Association of Renal Function and Direct-Acting Antiviral Agents for HCV: A Network Meta-Analysis
Abstract
1. Introduction
2. Methods
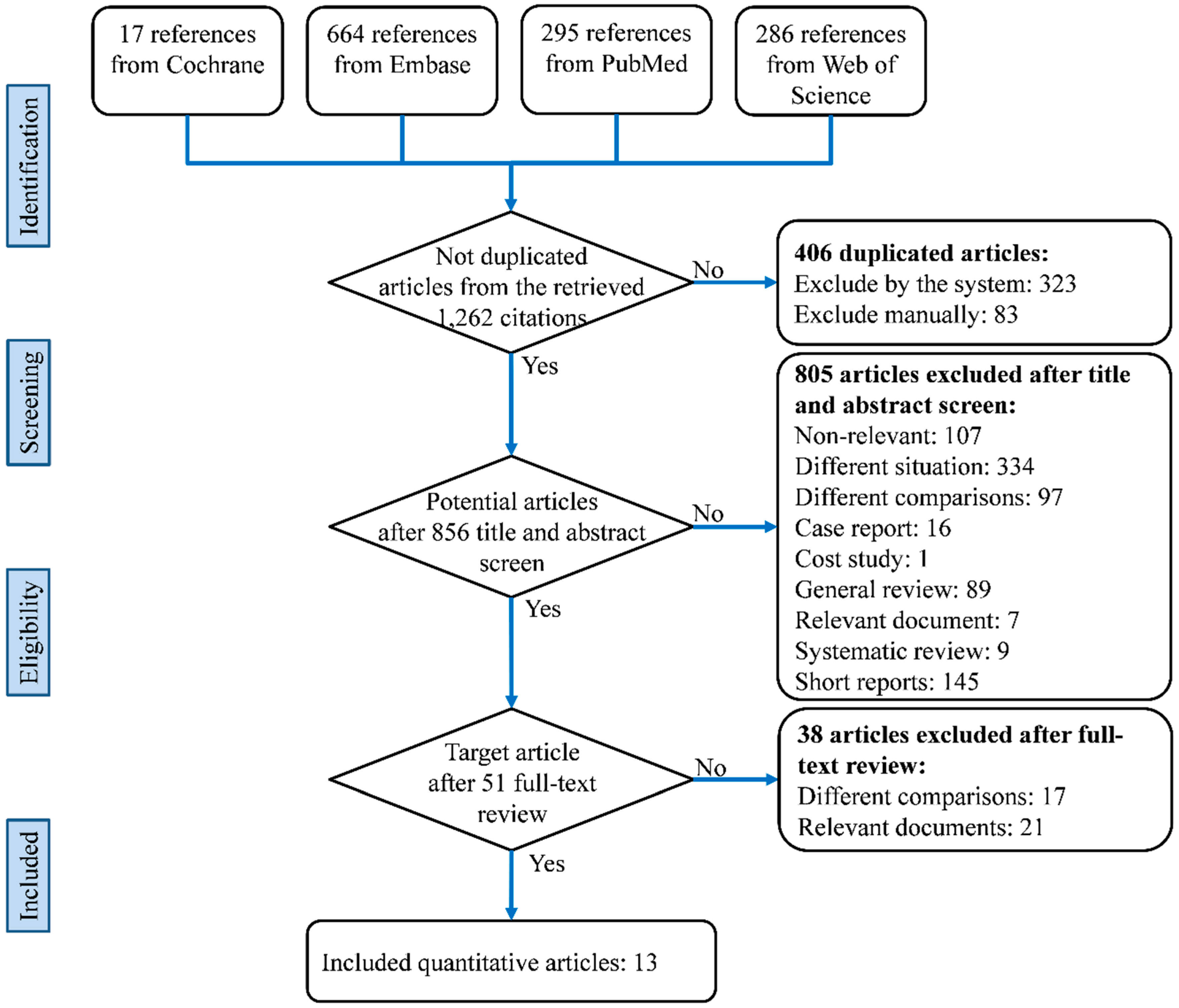
2.1. Literature Search and Selection
2.2. Quality Assessment
2.3. Data Extraction and Statistical Analysis
3. Results
3.1. Characteristics and Quality of the Included Studies
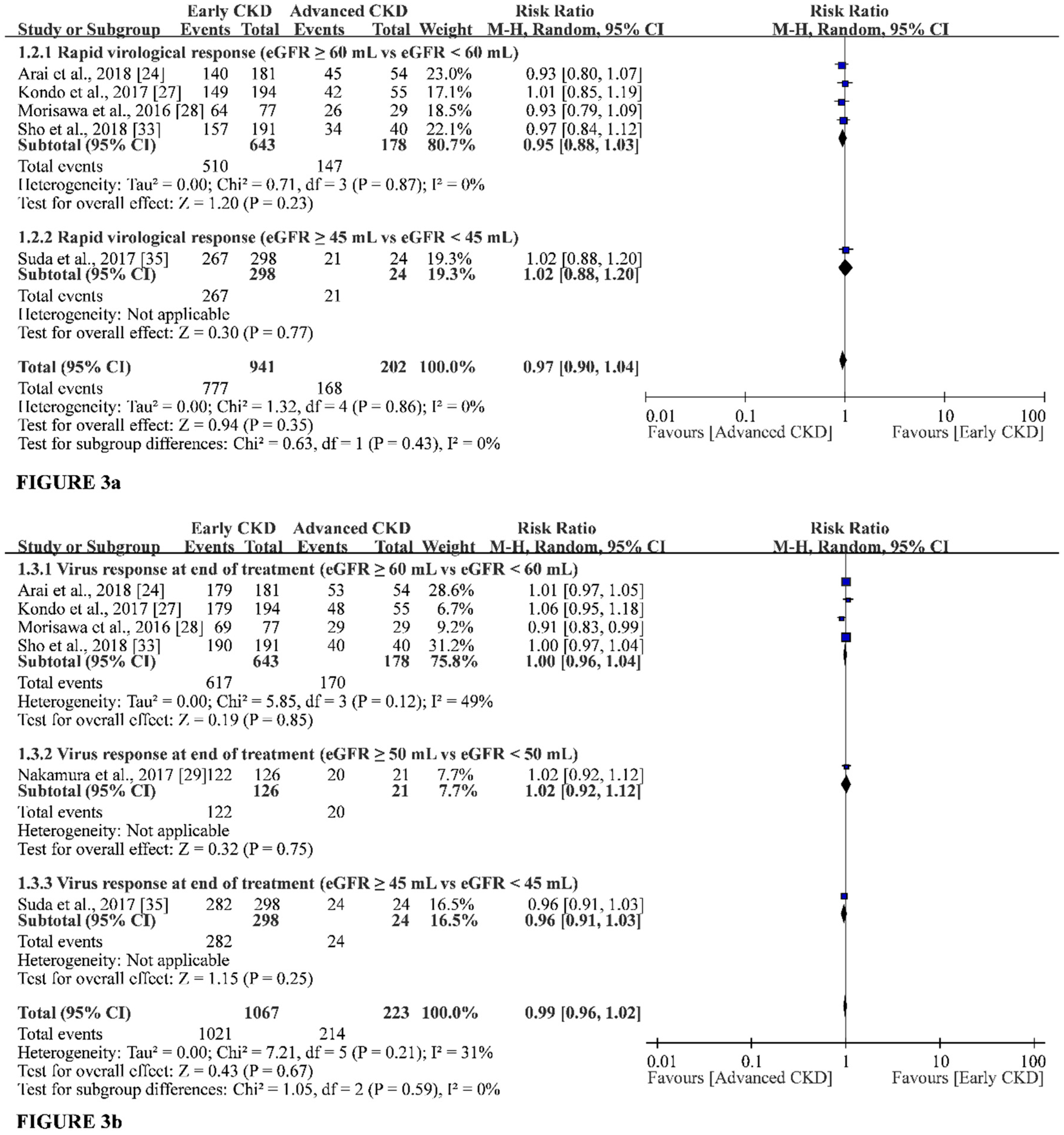
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohd Hanafiah, K.; Groeger, J.; Flaxman, A.D.; Wiersma, S.T. Global epidemiology of hepatitis c virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Hajarizadeh, B.; Grebely, J.; Dore, G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, H.Y.; Li, C.Y.; Lee, M.S.; Su, Y.C. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014, 85, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Gretch, D.R.; Yamabe, H.; Hart, J.; Bacchi, C.E.; Hartwell, P.; Couser, W.G.; Corey, L.; Wener, M.H.; Alpers, C.E.; et al. Membranoproliferative glomerulonephritis associated with hepatitis c virus infection. N. Engl. J. Med. 1993, 328, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, H.; Johnson, R.J.; Gretch, D.R.; Fukushi, K.; Osawa, H.; Miyata, M.; Inuma, H.; Sasaki, T.; Kaizuka, M.; Tamura, N.; et al. Hepatitis C virus infection and membranoproliferative glomerulonephritis in japan. J. Am. Soc. Nephrol. 1995, 6, 220–223. [Google Scholar] [PubMed]
- Li, W.C.; Lee, Y.Y.; Chen, I.C.; Wang, S.H.; Hsiao, C.T.; Loke, S.S. Age and gender differences in the relationship between hepatitis c infection and all stages of chronic kidney disease. J. Viral. Hepat. 2014, 21, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Verdesca, S.; Messa, P.; Martin, P. Hepatitis C virus infection increases the risk of developing chronic kidney disease: A systematic review and meta-analysis. Dig. Dis. Sci. 2015, 60, 3801–3813. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Resche Rigon, M.; Sene, D.; Terrier, B.; Karras, A.; Perard, L.; Schoindre, Y.; Coppere, B.; Blanc, F.; Musset, L.; et al. Rituximab plus peg-interferon-alpha/ribavirin compared with peg-interferon-alpha/ribavirin in hepatitis c-related mixed cryoglobulinemia. Blood 2010, 116, 326–334, quiz 504–325. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Cacoub, P. Renal involvement in HCV-related vasculitis. