Prescribing Hemodialysis or Hemodiafiltration: When One Size Does Not Fit All the Proposal of a Personalized Approach Based on Comorbidity and Nutritional Status
Abstract
1. Diet and Hemodialysis Prescription: A Necessary Integration
2. Tolerance beyond Depuration
- Dialysis tolerance (intradialysis hypotension, post-dialysis fatigue and subjective evaluation of the effect of dialysis on quality of life) [69].
- Dialysis frequency;
- Dialysis duration;
- Dialysis modality (hemodialysis (HD) or hemodiafiltration (HDF))
- Type of membrane (permeability; surface);
- Blood and dialysate flow (in HDF: pre- or post-dilutional modality);
- anticoagulation.
3. Arbitrary (or Unproven) Assumptions
- Different dialyzers in the same category are equivalent (high-, medium- or low-flux) in terms of performance and albumin leakage (while this is not entirely true, a detailed discussion is beyond the scope of this review);
- Loss of albumin is higher in the first minutes of HDF, supporting the choice of low-permeability membranes in the case of more frequent dialysis [74];
- Loss of albumin is also a marker of loss of other potentially useful nutrients, including vitamins; such a loss may contribute to malnutrition;
- Adsorption by dialysis membranes is not a relevant element in the removal of uremic toxins; if present, it is similar in similar categories of dialyzers [78].
4. Nutritional Markers and Integrated Scores
5. Patient Categorization
6. Good Nutritional Status, Good Clinical Condition, Low MIS
7. Poor Nutritional Status, Poor Clinical Condition, High MIS
8. Discrepant Measures of Nutritional Status, Clinical Condition, MIS
9. Vascular Access and Anticoagulation
10. Dialysis Initiation and Residual Renal Function
11. What This Review Did Not Address
12. Conclusions and Suggestions for Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nistor, I.; Palmer, S.C.; Craig, J.C.; Saglimbene, V.; Vecchio, M.; Covic, A.; Strippoli, G.F. Haemodiafiltration, haemofiltration and haemodialysis for end-stage kidney disease. Cochrane Database Syst. Rev. 2015, CD006258. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, T.A.; Zawada, E.T., Jr.; Stacy, W.K. Hemodialysis. Basic principles and practice. Postgrad. Med. 1985, 77, 95–101 & 104. [Google Scholar] [CrossRef] [PubMed]
- Misra, M. Basic mechanisms governing solute and fluid transport in hemodialysis. Hemodial. Int. 2008, 12 (Suppl. 2), S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Hingwala, J.; Tangri, N.; Rigatto, C.; Komenda, P. Improving the quality and efficiency of conventional in-center hemodialysis. Semin. Dial. 2015, 28, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.K. Phosphate is a uremic toxin. J. Ren. Nutr. 2008, 18, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Lorenzo, V. Parathyroid hormone, a uremic toxin. Semin. Dial. 2009, 22, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Van Laecke, S.; Glorieux, G. The middle-molecule hypothesis 30 years after: Lost and rediscovered in the universe of uremic toxicity? J. Nephrol. 2008, 21, 146–160. [Google Scholar] [PubMed]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J.; European Uremic Toxin Work Group. A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J. Uremic toxins: What are they? An integrated overview of pathobiology and classification. J. Ren. Nutr. 2008, 18, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Azar, A.T.; Wahba, K.; Mohamed, A.S.; Massoud, W.A. Association between dialysis dose improvement and nutritional status among hemodialysis patients. Am. J. Nephrol. 2007, 27, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Combe, C.; Chauveau, P.; Laville, M.; Fouque, D.; Azar, R.; Cano, N.; Canaud, B.; Roth, H.; Leverve, X.; Aparicio, M.; et al. Influence of nutritional factors and hemodialysis adequacy on the survival of 1610 French patients. Am. J. Kidney Dis. 2001, 37 (Suppl. 2), S81–S88. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Chen, S.-W.; Chiang, C.-H.; Wang, M.; Peng, S.-J.; Kan, Y.-T. Effects of increasing dialysis dose on serum albumin and mortality in hemodialysis patients. Am. J. Kidney Dis. 1996, 27, 380–386. [Google Scholar] [CrossRef]
- Oreopoulos, D.G. Beyond Kt/V: Redefining adequacy of dialysis in the 21st century. Int. Urol. Nephrol. 2002, 34, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Taskapan, H.; Esbaei, K.; Jassal, S.V.; Bargman, J.M.; Oreopoulos, D.G. K/DOQI guideline requirements for calcium, phosphate, calcium phosphate product, and parathyroid hormone control in dialysis patients: Can we achieve them? Int. Urol. Nephrol. 2006, 38, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Kimata, N.; Akiba, T.; Pisoni, R.L.; Albert, J.M.; Satayathum, S.; Cruz, J.M.; Akizawa, T.; Andreucci, V.E.; Young, E.W.; Port, F.K. Mineral metabolism and haemoglobin concentration among haemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2005, 20, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Casino, F.G.; Pedrini, L.A.; Santoro, A.; Mandolfo, S.; David, S.; De Cristofaro, V.; Teatini, U.; Lomonte, C.; Lopez, T. A simple approach for assessing equilibrated Kt/V beta 2-M on a routine basis. Nephrol. Dial. Transplant. 2010, 25, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Kazama, J.J. Japanese Society of Dialysis Therapy treatment guidelines for secondary hyperparathyroidism. Ther. Apher. Dial. 2007, 11 (Suppl. 1), S44–S47. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Barbieri, C.; Marcelli, D.; Bellocchio, F.; Bowry, S.; Mari, F.; Amato, C.; Gatti, E. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int. 2015, 88, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. Hemodialysis Treatment Time: As Important as it Seems? Semin. Dial. 2017, 30, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, J. Hemodialysis Time and Kt/V: Less May Be Better. Semin. Dial. 2017, 30, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Buoncristiani, U.; Canaud, B.; Köhler, H.; Petitclerc, T.; Zucchelli, P. Dialysis dose and frequency. Nephrol. Dial. Transplant. 2005, 20, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.S.; Charra, B.; Pierratos, A.; Work, J. In search of ideal hemodialysis: Is prolonged frequent dialysis the answer? Am. J. Kidney Dis. 1999, 34, 597–610. [Google Scholar] [CrossRef]
- Twardowski, Z.J. We should strive for optimal hemodialysis: A criticism of the hemodialysis adequacy concept. Hemodial. Int. 2003, 7, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Gillon, R. Medical ethics: Four principles plus attention to scope. BMJ 1994, 309, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Pullman, D. Ethics first aid: Reframing the role of “principlism” in clinical ethics education and practice. J. Clin. Ethics 2005, 16, 223–229. [Google Scholar] [PubMed]
- Piccoli, G.B.; Sofronie, A.C.; Coindre, J.P. The strange case of Mr. H. Starting dialysis at 90 years of age: Clinical choices impact on ethical decisions. BMC Med. Ethics 2017, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.A.; Branley, P.; Bulfone, L.; Collins, J.F.; Craig, J.C.; Fraenkel, M.B.; Harris, A.; Johnson, D.W.; Kesselhut, J.; Li, J.J.; et al. A randomized, controlled trial of early versus late initiation of dialysis. N. Engl. J. Med. 2010, 363, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Nacak, H.; Bolignano, D.; Van Diepen, M.; Dekker, F.; Van Biesen, W. Timing of start of dialysis in diabetes mellitus patients: A systematic literature review. Nephrol. Dial. Transplant. 2016, 31, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Altamimi, S.; Ashkar, M.; Balk, E.M.; Stel, V.S.; Wright, S.; Jaber, B.L. GFR at initiation of dialysis and mortality in CKD: A meta-analysis. Am. J. Kidney Dis. 2012, 59, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Rivara, M.B.; Mehrotra, R. Timing of Dialysis Initiation: What Has Changed Since IDEAL? Semin. Nephrol. 2017, 37, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.F.; Na, Y.; Rosansky, S.J.; Sontrop, J.M.; Macnab, J.J.; Glassock, R.J.; Eggers, P.W.; Jackson, K.; Moist, L. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. Can. Med. Assoc. J. 2011, 183, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Susantitaphong, P.; Isaranuwatchai, S.; Chenbhanich, J.; Eiam-Ong, S.; Jaber, B.L. Dialysis Therapy and Conservative Management of Advanced Chronic Kidney Disease in the Elderly: A Systematic Review. Nephron 2017. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, N.R.; Kumar, P. Conservative management of end-stage renal disease without dialysis: A systematic review. J. Palliat. Med. 2012, 15, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Sousa, C.; Teles, P.; Coelho, A.; Xavier, E. Frequency of intradialytic hypotensive episodes: Old problem, new insights. J. Am. Soc. Hypertens. 