The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Critically Ill Patients
Abstract
1. Introduction
2. Materials and Methods
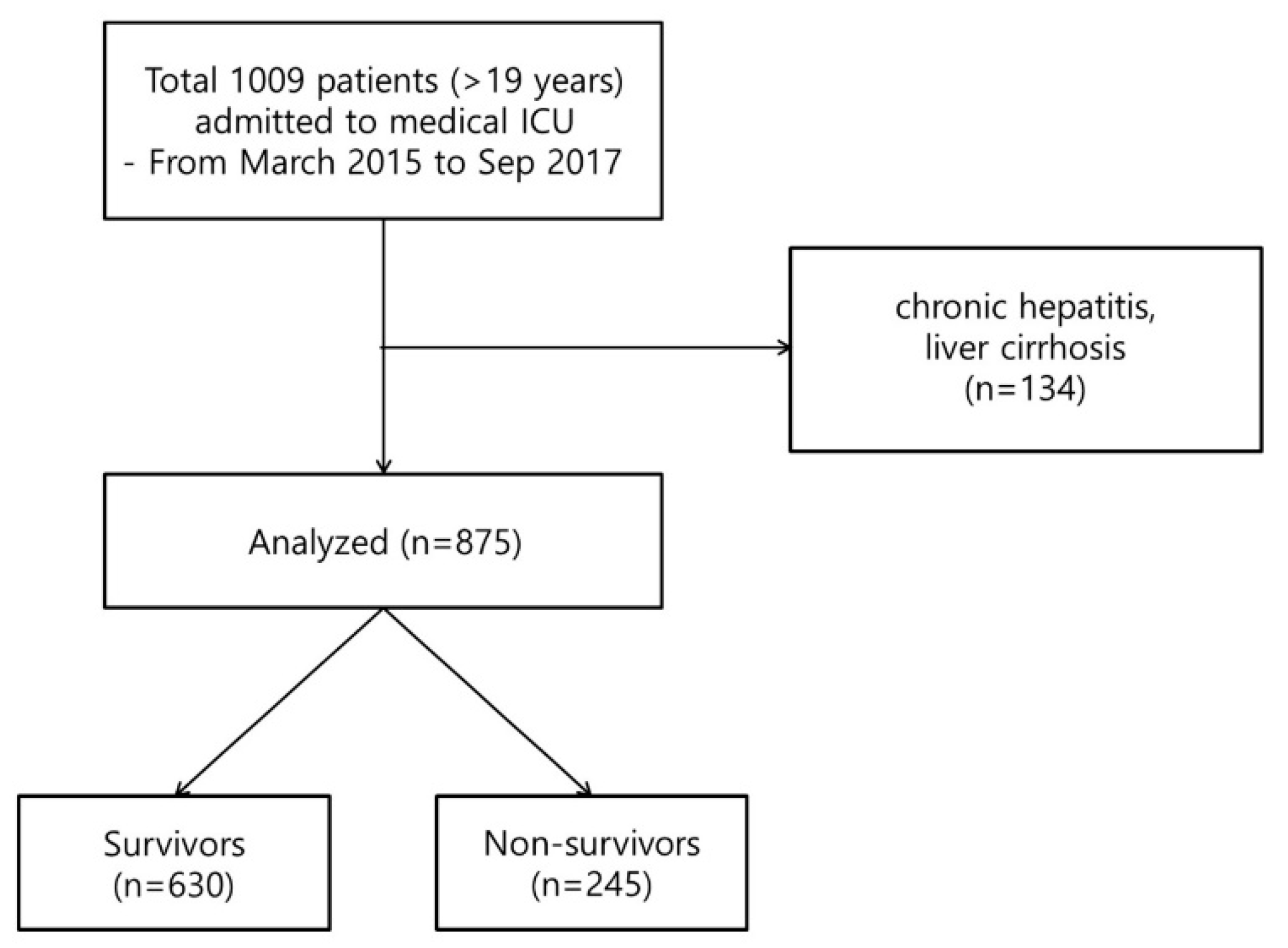
2.1. Patients and Study Design
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethics
3. Results
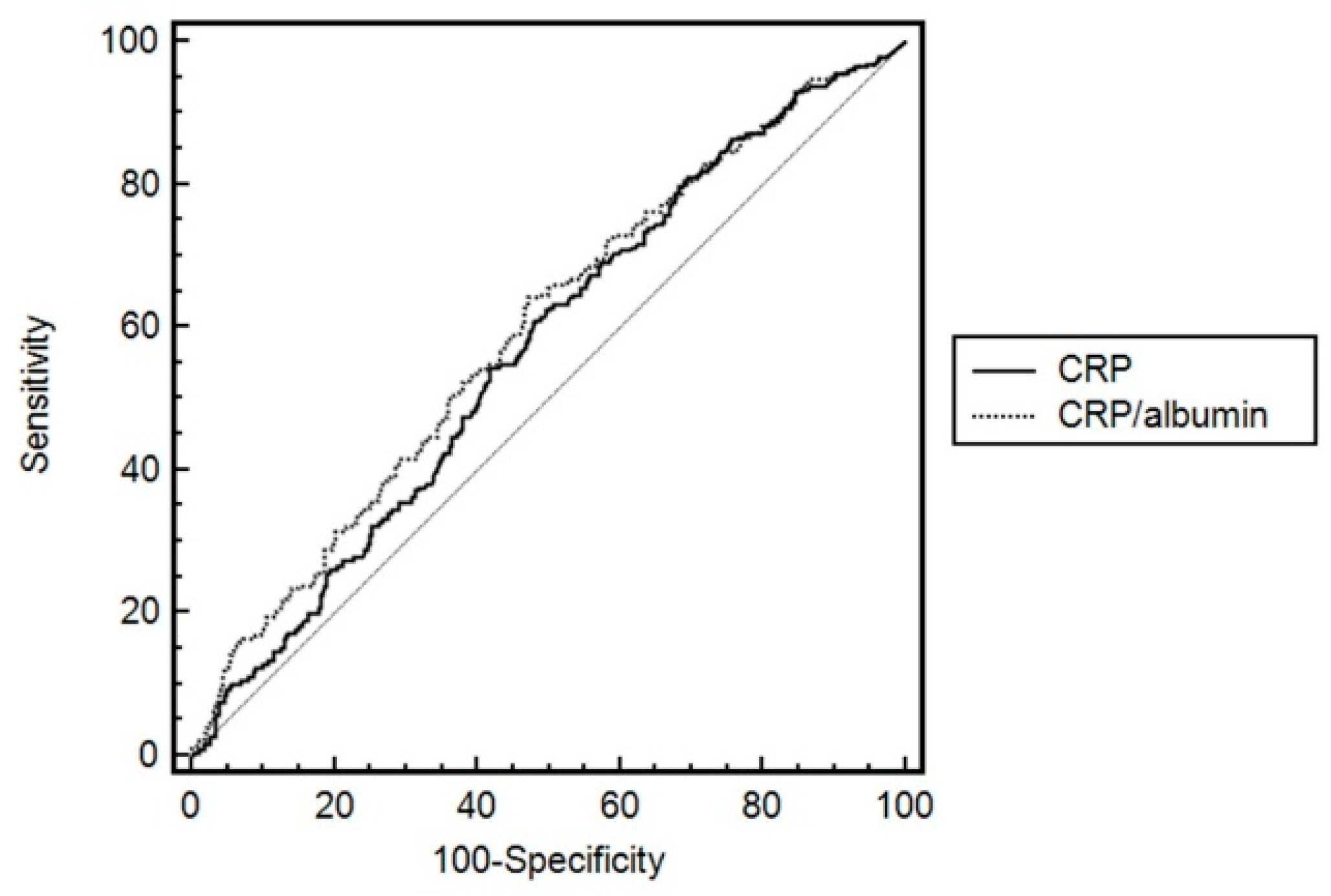
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Thijs, L.G.; Hack, C.E. Time course of cytokine levels in sepsis. Intensiv. Care Med. 1995, 21, S258–S263. [Google Scholar] [CrossRef]
- Ho, K.M.; Lee, K.Y.; Dobb, G.J.; Webb, S.A. C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: A prospective cohort study. Intensiv. Care Med. 2008, 34, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.M.; Dobb, G.J.; Lee, K.Y.; Towler, S.C.; Webb, S.A. C-reactive protein concentration as a predictor of intensive care unit readmission: A nested case-control study. J. Crit. Care 2006, 21, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, H.; Masetto, A.C.; Mesquita, E.T. C-reactive protein: An inflammatory marker with prognostic value in patients with decompensated heart failure. Arq. Bras. Cardiol. 2007, 88, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Devran, O.; Karakurt, Z.; Adiguzel, N.; Gungor, G.; Mocin, O.Y.; Balci, M.K.; Celik, E.; Salturk, C.; Takir, H.B.; Kargin, F.; et al. C-reactive protein as a predictor of mortality in patients affected with severe sepsis in intensive care unit. Multidiscip. Respir. Med. 2012, 7, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.M.; Lobo, F.R.; Bota, D.P.; Lopes-Ferreira, F.; Soliman, H.M.; Melot, C.; Vincent, J.L. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest 2003, 123, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Artero, A.; Zaragoza, R.; Camarena, J.J.; Sancho, S.; Gonzalez, R.; Nogueira, J.M. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J. Crit. Care 2010, 25, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, P.; Feldman, J. Association of serum albumin and mortality risk. J. Clin. Epidemiol. 1997, 50, 693–703. [Google Scholar] [CrossRef]