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Fissell, R.B.; Bragg-Gresham, J.L.; Woods, J.D.; Jadoul, M.; Gillespie, B.; Hedderwick, S.A.; Rayner, H.C.; Greenwood, R.N.; Akiba, T.; Young, E.W. Patterns of hepatitis c prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int. 2004, 65, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Dixit, V.; Messa, P. Impact of hepatitis c on survival in dialysis patients: A link with cardiovascular mortality? J. Viral Hepat. 2012, 19, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Ho, H.J.; Huang, Y.T.; Wang, H.H.; Wu, M.S.; Lin, J.T.; Wu, C.Y. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis c virus infection. Gut 2015, 64, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Lin, J.T.; Ho, H.J.; Kao, Y.H.; Huang, Y.T.; Hsiao, N.W.; Wu, M.S.; Liu, Y.Y.; Wu, C.Y. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 2014, 59, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Lunghi, G.; Ganeshan, S.V.; Martin, P.; Messa, P. Hepatitis c virus infection and the dialysis patient. Semin. Dial. 2007, 20, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Dixit, V.; Messa, P.; Martin, P. Antiviral therapy (pegylated interferon and ribavirin) of hepatitis c in dialysis patients: Meta-analysis of clinical studies. J. Viral Hepat. 2014, 21, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and sofosbuvir for untreated hcv genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Jacobson, I.M.; Hezode, C.; Asselah, T.; Ruane, P.J.; Gruener, N.; Abergel, A.; Mangia, A.; Lai, C.L.; Chan, H.L.; et al. Sofosbuvir and velpatasvir for hcv genotype 1, 2, 4, 5, and 6 infection. N. Engl. J. Med. 2015, 373, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Ghalib, R.; Reddy, K.R.; Pockros, P.J.; Ben Ari, Z.; Zhao, Y.; Brown, D.D.; Wan, S.; DiNubile, M.J.; Nguyen, B.Y.; et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis c virus genotype 1, 4, or 6 infection: A randomized trial. Ann. Intern. Med. 2015, 163, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.; Nelson, D.R.; Bruchfeld, A.; Liapakis, A.; Silva, M.; Monsour, H.; Martin, P.; Pol, S.; Londoño, M.C.; Hassanein, T.; et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis c virus genotype 1 infection and stage 4-5 chronic kidney disease (the c-surfer study): A combination phase 3 study. Lancet 2015, 386, 1537–1545. [Google Scholar] [CrossRef]