2015, 9, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. How can we prevent intradialytic hypotension? Curr. Opin. Nephrol. Hypertens. 2012, 21, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, Z.J. Treatment time and ultrafiltration rate are more important in dialysis prescription than small molecule clearance. Blood Purif. 2007, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Golper, T.A. Incremental dialysis: Review of recent literature. Curr. Opin. Nephrol. Hypertens. 2017. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Crowley, S.T.; Beddhu, S.; Chen, J.L.; Daugirdas, J.T.; Goldfarb, D.S.; Jin, A.; Kovesdy, C.P.; Leehey, D.J.; Moradi, H.; et al. Renal Replacement Therapy and Incremental Hemodialysis for Veterans with Advanced Chronic Kidney Disease. Semin. Dial. 2017, 30, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ramirez, S.; Anand, S.; Qian, J.; Zuo, L. Twice-Weekly Hemodialysis in China: Can It Be A Better Option for Initiation or Maintenance Dialysis Therapy? Semin. Dial. 2017, 30, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Basile, C.; Casino, F.G.; Kalantar-Zadeh, K. Is incremental hemodialysis ready to return on the scene? From empiricism to kinetic modelling. J. Nephrol. 2017, 30, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Park, J.T.; Kim, Y.L.; Kang, S.W.; Yang, C.W.; Kim, N.H.; Oh, Y.K.; Lim, C.S.; Kim, Y.S.; Lee, J.P.; et al. Comparison of outcomes between the incremental and thrice-weekly initiation of hemodialysis: A propensity-matched study of a prospective cohort in Korea. Nephrol. Dial. Transplant. 2017, 32, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Bolasco, P.; Cupisti, A.; Locatelli, F.; Caria, S.; Kalantar-Zadeh, K. Dietary Management of Incremental Transition to Dialysis Therapy: Once-Weekly Hemodialysis Combined with Low-Protein Diet. J. Ren. Nutr. 2016, 26, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Nesrallah, G.E.; Mustafa, R.A.; Clark, W.F.; Bass, A.; Barnieh, L.; Hemmelgarn, B.R.; Klarenbach, S.; Quinn, R.R.; Hiremath, S.; Ravani, P.; et al. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. Can. Med. Assoc. J. 2014, 186, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Qian, Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Hladunewich, M.A.; Hou, S.; Odutayo, A.; Cornelis, T.; Pierratos, A.; Goldstein, M.; Tennankore, K.; Keunen, J.; Hui, D.; Chan, C.T. Intensive hemodialysis associates with improved pregnancy outcomes: A Canadian and United States cohort comparison. J. Am. Soc. Nephrol. 2014, 25, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Minelli, F.; Versino, E.; Cabiddu, G.; Attini, R.; Vigotti, F.N.; Rolfo, A.; Giuffrida, D.; Colombi, N.; Pani, A.; et al. Pregnancy in dialysis patients in the new millennium: A systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol. Dial. Transplant. 2016, 31, 1915–1934. [Google Scholar] [CrossRef] [PubMed]
- Tennankore, K.K.; Na, Y.; Wald, R.; Chan, C.T.; Perl, J. Short daily-, nocturnal- and conventional-home hemodialysis have similar patient and treatment survival. Kidney Int. 2018, 93, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Pierratos, A. Daily nocturnal hemodialysis—A paradigm shift worthy of disrupting current dialysis practice. Nat. Clin. Pract. Nephrol. 2008, 4, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Kjellstrand, C.; Buoncristiani, U.; Ting, G.; Traeger, J.; Piccoli, G.B.; Sibai-Galland, R.; Young, B.A.; Blagg, C.R. Survival with short-daily hemodialysis: Association of time, site, and dose of dialysis. Hemodial. Int. 2010, 14, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Cabiddu, G.; Moio, M.R.; Fois, A.; Cao, R.; Molfino, I.; Kaniassi, A.; Lippi, F.; Froger, L.; Pani, A.; et al. Efficiency and nutritional parameters in an elderly high risk population on hemodialysis and hemodiafiltration in Italy and France: Different treatments with similar names? BMC Nephrol. 2018, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Sum, S.S.; Marcus, A.F.; Blair, D.; Olejnik, L.A.; Cao, J.; Parrott, J.S.; Peters, E.N.; Hand, R.K.; Byham-Gray, L.D. Comparison of Subjective Global Assessment and Protein Energy Wasting Score to Nutrition Evaluations Conducted by Registered Dietitian Nutritionists in Identifying Protein Energy Wasting Risk in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2017, 27, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Cuppari, L.; Campbell, K.L.; Avesani, C.M. Nutritional assessment of elderly patients on dialysis: Pitfalls and potentials for practice. Nephrol. Dial. Transplant. 2017. [Google Scholar] [CrossRef] [PubMed]
- Riella, M.C. Nutritional evaluation of patients receiving dialysis for the management of protein-energy wasting: What is old and what is new? J. Ren. Nutr. 2013, 23, 195–198. [Google Scholar] [CrossRef] [PubMed]
- van Zuijdewijn, C.L.D.R.; ter Wee, P.M. Assessment of Protein-Energy Wasting: Quest for the Gold Standard. J. Ren. Nutr. 2016, 26, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.; Bernardini, J.; Piraino, B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am. J. Kidney Dis. 2001, 37, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Cillo, N.; Cirillo, M.; De Santo, N.G. Charlson Comorbidity Index is a predictor of outcomes in incident hemodialysis patients and correlates with phase angle and hospitalization. Int. J. Artif. Organs 2004, 27, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Leavey, S.F.; Strawderman, R.L.; Jones, C.A.; Port, F.K.; Held, P.J. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am. J. Kidney Dis. 1998, 31, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Farage, N.E.; Azevedo, D.L.; Viana, G.G.; Mattos, J.P.; Velarde, L.G.; Fouque, D. Impact of serum albumin and body-mass index on survival in hemodialysis patients. Int. Urol. Nephrol. 2007, 39, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Johansen, K.L.; Lew, N.; Lazarus, J.M.; Lowrie, E.G. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000, 57, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Tordoir, J.H. Dialysis: Vascular access type defines survival in patients on dialysis. Nat. Rev. Nephrol. 2011, 7, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Perl, J.; Wald, R.; McFarlane, P.; Bargman, J.M.; Vonesh, E.; Na, Y.; Jassal, S.V.; Moist, L. Hemodialysis vascular access modifies the association between dialysis modality and survival. J. Am. Soc. Nephrol. 2011, 22, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Heimbürger, O.; Stenvinkel, P.; Qureshi, A.R.; Lindholm, B. Association between residual renal function, inflammation and patient survival in new peritoneal dialysis patients. Nephrol. Dial. Transplant. 2003, 18, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Yang, J.Y.; Jung, W.J.; Shin, M.J.; Yang, B.Y.; Song, S.H.; Kwak, I.S.; Seong, E.Y. Significance of residual renal function for phosphate control in chronic hemodialysis patients. Kidney Res. Clin. Pract. 2014, 33, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hecking, M.; Karaboyas, A.; Antlanger, M.; Saran, R.; Wizemann, V.; Chazot, C.; Rayner, H.; Hörl, W.H.; Pisoni, R.L.; Robinson, B.M.; et al. Significance of interdialytic weight gain versus chronic volume overload: Consensus opinion. Am. J. Nephrol. 2013, 38, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.; Cohen, S.D.; Weisbord, S.D. Quality of life in patients with end-stage renal disease treated with hemodialysis: Survival is not enough! J. Nephrol. 2008, 21, S54–S58. [Google Scholar] [PubMed]
- Sousa-Martins, P.; Moura, A.; Madureira, J.; Alija, P.; Oliveira, J.G.; Lopez, M.; Filgueiras, M.; Amado, L.; Sameiro-Faria, M.; Miranda, V.; et al. Risk factors for mortality in end-stage kidney disease patients under online-hemodiafiltration: Three-year follow-up study. Biomarkers 2016, 21, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Imamović, G.; Hrvačević, R.; Kapun, S.; Marcelli, D.; Bayh, I.; Grassmann, A.; Scatizzi, L.; Maslovarić, J.; Canaud, B. Survival of incident patients on high-volume online hemodiafiltration compared to low-volume online hemodiafiltration and high-flux hemodialysis. Int. Urol. Nephrol. 2014, 46, 1191–2001. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Quiroga, B.; Abad, S.; Aragoncillo, I.; Arroyo, D.; Panizo, N.; López-Gómez, J.M. Albumin leakage in online hemodiafiltration, more convective transport, more losses? Ther. Apher. Dial. 2015, 19, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Florens, N.; Juillard, L. Large Middle Molecule and Albumin Removal: Why Should We Not Rest on Our Laurels? Contrib. Nephrol. 2017, 191, 178–187. [Google Scholar] [PubMed]