- Kim, M.H.; Ahn, J.Y.; Song, J.E.; Choi, H.; Ann, H.W.; Kim, J.K.; Kim, J.H.; Jeon, Y.D.; Kim, S.B.; Jeong, S.J.; et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Zampieri, F.G.; Forte, D.N.; Azevedo, L.C.; Park, M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Wei, X.; Sheng, H.; Chi, P.; Liu, Y.; Huang, X.; Xiang, Y.; Zhu, Q.; Xing, S.; Liu, W. C-reactive protein/albumin and neutrophil/lymphocyte ratios and their combination predict overall survival in patients with gastric cancer. Oncol. Lett. 2017, 14, 7417–7424. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Kono, Y.; Murakami, Y.; Shishido, Y.; Kuroda, H.; Matsunaga, T.; Fukumoto, Y.; Osaki, T.; Ashida, K.; Fujiwara, Y. Prognostic significance of the preoperative ratio of C-reactive protein to albumin and neutrophil-lymphocyte ratio in gastric cancer patients. World J. Surg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.L.; Wang, F.H.; Zhang, D.S.; Qiu, M.Z.; Ren, C.; Jin, Y.; Zhou, Y.X.; Wang, D.S.; He, M.M.; Bai, L.; et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: The C-reactive protein/albumin ratio. BMC Cancer 2015, 15, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Tanaka, K.; Fushiya, N.; Koike, K.; Nishino, H.; Matsushima, M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann. Surg. Oncol. 2015, 22, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, J.; Guo, L.; Zuo, Q. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol. 2016, 37, 12525–12533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Model building strategy for logistic regression: Purposeful selection. Ann. Transl. Med. 2016, 4, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Residuals and regression diagnostics: Focusing on logistic regression. Ann. Transl. Med. 2016, 4, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Povoa, P.; Almeida, E.; Moreira, P.; Fernandes, A.; Mealha, R.; Aragao, A.; Sabino, H. C-reactive protein as an indicator of sepsis. Intensiv. Care Med. 1998, 24, 1052–1056. [Google Scholar] [CrossRef]
