2.56 (1.66–3.95) | <0.01 | 1.53 (1.04–2.25) | 0.03 | 0.85 |
| Females | 5.22 (2.07–13.18) | <0.01 | 1.70 (0.65–4.42) | 0.28 | ||
| Elevated BP | Males | 1.62 (1.07–2.45) | 0.02 | 0.81 (0.57–1.16) | 0.25 | 0.89 |
| Females | 3.46 (1.93–6.21) | <0.01 | 0.85 (0.43–1.69) | 0.63 | ||
| Elevated FPG | Males | 2.56 (1.84–3.55) | <0.01 | 1.20 (0.85–1.67) | 0.29 | 1.00 |
| Females | 2.98 (1.66–5.34) | <0.01 | 1.20 (0.56–2.57) | 0.64 |
| Metrics | Population | Crude IRR (95% CI) | p | Adjusted IRR * (95% CI) | p | Pinteraction |
|---|---|---|---|---|---|---|
| Smoking | Young adults | 1.67 (0.89–3.14) | 0.11 | 1.10 (0.55–2.18) | 0.78 | 0.48 |
| Older adults | 1.35 (0.95–1.92) | 0.09 | 1.22 (0.84–1.75) | 0.29 | ||
| High BMI | Young adults | 4.42 (1.64–11.92) | <0.01 | 2.28 (0.84–6.16) | 0.10 | 0.01 |
| Older adults | 1.07 (0.78–1.47) | 0.66 | 1.15 (0.86–1.52) | 0.34 | ||
| Physical inactivity | Young adults | 7.08 (1.56–32.16) | 0.01 | 4.34 (0.87–21.55) | 0.07 | 0.03 |
| Older adults | 1.91 (1.17–3.12) | 0.01 | 1.63 (1.01–2.64) | 0.046 | ||
| Unhealthy dietary pattern | Young adults | 0.93 (0.21–4.11) | 0.93 | 0.91 (0.21–3.95) | 0.90 | 0.31 |
| Older adults | 1.27 (0.66–2.44) | 0.47 | 1.26 (0.67–2.37) | 0.47 | ||
| Elevated TC | Young adults | 2.69 (1.27–5.66) | 0.01 | 1.12 (0.51–2.48) | 0.77 | 0.51 |
| Older adults | 1.47 (0.98–2.21) | 0.06 | 1.67 (1.11–2.51) | 0.02 | ||
| Elevated BP | Young adults | 3.69 (1.92–7.12) | <0.01 | 1.56 (0.67–3.62) | 0.29 | <0.01 |
| Older adults | 0.78 (0.50–1.22) | 0.28 | 0.61 (0.40–0.92) | 0.02 | ||
| Elevated FPG | Young adults | 3.60 (1.77–7.30) | <0.01 | 1.72 (0.77–3.83) | 0.18 | 0.04 |
| Older adults | 1.29 (0.92–1.80) | 0.14 | 1.05 (0.74–1.47) | 0.79 |
| Participants | Ideal Metrics Number | IHD Cases/Participants | Crude OR (95% CI) | p | Adjusted * OR (95% CI) | p |
|---|---|---|---|---|---|---|
| Males | 0–2 | 150/1741 | Reference | NA | Reference | NA |
| 3–4 | 44/1147 | 0.34 (0.21–0.56) | <0.01 | 0.65 (0.42–1.01) | 0.06 | |
| 5–7 | 4/285 | 0.06 (0.01–0.51) | 0.01 | 0.47 (0.05–4.30) | 0.50 | |
| One more ideal metric | NA | 0.61 (0.53–0.70) | <0.01 | 0.86 (0.73–1.02) | 0.09 | |
| Females | 0–2 | 99/1601 | Reference | NA | Reference | NA |
| 3–4 | 29/1639 | 0.22 (0.11–0.45) | <0.01 | 0.41 (0.19–0.88) | 0.02 | |
| 5–7 | 0/589 | NA | NA | NA | NA | |
| One more ideal metric | NA | 0.52 (0.45–0.60) | <0.01 | 0.73 (0.59–0.91) | 0.01 | |
| Young | 0–2 | 54/1708 | Reference | NA | Reference | NA |
| 3–4 | 15/2028 | 0.18 (0.09–0.37) | <0.01 | 0.35 (0.15–0.83) | 0.02 | |
| 5–7 | 0/792 | NA | NA | NA | NA | |
| One more ideal metric | NA | 0.49 (0.40–0.60) | <0.01 | 0.69 (0.52–0.91) | 0.01 | |
| Older | 0–2 | 195/1634 | Reference | NA | Reference | NA |
| 3–4 | 58/758 | 0.61 (0.39–0.95) | 0.03 | 0.68 (0.44–1.03) | 0.07 | |
| 5–7 | 4/82 | 0.35 (0.04–2.91) | 0.32 | 0.55 (0.06–5.09) | 0.59 | |
| One more ideal metric | NA | 0.83 (0.71–0.97) | 0.02 | 0.89 (0.76–1.05) | 0.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Wang, Z. Could Cardiovascular Health Metrics Account for Age and Sex Disparities in Self-Reported Ischemic Heart Disease Prevalence? J. Clin. Med. 2018, 7, 369. https://doi.org/10.3390/jcm7100369
Peng Y, Wang Z. Could Cardiovascular Health Metrics Account for Age and Sex Disparities in Self-Reported Ischemic Heart Disease Prevalence? Journal of Clinical Medicine. 2018; 7(10):369. https://doi.org/10.3390/jcm7100369
Chicago/Turabian StylePeng, Yang, and Zhiqiang Wang. 2018. "Could Cardiovascular Health Metrics Account for Age and Sex Disparities in Self-Reported Ischemic Heart Disease Prevalence?" Journal of Clinical Medicine 7, no. 10: 369. https://doi.org/10.3390/jcm7100369
APA StylePeng, Y., & Wang, Z. (2018). Could Cardiovascular Health Metrics Account for Age and Sex Disparities in Self-Reported Ischemic Heart Disease Prevalence? Journal of Clinical Medicine, 7(10), 369. https://doi.org/10.3390/jcm7100369