- Krieter, D.H.; Canaud, B. High permeability of dialysis membranes: What is the limit of albumin loss. Nephrol. Dial. Transplant. 2003, 18, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Tsuchida, K.; Hirose, D.; Michiwaki, H.; Hann, M.; Kanayama, H.O.; Doi, T.; Minakuchi, J. The effect of albumin leakage in hemodialysis patients on redox status of serum albumin. J. Artif. Organs 2016, 19, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Tsuchida, K.; Ishihara, N.; Minagawa, N.; Ichien, G.; Yamada, S.; Hirose, D.; Michiwaki, H.; Kanayama, H.O.; Doi, T.; et al. Implications of Albumin Leakage for Survival in Maintenance Hemodialysis Patients: A 7-year Observational Study. Ther. Apher. Dial. 2017, 21, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Minakuchi, J. Albumin loss under the use of the high-performance membrane. Contrib. Nephrol. 2011, 173, 76–83. [Google Scholar] [PubMed]
- Santoro, A.; Guadagni, G. Dialysis membrane: From convection to adsorption. NDT Plus 2010, 3 (Suppl. 1), i36–i39. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Sirolli, V.; Lupisella, S.; Levi-Mortera, S.; Pavone, B.; Pieroni, L.; Amoroso, L.; Di Vito, R.; Bucci, S.; Bernardini, S.; et al. Proteomic investigations on the effect of different membrane materials on blood protein adsorption during haemodialysis. Blood Transfus. 2012, 10 (Suppl. 2), S101–S112. [Google Scholar] [PubMed]
- Marcason, W. Should Albumin and Prealbumin Be Used as Indicators for Malnutrition? J. Acad. Nutr. Diet. 2017, 117, 1144. [Google Scholar] [CrossRef] [PubMed]
- Santin, F.; Rodrigues, J.; Brito, F.B.; Avesani, C.M. Performance of subjective global assessment and malnutrition inflammation score for monitoring the nutritional status of older adults on hemodialysis. Clin. Nutr. 2018, 37, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.B.; Silva, L.F.; Lopes, G.B.; Penalva, M.A.; Matos, C.M.; Robinson, B.M.; Lopes, A.A. Additional Contribution of the Malnutrition-Inflammation Score to Predict Mortality and Patient-Reported Outcomes as Compared With Its Components in a Cohort of African Descent Hemodialysis Patients. J. Ren. Nutr. 2017, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Wolfenden, R.C.; Wells, L.M. Is subjective global assessment a reliable measure of nutritional status in hemodialysis? J. Ren. Nutr. 2004, 14, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Gurreebun, F.; Hartley, G.H.; Brown, A.L.; Ward, M.C.; Goodship, T.H. Nutritional screening in patients on hemodialysis: Is subjective global assessment an appropriate tool? J. Ren. Nutr. 2007, 17, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Eriguchi, R.; Obi, Y.; Streja, E.; Tortorici, A.R.; Rhee, C.M.; Soohoo, M.; Kim, T.; Kovesdy, C.P.; Kalantar-Zadeh, K. Longitudinal Associations among Renal Urea Clearance-Corrected Normalized Protein Catabolic Rate, Serum Albumin, and Mortality in Patients on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Sreedhara, R.; Avram, M.M.; Blanco, M.; Batish, R.; Avram, M.M.; Mittman, N. Prealbumin is the best nutritional predictor of survival in hemodialysis and peritoneal dialysis. Am. J. Kidney Dis. 1996, 28, 937–942. [Google Scholar] [CrossRef]
- Takahashi, R.; Ito, Y.; Takahashi, H.; Ishii, H.; Kasuga, H.; Mizuno, M.; Suzuki, Y.; Yuzawa, Y.; Maruyama, S.; Murohara, T.; et al. Combined values of serum albumin, C-reactive protein and body mass index at dialysis initiation accurately predicts long-term mortality. Am. J. Nephrol. 2012, 36, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Accuracy and limitations of the diagnosis of malnutrition in dialysis patients. Semin. Dial. 2012, 25, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Inoue, K.; Shimizu, K.; Hiraga, K.; Takahashi, E.; Otaki, K.; Yoshikawa, T.; Furuta, K.; Tokunaga, C.; Sakakibara, T.; et al. Comparison of Nutritional Risk Scores for Predicting Mortality in Japanese Chronic Hemodialysis Patients. J. Ren. Nutr. 2017, 27, 201–206. [Google Scholar] [CrossRef] [PubMed]
- De Roij van Zuijdewijn, C.L.; ter Wee, P.M.; Chapdelaine, I.; Bots, M.L.; Blankestijn, P.J.; van den Dorpel, M.A.; Nubé, M.J.; Grooteman, M.P. A Comparison of 8 Nutrition-Related Tests to Predict Mortality in Hemodialysis Patients. J. Ren. Nutr. 2015, 25, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, J.; Yamagata, K.; Nishi, S.; Nakai, S.; Masakane, I.; Iseki, K.; Tsubakihara, Y. Significance of the decreased risk of dialysis-related amyloidosis now proven by results from Japanese nationwide surveys in 1998 and 2010. Nephrol. Dial. Transplant. 2016, 31, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Minakuchi, J. Effect of large-size dialysis membrane and hemofiltration/hemodiafiltration methods on long-term dialysis patients. Contrib. Nephrol. 2011, 168, 179–187. [Google Scholar] [PubMed]
- Fujimori, A. Beta-2-microglobulin as a uremic toxin: The Japanese experience. Contrib. Nephrol. 2011, 168, 129–133. [Google Scholar] [PubMed]
- Thomas, G.; Jaber, B.L. Convective therapies for removal of middle molecular weight uremic toxins in end-stage renal disease: A review of the evidence. Semin. Dial. 2009, 22, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.B.; Massy, Z.A. Beta2-microglobulin. Semin. Dial. 2009, 22, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Koehler, K.; Bowry, S.; Stuard, S. What Is the Optimal Target Convective Volume in On-Line Hemodiafiltration Therapy? Electrolyte Blood Press 2016, 14, 1–4. [Google Scholar]
- Canaud, B. Online hemodiafiltration. Technical options and best clinical practices. Contrib. Nephrol. 2007, 158, 110–122. [Google Scholar] [PubMed]
- Ahrenholz, P.G.; Winkler, R.E.; Michelsen, A.; Lang, D.A.; Bowry, S.K. Dialysis membrane-dependent removal of middle molecules during hemodiafiltration: The beta2-microglobulin/albumin relationship. Clin. Nephrol. 2004, 62, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Schiffl, H. High-flux dialyzers, backfiltration, and dialysis fluid quality. Semin. Dial. 2011, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schiffl, H. Prospective randomized cross-over long-term comparison of online haemodiafiltration and ultrapure high-flux haemodialysis. Eur. J. Med. Res. 2007, 12, 26–33. [Google Scholar] [PubMed]
- Van Biesen, W.; Vanholder, R.; Schepers, E.; Glorieux, G.; Dhondt, A.; Eloot, S. The Place of Large Pore Membranes in the Treatment Portfolio of Patients on Hemodialysis. Contrib. Nephrol. 2017, 191, 168–177. [Google Scholar] [PubMed]
- Macías, N.; Vega, A.; Abad, S.; Santos, A.; Cedeño, S.; Linares, T.; García-Prieto, A.M.; Aragoncillo, I.; Yuste, C.; López-Gómez, J.M. Is High-Volume Online Hemodiafiltration Associated with Malnutrition? Ther. Apher. Dial. 2017, 21, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Hsu, C.W.; Hu, C.C.; Yen, T.H.; Huang, W.H. Association Between Hemodiafiltration and Hypoalbuminemia in Middle-Age Hemodialysis Patients. Medicine 2016, 95, e3334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Mao, J.R.; Chen, H.; Su, W.; Zhang, Y.; Zhang, L.; Chen, D.Q.; Zhao, Y.Y.; Vaziri, N.D. Removal of uremic retention products by hemodialysis is coupled with indiscriminate loss of vital metabolites. Clin. Biochem. 2017, 50, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Cobo, G.; Lindholm, B.; Stenvinkel, P. Inflammation and Protein-Energy Wasting in the Uremic Milieu. Contrib. Nephrol. 2017, 191, 58–71. [Google Scholar] [PubMed]
- Piccoli, G.B.; Moio, M.R.; Fois, A.; Sofronie, A.; Gendrot, L.; Cabiddu, G.; D’Alessandro, C.; Cupisti, A. The Diet and Haemodialysis Dyad: Three Eras, Four Open Questions and Four Paradoxes. A Narrative Review, Towards a Personalized, Patient-Centered Approach. Nutrients 2017, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Minakuchi, J. Clinical benefits of predilution on-line hemodiafiltration. Contrib. Nephrol. 2007, 158, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Park, S. Confronting Practical Problems for Initiation of On-line Hemodiafiltration Therapy. Blood Purif. 2013, 35 (Suppl. 1), 18–22. [Google Scholar] [CrossRef] [PubMed]
- Morfin, J.A.; Fluck, R.J.; Weinhandl, E.D.; Kansal, S.; McCullough, P.A.; Komenda, P. Intensive Hemodialysis and Treatment Complications and Tolerability. Am. J. Kidney Dis. 2016, 68, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pei, X.; Zhao, W. Timing of Dialysis Initiation and Mortality Risk in Chronic Kidney Disease: A Meta-Analysis. Ther. Apher. Dial. 2018. [Google Scholar] [CrossRef] [PubMed]
- A “New Normal”: Life on Dialysis—The First 90 Days. Available online: https://www.kidney.org/sites/default/files/docs/11-10-0307_dialysistransitionbk2_oct07_lr_bm.pdf (accessed on 6 August 2018).