- Sierra, R.; Rello, J.; Bailen, M.A.; Benitez, E.; Gordillo, A.; Leon, C.; Pedraza, S. C-reactive protein used as an early indicator of infection in patients with systemic inflammatory response syndrome. Intensiv. Care Med. 2004, 30, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Gogos, C.A.; Giali, S.; Paliogianni, F.; Dimitracopoulos, G.; Bassaris, H.P.; Vagenakis, A.G. Interleukin-6 and C-reactive protein as early markers of sepsis in patients with diabetic ketoacidosis or hyperosmosis. Diabetologia 2001, 44, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S.; Kuhmonen, J.; Siironen, J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir. 2012, 154, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, J.; Povoa, P.; Coelho, L.; Almeida, E.; Moreira, P.; Fernandes, A.; Mealha, R.; Sabino, H. Is C-reactive protein a good prognostic marker in septic patients? Intensiv. Care Med. 2009, 35, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Al-Subaie, N.; Reynolds, T.; Myers, A.; Sunderland, R.; Rhodes, A.; Grounds, R.M.; Hall, G.M. C-reactive protein as a predictor of outcome after discharge from the intensive care: A prospective observational study. Br. J. Anaesth. 2010, 105, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Vardas, K.; Ilia, S.; Sertedaki, A.; Charmandari, E.; Briassouli, E.; Goukos, D.; Apostolou, K.; Psarra, K.; Botoula, E.; Tsagarakis, S.; et al. Increased glucocorticoid receptor expression in sepsis is related to heat shock proteins, cytokines, and cortisol and is associated with increased mortality. Intensiv. Care Med. Exp. 2017, 5, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Tavladaki, T.; Spanaki, A.M.; Dimitriou, H.; Kondili, E.; Choulaki, C.; Georgopoulos, D.; Briassoulis, G. Similar metabolic, innate immunity, and adipokine profiles in adult and pediatric sepsis versus systemic inflammatory response syndrome—A pilot study. Pediatr. Crit. Care Med. 2017, 18, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Dubois, M.J.; Navickis, R.J.; Wilkes, M.M. Hypoalbuminemia in acute illness: Is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann. Surg. 2003, 237, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Wagner, D.P.; Draper, E.A.; Zimmerman, J.E.; Bergner, M.; Bastos, P.G.; Sirio, C.A.; Murphy, D.J.; Lotring, T.; Damiano, A.; et al. The apache iii prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991, 100, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- Randle, N.W.; Hubert-Hartmann, K.; Mulheran, L.C. Serum albumin levels: Relationship to length of hospital stay. Hosp. Pharm. 1984, 19, 802–805. [Google Scholar] [PubMed]
- Moshage, H.J.; Janssen, J.A.; Franssen, J.H.; Hafkenscheid, J.C.; Yap, S.H. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J. Clin. Investig. 1987, 79, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- De Mutsert, R.; Grootendorst, D.C.; Indemans, F.; Boeschoten, E.W.; Krediet, R.T.; Dekker, F.W. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J. Ren. Nutr. 2009, 19, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, S.H.; Park, K.N.; Oh, S.H.; Kim, Y.M.; Kim, H.J.; Youn, C.S. High-sensitivity C-reactive protein/albumin ratio as a predictor of in-hospital mortality in older adults admitted to the emergency department. Clin. Exp. Emerg. Med. 2017, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhou, Y.; Xu, Z.; Yang, Y.; Kuang, D.; You, H.; Ma, S.; Hao, C.; Gu, Y.; Lin, S.; et al. The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol. 2011, 12, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Ates, I.; Akpinar, M.Y.; Yuksel, M.; Kuzu, U.B.; Kacar, S.; Coskun, O.; Kayacetin, E. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 424–430. [Google Scholar] [CrossRef]