- Machowska, A.; Alscher, M.D.; Vanga, S.R.; Koch, M.; Aarup, M.; Qureshi, A.R.; Lindholm, B.; Rutherford, P. Offering Patients Therapy Options in Unplanned Start (OPTiONS): Implementation of an educational program is feasible and effective. BMC Nephrol. 2017, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, R.; Roshanravan, B. Management of Physical Frailty in Patients Requiring Hemodialysis Therapy. Contrib. Nephrol. 2018, 196, 101–109. [Google Scholar] [PubMed]
- Johansen, K.L. The Frail Dialysis Population: A Growing Burden for the Dialysis Community. Blood Purif. 2015, 40, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Jaber, B.L.; Lee, Y.; Collins, A.J.; Hull, A.R.; Kraus, M.A.; McCarthy, J.; Miller, B.W.; Spry, L.; Finkelstein, F.O.; FREEDOM Study Group. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: Interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am. J. Kidney Dis. 2010, 56, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Bechis, F.; Pozzato, M.; Ettari, G.; Alloatti, S.; Vischi, M.; Mezza, E.; Iacuzzo, C.; Quaglia, M.; Burdese, M.; et al. Daily Dialysis: Toward a New Standard in Well-Being. Hemodial. Int. 2001, 5, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Lévesque, R.; Krieter, D.; Desmeules, S.; Chalabi, L.; Moragués, H.; Morena, M.; Cristol, J.P. On-line hemodiafiltration as routine treatment of end-stage renal failure: Why pre- or mixed dilution mode is necessary in on-line hemodiafiltration today? Blood Purif. 2004, 22 (Suppl. 2), 40–48. [Google Scholar] [CrossRef] [PubMed]
- Blankestijn, P.J.; Davenport, A.; Basile, C.; Locatelli, F.; Maduell, F.; Mitra, S.; Ronco, C.; Shroff, R.; Tattersall, J.; Wanner, C. Optimization of the convection volume in online post-dilution haemodiafiltration: Practical and technical issues. Clin. Kidney J. 2015, 8, 191–198. [Google Scholar]
- Ikizler, T.A. A patient with CKD and poor nutritional status. Clin. J. Am. Soc. Nephrol. 2013, 8, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Rocchetti, M.T.; Scatena, A.; Rosati, A.; Migliori, M.; Pizzarelli, F.; Gesualdo, L.; REDERT Study Group. Long term variation of serum levels of uremic toxins in patients treated by post-dilution high volume on-line hemodiafiltration in comparison to standard low-flux bicarbonate dialysis: Results from the REDERT study. J. Nephrol. 2017, 30, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Potier, J.; Le Roy, F.; Faucon, J.P.; Besselièvre, T.; Renaudineau, E.; Farquet, C.; Soihan, P.; Touzard, D.; Djema, A.; Ilinca, T. Elevated removal of middle molecules without significant albumin loss with mixed-dilution hemodiafiltration for patients unable to provide sufficient blood flow rates. Blood Purif. 2013, 36, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Casino, F.G.; Basile, C. How to set the stage for a full-fledged clinical trial testing ‘incremental haemodialysis’. Nephrol. Dial. Transplant. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.T.; Fishbane, S.; Obi, Y.; Kalantar-Zadeh, K. Preservation of residual kidney function in hemodialysis patients: Reviving an old concept. Kidney Int. 2016, 90, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Toth-Manikowski, S.M.; Mullangi, S.; Hwang, S.; Shafi, T. Incremental short daily home hemodialysis: A case series. BMC Nephrol. 2017, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Shafi, T.; Mullangi, S.; Toth-Manikowski, S.M.; Hwang, S.; Michels, W.M. Residual Kidney Function: Implications in the Era of Personalized Medicine. Semin. Dial. 2017, 30, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, C. Advances in Understanding and Management of Residual Renal Function in Patients with Chronic Kidney Disease. Kidney Dis. 2017, 2, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, J.; Grantham, J.J. Residual renal function: A paradigm shift. Kidney Int. 2017, 91, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Shafi, T.; Jaar, B.G.; Plantinga, L.C.; Fink, N.E.; Sadler, J.H.; Parekh, R.S.; Powe, N.R.; Coresh, J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am. J. Kidney Dis. 2010, 56, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Belmouaz, M.; Diolez, J.; Bauwens, M.; Duthe, F.; Ecotiere, L.; Desport, E.; Bridoux, F. Comparison of hemodialysis with medium cut-off dialyzer and on-line hemodiafiltration on the removal of small and middle-sized molecules. Clin. Nephrol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Block, G.; Humphreys, M.H.; Kopple, J.D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003, 63, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D. Obesity paradox in patients on maintenance dialysis. Contrib. Nephrol. 2006, 151, 57–69. [Google Scholar] [PubMed]
- Rangel, A.V.; Kim, J.C.; Kaushik, M.; Garzotto, F.; Neri, M.; Cruz, D.N.; Ronco, C. Backfiltration: Past, present and future. Contrib. Nephrol. 2011, 175, 35–45. [Google Scholar] [PubMed]
- Kirsch, A.H.; Lyko, R.; Nilsson, L.G.; Beck, W.; Amdahl, M.; Lechner, P.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2017, 32, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lorenzin, A.; Neri, M.; Clark, W.R.; Garzotto, F.; Brendolan, A.; Nalesso, F.; Marchionna, N.; Zanella, M.; Sartori, M.; Fiore, G.B.; et al. Modeling of Internal Filtration in Theranova Hemodialyzers. Contrib. Nephrol. 2017, 191, 127–141. [Google Scholar] [PubMed]
- Calabia, J.; Arcos, E.; Carrero, J.J.; Comas, J.; Vallés, M. Does the obesity survival paradox of dialysis patients differ with age? Blood Purif. 2015, 39, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, T.; Mehrotra, R.; Park, J.; Streja, E.; Dukkipati, R.; Nissenson, A.R.; Ma, J.Z.; Kovesdy, C.P.; Kalantar-Zadeh, K. Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. Am. J. Kidney Dis. 2014, 63, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Androga, L.; Sharma, D.; Amodu, A.; Abramowitz, M.K. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int. Rep. 2017, 2, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Kittiskulnam, P.; Carrero, J.J.; Chertow, G.M.; Kaysen, G.A.; Delgado, C.; Johansen, K.L. Sarcopenia among patients receiving hemodialysis: Weighing the evidence. J. Cachexia Sarcopenia Muscle 2017, 8, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Brunani, A.; Savia, G.; Pillon, L.; Favaro, E.; Berselli, M.E.; Cavagnini, F. Discriminating between body fat and fluid changes in the obese adult using bioimpedance vector analysis. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; De Nicola, L.; Pecoraro, P.; Trio, R.; Di Paola, F.; Iodice, C.; Bellizzi, V.; Memoli, B. Abnormalities of bioimpedance measures in overweight and obese hemodialyzed patients. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Torun, D.; Micozkadioglu, H.; Torun, N.; Ozelsancak, R.; Sezer, S.; Adam, F.U.; Ozdemir, F.N.; Haberal, M. Increased body mass index is not a reliable marker of good nutrition in hemodialysis patients. Ren. Fail. 2007, 29, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, A.K.; Fleischmann, E.H.; Bower, J.D. Impact of lower delivered Kt/V on the survival of overweight patients on hemodialysis. Kidney Int. 1999, 56, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A. Differences in prescribed Kt/V and delivered haemodialysis dose—Why obesity makes a difference to survival for haemodialysis patients when using a ‘one size fits all’ Kt/V target. Nephrol. Dial. Transplant. 2013, 28 (Suppl. 4), iv219–iv223. [Google Scholar] [CrossRef] [PubMed]
- Segall, L.; Moscalu, M.; Hogaş, S.; Mititiuc, I.; Nistor, I.; Veisa, G.; Covic, A. Protein-energy wasting, as well as overweight and obesity, is a long-term risk factor for mortality in chronic hemodialysis patients. Int. Urol. Nephrol. 2014, 46, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A. Using and interpreting serum albumin and prealbumin as nutritional markers in patients on chronic dialysis. Semin. Dial. 2014, 27, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.A.; Nissenson, A.; Chertow, G.M.; Farid, M.; Singh, I.; Van Oijen, M.G.; Esrailian, E.; Solomon, M.D.; Spiegel, B.M. The relationship between laboratory-based outcome measures and mortality in end-stage renal disease: A systematic review. Hemodial. Int. 2009, 13, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Herselman, M.; Esau, N.; Kruger, J.M.; Labadarios, D.; Moosa, M.R. Relationship between serum protein and mortality in adults on long-term hemodialysis: Exhaustive review and meta-analysis. Nutrition 2010, 26, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Sinuani, I.; Azar, A.; Shapiro, G.; Feldman, L.; Stav, K.; Sandbank, J.; Averbukh, Z. Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition 2015, 31, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Obi, Y.; Streja, E.; Rhee, C.M.; Catabay, C.J.; Vaziri, N.