- Briassoulis, G.; Venkataraman, S.; Thompson, A. Cytokines and metabolic patterns in pediatric patients with critical illness. Clin. Dev. Immunol. 2010, 2010, 354047–354058. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Ma, Y.; Deng, F.; Ju, W.B.; Sun, X.Y.; Wang, H. The prognostic value of C-reactive protein/albumin ratio in human malignancies: An updated meta-analysis. Onco Targets Ther. 2017, 10, 3059–3070. [Google Scholar] [CrossRef] [PubMed]
- Spanaki, A.M.; Tavladaki, T.; Dimitriou, H.; Kozlov, A.V.; Duvigneau, J.C.; Meleti, E.; Weidinger, A.; Papakonstantinou, E.; Briassoulis, G. Longitudinal profiles of metabolism and bioenergetics associated with innate immune hormonal inflammatory responses and amino-acid kinetics in severe sepsis and systemic inflammatory response syndrome in children. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
| Variables | Non-Survivors | Survivors | p-Value |
|---|---|---|---|
| (n = 245) | (n = 630) | ||
| Age, years of age | 68.2 ± 13.4 | 65.5 ± 15.0 | 0.014 |
| Male (%) | 311 (61.6) | 137 (60.6) | 0.805 |
| BMI, kg/m2 | 22.1 ± 4.7 | 22.0 ± 4.9 | 0.725 |
| Disease severity | |||
| APACHE II score | 29.2 ± 9.5 | 22.7 ± 8.3 | <0.001 |
| Underlying diseases | |||
| Diabetes mellitus | 67 (27.3) | 193 (30.6) | 0.339 |
| Chronic lung disease † | 36 (14.7) | 113 (17.9) | 0.252 |
| Hypertension | 134 (54.7) | 351 (55.7) | 0.785 |
| Heart failure | 30 (12.2) | 81 (12.9) | 0.807 |
| Coronary artery disease | 41 (16.7) | 83 (13.2) | 0.175 |
| Cancer | 88 (35.9) | 141 (22.4) | <0.001 |
| Acute renal failure | 97 (39.6) | 166 (26.3) | <0.001 |
| ARDS | 32 (13.1) | 60 (9.5) | 0.126 |
| Sepsis | 182 (75.1) | 426 (67.6) | 0.031 |
| Laboratory parameters | |||
| WBC (103/µL) | 13.8 ± 11.8 | 14.7 ± 9.5 | 0.675 |
| Hct (%) | 28.2 ± 7.3 | 29.7 ± 6.5 | 0.004 |
| Platelets, 103/mm3 | 125.6 ± 105.5 | 180.4 ± 121.8 | <0.001 |
| Albumin, g/dL | 2.4 ± 0.6 | 2.6 ± 0.5 | <0.001 |
| Creatinine, mg/dL | 2.1 ± 1.9 | 2.7 ± 15.0 | 0.579 |
| Procalcitonin, ng/mL | 1.8 ± 2.2 | 1.5 ± 2.0 | 0.781 |
| CRP, mg/L | 125.2 ± 108.1 | 111.1 ± 96.7 | 0.045 |
| CRP/Albumin ratio | 50.9 ± 54.3 | 43.9 ± 46.9 | 0.032 |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.00 | 0.99–1.02 | 0.601 |