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum uric acid, protein intake and mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2017, 32, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Lines, S.W.; Richardson, V.R.; Thomas, B.; Dunn, E.J.; Wright, M.J.; Carter, A.M. Complement and cardiovascular disease—The missing link in haemodialysis patients? Nephron 2016, 132, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Marçais, C.; Maucort-Boulch, D.; Drai, J.; Dantony, E.; Carlier, M.C.; Blond, E.; Genet, L.; Kuentz, F.; Lataillade, D.; Legrand, E.; et al. Circulating Klotho Associates with Cardiovascular Morbidity and Mortality During Hemodialysis. J. Clin. Endocrinol. Metab. 2017, 102, 3154–3161. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.; Galassi, A.; Capelli, I.; Schillaci, R.; La Manna, G.; Cozzolino, M. Klotho-FGF23, Cardiovascular Disease, and Vascular Calcification: Black or White? Curr. Vasc. Pharmacol. 2018, 16, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Artunc, F.; Nowak, A.; Müller, C.; Peter, A.; Heyne, N.; Häring, H.U.; Friedrich, B. Mortality prediction using modern peptide biomarkers in hemodialysis patients—A comparative analysis. Kidney Blood Press Res. 2014, 39, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Marthi, A.; Donovan, K.; Haynes, R.; Wheeler, D.C.; Baigent, C.; Rooney, C.M.; Landray, M.J.; Moe, S.M.; Yang, J.; Holland, L.; et al. Fibroblast Growth Factor-23 and Risks of Cardiovascular and Noncardiovascular Diseases: A Meta-Analysis. J. Am. Soc. Nephrol. 2018, 29, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Krzanowski, M.; Krzanowska, K.; Dumnicka, P.; Gajda, M.; Woziwodzka, K.; Fedak, D.; Grodzicki, T.; Litwin, J.A.; Sułowicz, W. Elevated Circulating Osteoprotegerin Levels in the Plasma of Hemodialyzed Patients with Severe Artery Calcifications. Ther. Apher. Dial. 2018. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Kao, W.H.; Crainiceanu, C.; Sozio, S.M.; Oberai, P.C.; Shafi, T.; Coresh, J.; Powe, N.R.; Plantinga, L.C.; Jaar, B.G.; et al. Biomarkers of vascular calcification and mortality in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2014, 9, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, T.; Sato, K.; Kawakami, S.; Kiyomoto, M.; Enomoto, M.; Suzuki, T.; Genei, H.; Nakada, H.; Iino, Y.; Katayama, Y. Effects of reduced dialysis fluid flow in hemodialysis. J. Nippon Med. Sch. 2013, 80, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Ponce, P.; Marcelli, D.; Scholz, C.; Wehmeyer, W.; Gonçalves, P.; Grassmann, A.; Brand, K.; Canaud, B. Does the extracorporeal blood flow affect survival of the arteriovenous vascular access? Hemodial. Int. 2015, 19, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Mandolfo, S.; Borlandelli, S.; Imbasciati, E.; Badalamenti, S.; Graziani, G.; Sereni, L.; Varesani, M.; Wratten, M.L.; Corsi, A.; Elli, A. Pilot study to assess increased dialysis efficiency in patients with limited blood flow rates due to vascular access problems. Hemodial. Int. 2008, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mandolfo, S.; Borlandelli, S.; Ravani, P.; Imbasciati, E. How to improve dialysis adequacy in patients with vascular access problems. J. Vasc. Access. 2006, 7, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.J.; Paulson, W.D.; Schwab, S.J. Vascular access as a determinant of adequacy of dialysis. Semin. Nephrol. 2005, 25, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Thamer, M.; Zhang, Q.; Zhang, Y.; Allon, M. Vascular Access Type and Clinical Outcomes among Elderly Patients on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Quinn, R.; Oliver, M.; Kamar, F.; Kabani, R.; Devoe, D.; Mysore, P.; Pannu, N.; MacRae, J.; Manns, B.; et al. Multi-Disciplinary Vascular Access Care and Access Outcomes in People Starting Hemodialysis Therapy. Clin. J. Am. Soc. Nephrol. 2017, 12, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Allon, M. Reassessing Recommendations for Choice of Vascular Access. Clin. J. Am. Soc. Nephrol. 2017, 12, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.K.; Myers, E.R.; Rosas, S.E.; O’Hare, A.M.; Colón-Emeric, C.S. Choice of Hemodialysis Access in Older Adults: A Cost-Effectiveness Analysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Hod, T.; Goldfarb-Rumyantzev, A.S.; Patibandla, B.K.; Narra, A.; Brown, R.S. Second vascular access after failure of the first fistula in the elderly. Clin. Nephrol. 2016, 86, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, H.; Shintaku, S.; Moriishi, M. Vascular access in super-aged patients. J. Vasc. Access. 2015, 16 (Suppl. 10), S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Moureau, F.; Nguyen, P. Anticoagulation in Chronic Hemodialysis: Progress Toward an Optimal Approach. Semin. Dial. 2015, 28, 474–489. [Google Scholar] [PubMed]
- Harel, Z.; Chertow, G.M.; Shah, P.S.; Harel, S.; Dorian, P.; Yan, A.T.; Saposnik, G.; Sood, M.M.; Molnar, A.O.; Perl, J.; et al. Warfarin and the Risk of Stroke and Bleeding in Patients with Atrial Fibrillation Receiving Dialysis: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2017, 33, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Streja, E.; Soohoo, M.; Hanna, M.; Savoj, J.; Kalantar-Zadeh, K.; Lau, W.L. Warfarin Use and Increased Mortality in End-Stage Renal Disease. Am. J. Nephrol. 2017, 46, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Hassan, Z.A.; Chalmin, F.; Vido, S.; Berrada, M.; Verhelst, D.; Donnadieu, P.; Moranne, O.; Esnault, V.L. Vitamin E-Coated and Heparin-Coated Dialyzer Membranes for Heparin-Free Hemodialysis: A Multicenter, Randomized, Crossover Trial. Am. J. Kidney Dis. 2016, 68, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.; Poesen, R.; Evenepoel, P. Heparin-coated dialyzer membranes: Is non-inferiority good enough? Kidney Int. 2014, 86, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Joshi, P.; Kaplan, M.; Lefkovitz, J.; Poenariu, A.; Dworkin, L.D.; Michaud, D.S. Rapid Change in Renal Function Decline Is Associated with Lower Survival and Worse Residual Renal Function Preservation in Peritoneal Dialysis Patients. Perit. Dial. Int. 2017, 37, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Schiffl, H.; Lang, S.M.; Fischer, R. Effects of high efficiency post-dilution on-line hemodiafiltration or conventional hemodialysis on residual renal function and left ventricular hypertrophy. Int. Urol. Nephrol. 2013, 45, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, T.; Koutoku, N. Preservation of residual renal function with HDF. Contrib. Nephrol. 2011, 168, 204–212. [Google Scholar] [PubMed]
- Davenport, A. Measuring residual renal function for hemodialysis adequacy: Is there an easier option? Hemodial. Int. 2017, 21 (Suppl. 2), S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Mor, V.; Mehrotra, R.; Trivedi, A.N. Initial Session Duration and Mortality Among Incident Hemodialysis Patients. Am. J. Kidney Dis. 2017, 70, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Sada, K.E.; Hinamoto, N.; Kimachi, M.; Yamamoto, Y.; Onishi, Y.; Fukuhara, S. Shorter dialysis session length was not associated with lower mental health and physical functioning in elderly hemodialysis patients: Results from the Japan Dialysis Outcome and Practice Patterns Study (J-DOPPS). PLoS ONE 2017, 12, e0184019. [Google Scholar] [CrossRef] [PubMed]
- Mourad, G.; Minguet, J.; Pernin, V.; Garrigue, V.; Peraldi, M.N.; Kessler, M.; Jacquelinet, C.; Couchoud, C.; Duny, Y.; Daurès, J.P. Similar patient survival following kidney allograft failure compared with non-transplanted patients. Kidney Int. 2014, 86, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Markell, M.; Stefanov, D.G.; Timpo, E.; Jindal, R.M.; Nee, R.; Sumrani, N.; John, D.; Tedla, F.; Salifu, M.O. Mortality after Renal Allograft Failure and Return to Dialysis. Am. J. Nephrol. 2017, 45, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.Z.; Ichii, H.; Lineen, J.; Foster, C.E., 3rd; Mathe, Z.; Schiff, J.; Kim, S.J.; Pahl, M.V.; Amin, A.N.; Kalantar-Zadeh, K.; et al. Timing of return to dialysis in patients with failing kidney transplants. Semin. Dial. 2013, 26, 667–674. [Google Scholar] [CrossRef] [PubMed]
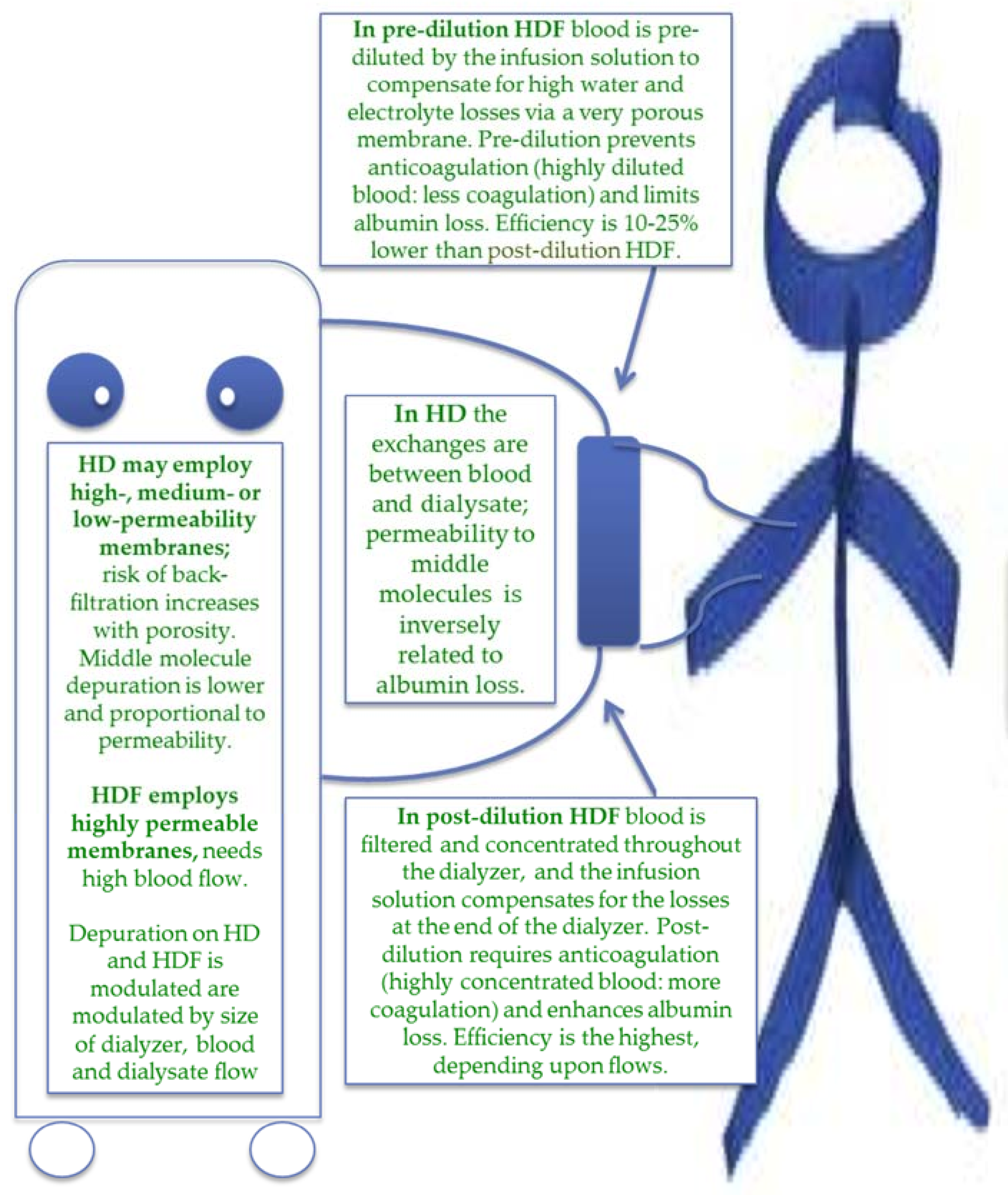

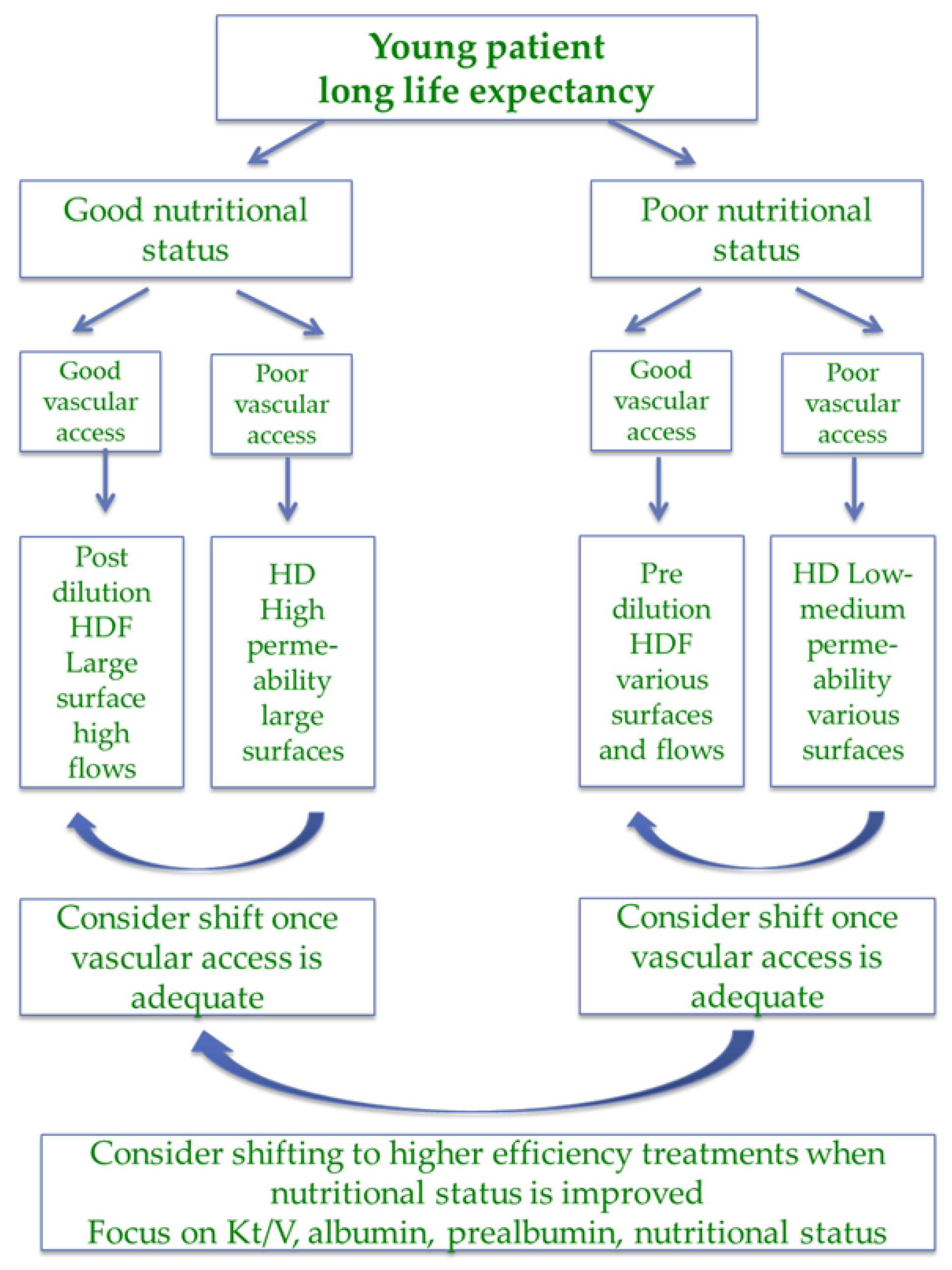
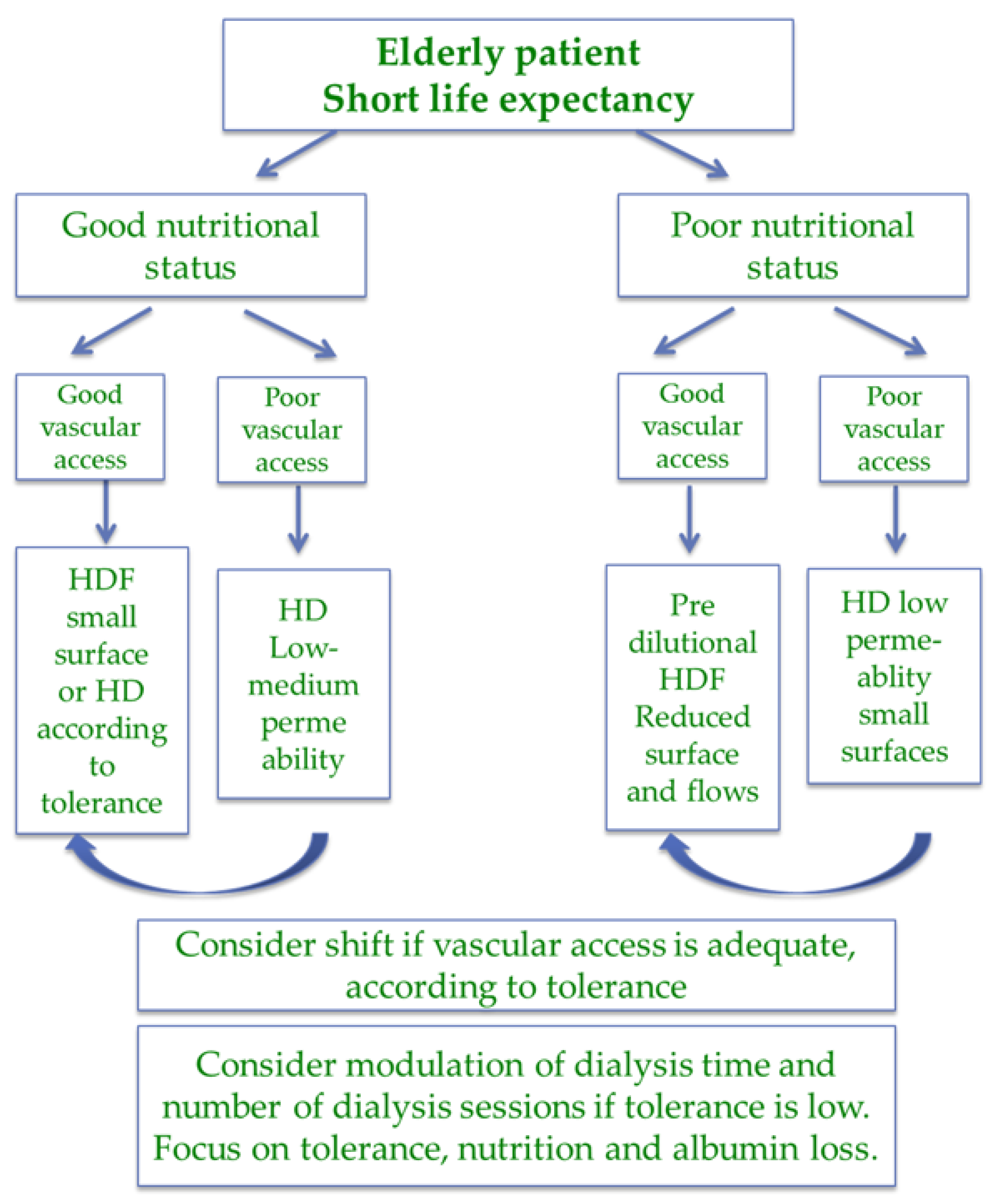
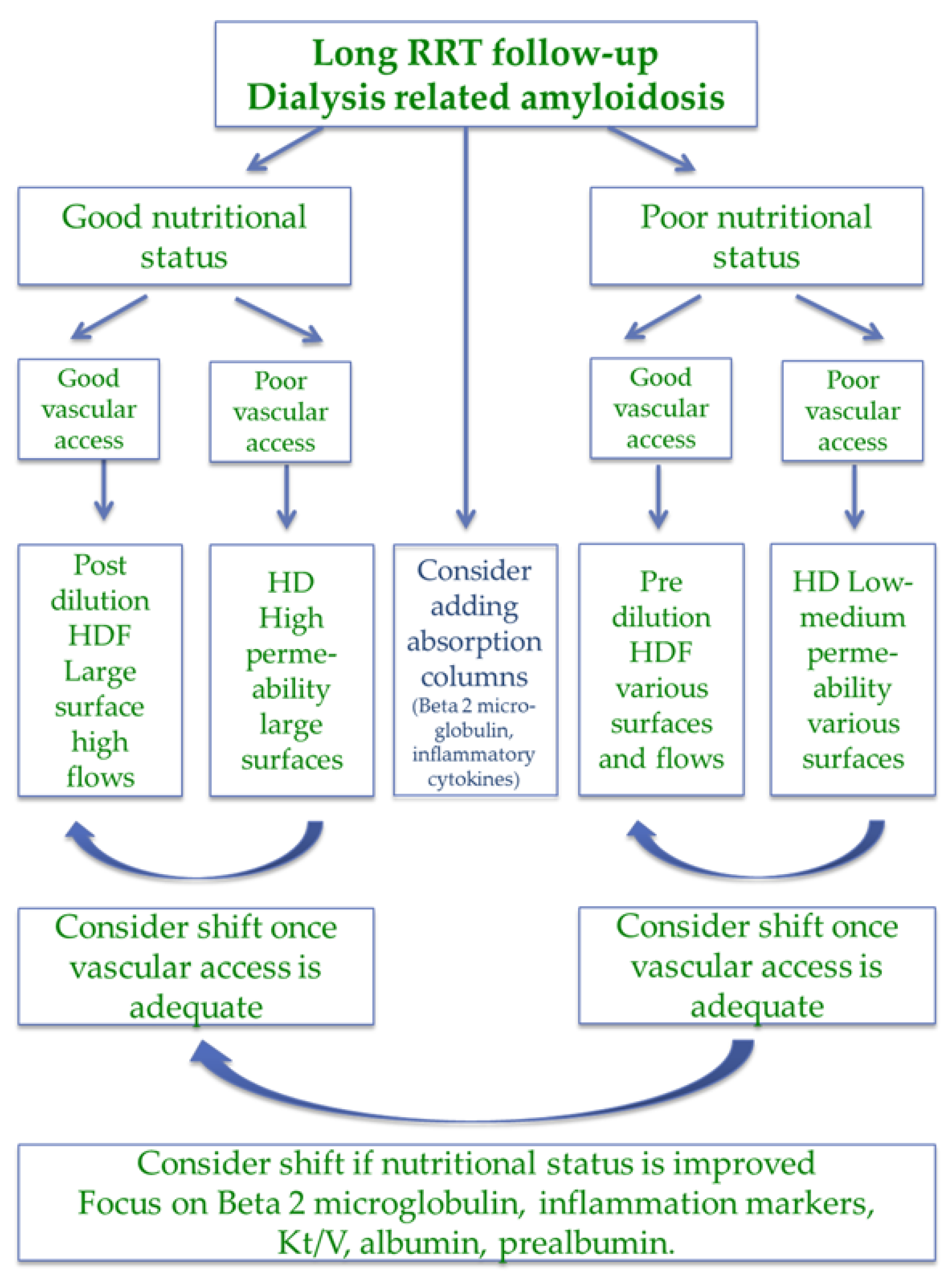
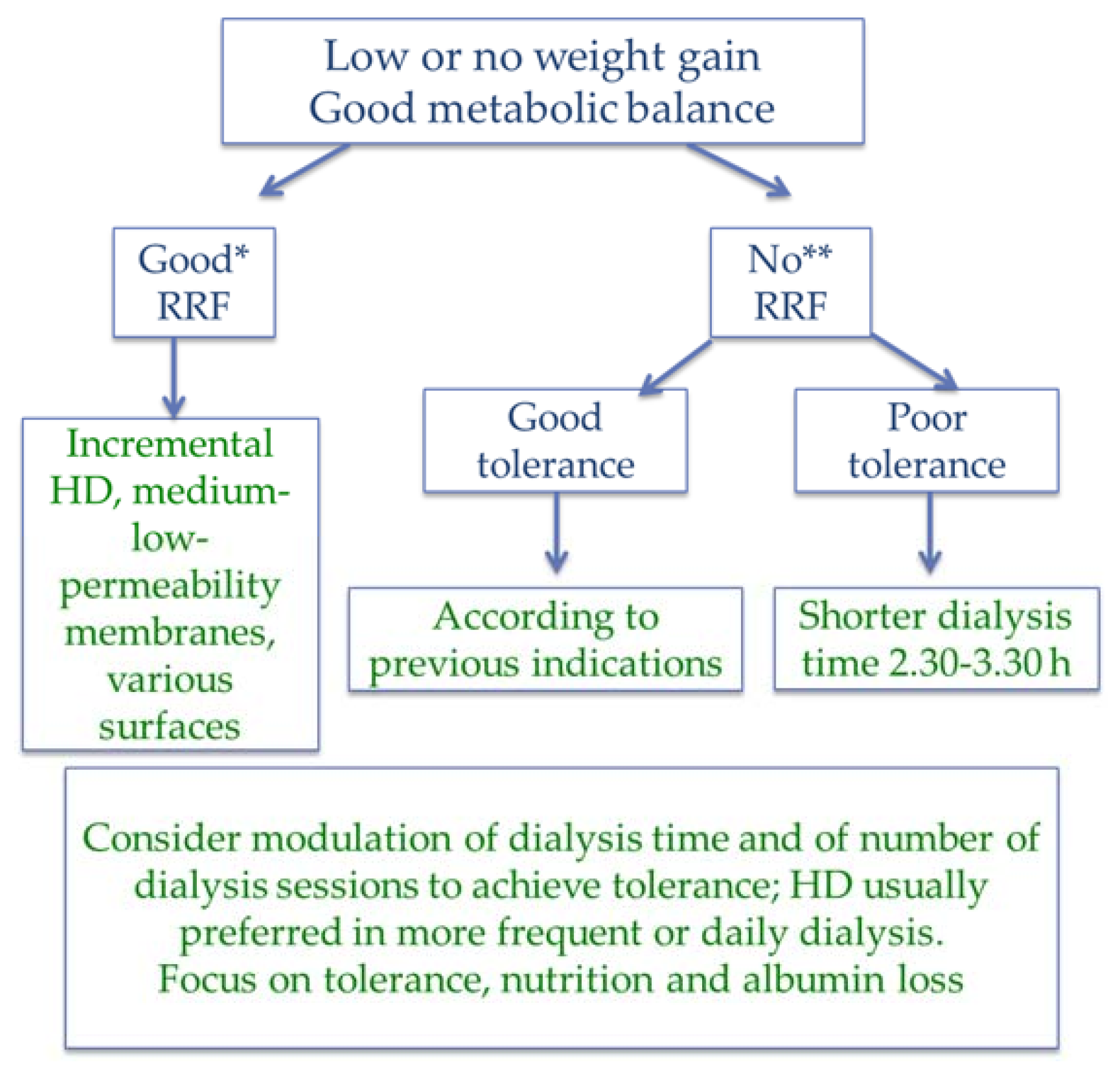
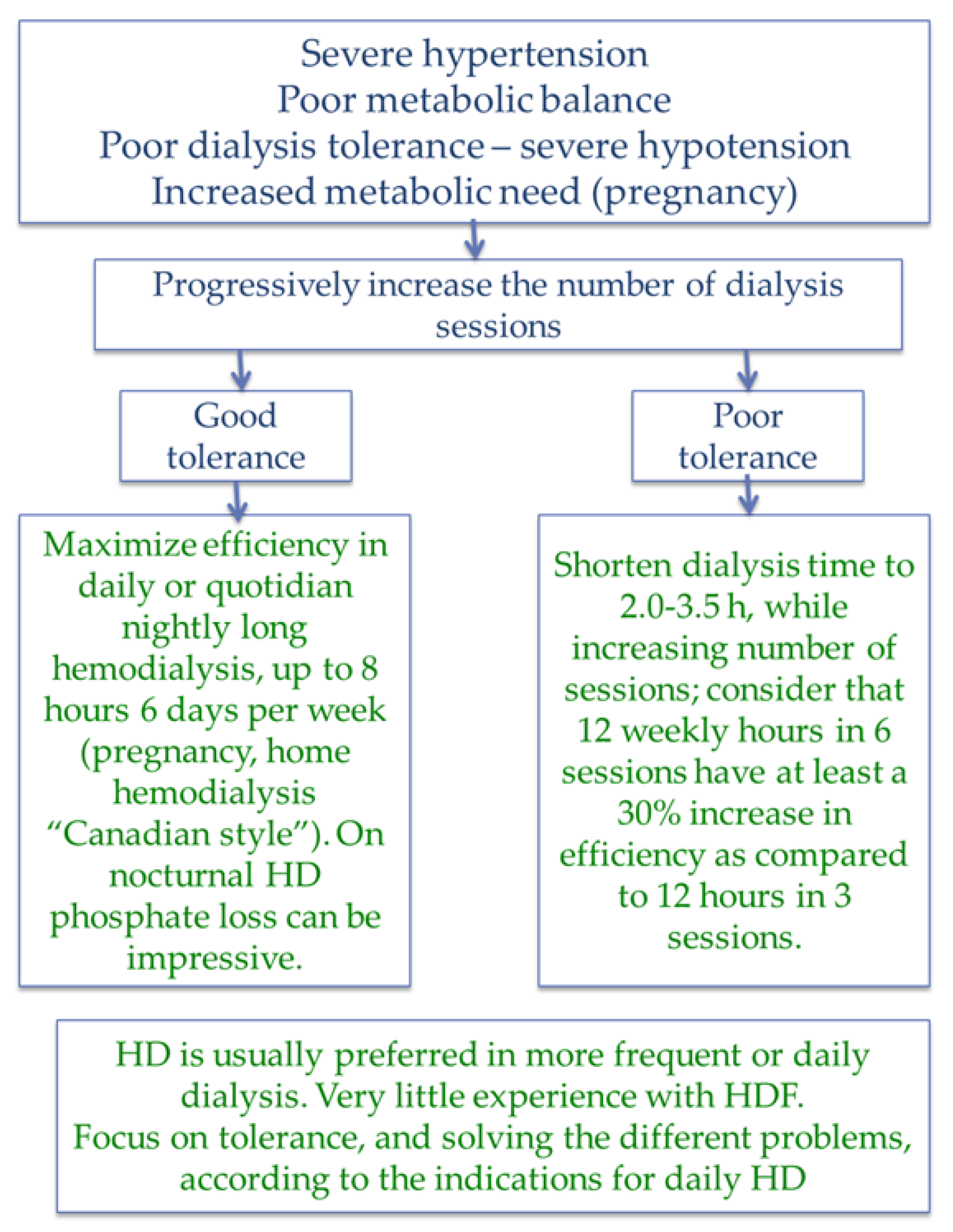
| Item | Magic numbers | Pros | Cons |
|---|---|---|---|
| Albumin | Normal ≥35 g/L to ≥40 g/L, may differ according to European or U.S. standards. | Simple, readily available, low cost, validated. | Depends on hydration, sensitivity to losses (especially in HDF or HD with high-permeability membranes). Validated in HD with thrice-weekly schedules. |
| Pre-albumin | Normal (depending on laboratory); in general 0.18–0.35 g/L. | Influences the evaluation of albumin levels. | Relatively expensive, but not fully validated, high variability. Little information for elderly patients. |
| Cholesterol | Usual threshold for malnutrition: <150 mg/dL. | Simple, readily available, low cost, validated. | Several metabolic interferences, not evaluable in the case of specific treatments. |