| Sex, F/M (%) | |||
| Male | Reference | Reference | Reference |
| Female | 1.18 | 0.83–1.66 | 0.358 |
| BMI | 0.99 | 0.96–1.01 | 0.254 |
| APACHE II score | 1.06 | 1.04–1.08 | <0.001 |
| Underlying diseases | |||
| Cancer | 1.59 | 1.11–2.30 | 0.012 |
| CRP/Albumin | 1.01 | 1.00–1.02 | 0.001 |
| Variables | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.00 | 0.99–1.01 | 0.992 |
| Sex | |||
| Male | Reference | Reference | Reference |
| Female | 1.23 | 0.93–1.61 | 0.142 |
| BMI | |||
| APACHE II score | 1.05 | 1.04–1.07 | <0.001 |
| Underlying diseases | |||
| Cancer | 1.30 | 0.98–1.71 | 0.071 |
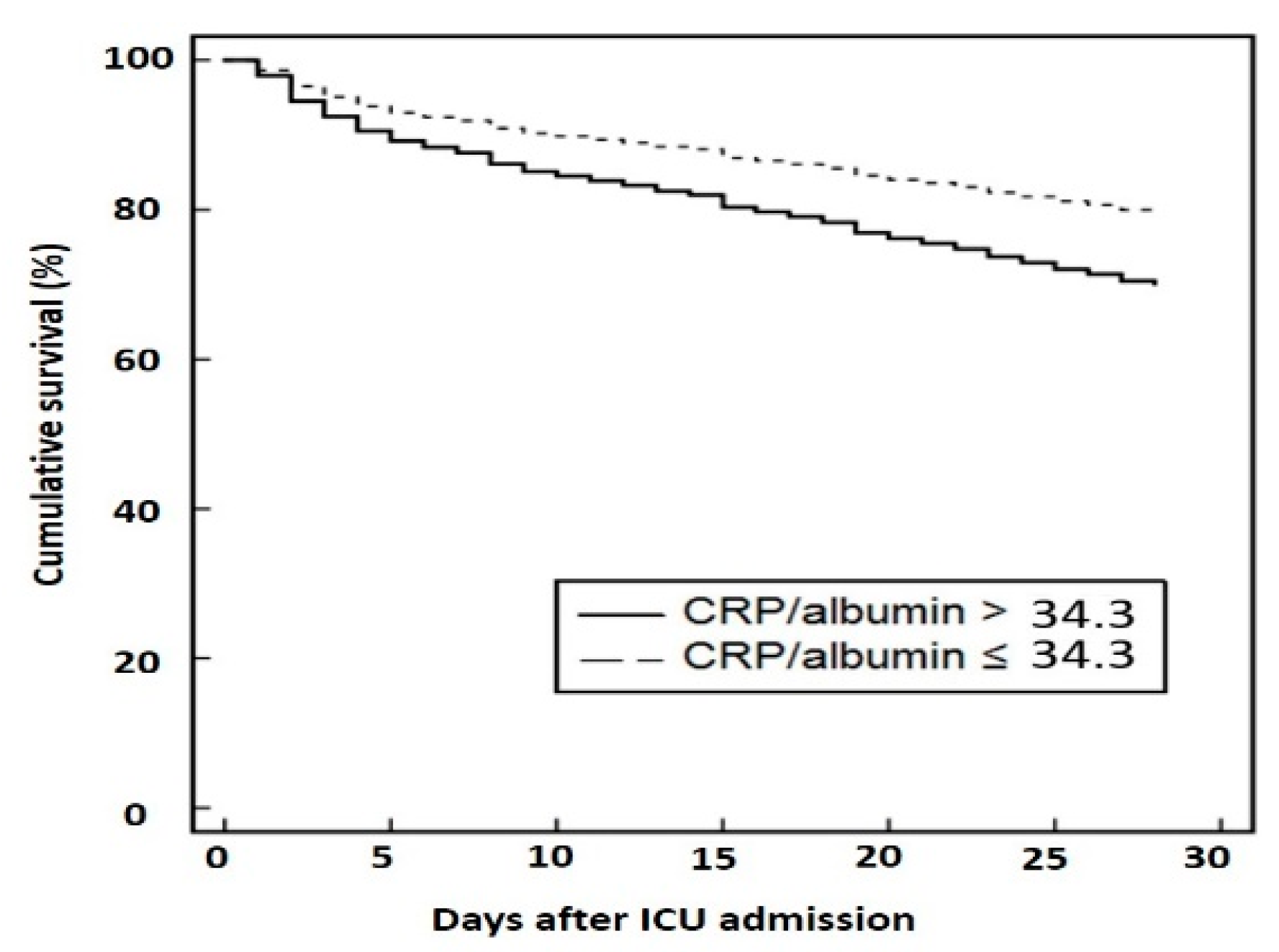
| CRP/Albumin | |||
| Low (≤34.3) | Reference | Reference | Reference |
| High (>34.3) | 1.68 | 1.27–2.21 | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.E.; Chung, K.S.; Song, J.H.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; Chang, J.; et al. The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Critically Ill Patients. J. Clin. Med. 2018, 7, 333. https://doi.org/10.3390/jcm7100333
Park JE, Chung KS, Song JH, Kim SY, Kim EY, Jung JY, Kang YA, Park MS, Kim YS, Chang J, et al. The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Critically Ill Patients. Journal of Clinical Medicine. 2018; 7(10):333. https://doi.org/10.3390/jcm7100333
Chicago/Turabian StylePark, Ji Eun, Kyung Soo Chung, Joo Han Song, Song Yee Kim, Eun Young Kim, Ji Ye Jung, Young Ae Kang, Moo Suk Park, Young Sam Kim, Joon Chang, and et al. 2018. "The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Critically Ill Patients" Journal of Clinical Medicine 7, no. 10: 333. https://doi.org/10.3390/jcm7100333
APA StylePark, J. E., Chung, K. S., Song, J. H., Kim, S. Y., Kim, E. Y., Jung, J. Y., Kang, Y. A., Park, M. S., Kim, Y. S., Chang, J., & Leem, A. Y. (2018). The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Critically Ill Patients. Journal of Clinical Medicine, 7(10), 333. https://doi.org/10.3390/jcm7100333