| Kt/V | Threshold for adequate dialysis depends on the formula chosen; adequate dialysis is usually defined as a level >1.2–1.4 in thrice-weekly dialysis. | Simple, readily available, validated, low cost. | Depends on formula, day of the week (first vs. midweek dialysis), baseline urea level; post-dialysis sample may be affected by urea rebound; may be higher in malnourished patients (low volumes). No fully validated formula for less and more frequent dialysis. |
| n-PCR | Threshold for adequate protein intake depends on the formula chosen; adequate intake usually >1.2 g/kg/day in thrice-weekly dialysis. | Simple, readily available, validated, low cost. | The best protein intake in elderly patients is not clear; data were established for relatively young patients when ideal intake was set at 1 g/day in the overall population (presently 0.8); does not distinguish between catabolism and intake. |
| Item | Number Definition | Advantages of the Definition | Disadvantages/Limits of Standardization |
|---|---|---|---|
| Permeability | Usually defined as high, medium, or low with respect to middle-molecule depuration; different cut-points available, no fully agreed definition. | Clear and easy definition; all types of membranes can be used in HD, and only high-permeability membranes in HDF. Back-filtration in HD is proportional to permeability. | Differences are less sharp for new membranes; research to improve selectivity, differences between membranes in the same category may be relevant. |
| Membrane size | In square meters: usually related to body surface (lower/higher/equal). | Clear and easy; several surfaces usually available for each membrane type. | Membrane size is related to membrane type and anticoagulation; effect of size on depuration depends on membrane performance. |
| Blood flow | No fully agreed standard; European reference 300–350 mL/min; in other settings target flow may be as high as 450 mL/min. | Clear and easy definition; good blood flow is also a marker of correct functioning of the vascular access. | Target may vary according to vascular access and type of treatment (lower in long-hour dialysis). Highly dependent on vascular access. |
| Dialysate flow | No fully agreed standard; European reference 500 mL/min., may be as high as 800 mL/min. | Clear and easy definition; agreed international standard. | Prescription can be adjusted (higher in HDF, lower in some types of daily dialysis). |
| Reinfusion (HDF) | No fully agreed standard; European reference 24 L/session on HDF. | Clear relationship between exchanges and middle-molecule depuration. | Standards are different across the world; pre-/post-dilution protocols are different; loss of albumin may increase with high exchanges. |
| Number of dialysis sessions | Thrice-weekly; incremental: 1–2 per week with progressive increase; intensive: 4–7 per week. “daily dialysis” at least 5 per week. | Clear, simple, validated. | All frequencies that differ from thrice-weekly are less validated, protocols are highly center-dependent. |
| Dialysis duration | Standard: 4 h thrice-weekly; shorter in “short” daily dialysis; various combinations of 2–8 h and 1–7 sessions. | Clear, simple, validated. | All durations that differ from 4 h are less validated, protocols are highly center-dependent. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccoli, G.B.; Nielsen, L.; Gendrot, L.; Fois, A.; Cataldo, E.; Cabiddu, G. Prescribing Hemodialysis or Hemodiafiltration: When One Size Does Not Fit All the Proposal of a Personalized Approach Based on Comorbidity and Nutritional Status. J. Clin. Med. 2018, 7, 331. https://doi.org/10.3390/jcm7100331
Piccoli GB, Nielsen L, Gendrot L, Fois A, Cataldo E, Cabiddu G. Prescribing Hemodialysis or Hemodiafiltration: When One Size Does Not Fit All the Proposal of a Personalized Approach Based on Comorbidity and Nutritional Status. Journal of Clinical Medicine. 2018; 7(10):331. https://doi.org/10.3390/jcm7100331
Chicago/Turabian StylePiccoli, Giorgina Barbara, Louise Nielsen, Lurilyn Gendrot, Antioco Fois, Emanuela Cataldo, and Gianfranca Cabiddu. 2018. "Prescribing Hemodialysis or Hemodiafiltration: When One Size Does Not Fit All the Proposal of a Personalized Approach Based on Comorbidity and Nutritional Status" Journal of Clinical Medicine 7, no. 10: 331. https://doi.org/10.3390/jcm7100331
APA StylePiccoli, G. B., Nielsen, L., Gendrot, L., Fois, A., Cataldo, E., & Cabiddu, G. (2018). Prescribing Hemodialysis or Hemodiafiltration: When One Size Does Not Fit All the Proposal of a Personalized Approach Based on Comorbidity and Nutritional Status. Journal of Clinical Medicine, 7(10), 331. https://doi.org/10.3390/jcm7100331