- Kohli, A.; Alshati, A.; Georgie, F.; Manch, R.; Gish, R.G. Direct-acting antivirals for the treatment of chronic hepatitis c in patients with chronic kidney disease. Therap. Adv. Gastroenterol. 2016, 9, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, J.J.; Doi, S.A. Metaxl User Guide. Version: 2016. Available online: http://www.epigear.com/index_files/MetaXL%20User%20Guide.pdf (accessed on 28 September 2018).
- Arai, T.; Atsukawa, M.; Tsubota, A.; Ikegami, T.; Shimada, N.; Kato, K.; Abe, H.; Okubo, T.; Itokawa, N.; Kondo, C.; et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir combination therapy for genotype 1b chronic hepatitis c patients complicated with chronic kidney disease. Hepatol. Res. 2018, 48, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.; Lawitz, E.; Pugatch, D.; Papatheodoridis, G.; Bräu, N.; Brown, A.; Pol, S.; Leroy, V.; Persico, M.; Moreno, C.; et al. Glecaprevir and pibrentasvir in patients with hcv and severe renal impairment. N. Engl. J. Med. 2017, 377, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Imamura, M.; Ikeda, H.; Suzuki, M.; Arataki, K.; Moriishi, M.; Mori, N.; Kokoroishi, K.; Katamura, Y.; Ezaki, T.; et al. Pharmacokinetics, efficacy and safety of daclatasvir plus asunaprevir in dialysis patients with chronic hepatitis c: Pilot study. J. Viral Hepat. 2016, 23, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Kondo, C.; Atsukawa, M.; Tsubota, A.; Shimada, N.; Abe, H.; Asano, T.; Yoshizawa, K.; Okubo, T.; Chuganji, Y.; Aizawa, Y.; et al. Daclatasvir and asunaprevir for genotype 1b chronic hepatitis c patients with chronic kidney disease. Hepatol. Res. 2017, 47, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, N.; Koshima, Y.; Satoh, J.I.; Maruyama, Y.; Kuriyama, S.; Yokoo, T.; Amemiya, M. Usefulness of combination therapy with daclatasvir plus asunaprevir in chronic hepatitis c patients with chronic kidney disease. Clin. Exp. Nephrol. 2017, 21, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Imamura, M.; Kawakami, Y.; Teraoka, Y.; Daijo, K.; Honda, F.; Morio, K.; Kobayashi, T.; Nakahara, T.; Nagaoki, Y.; et al. Efficacy and safety of daclatasvir plus asunaprevir therapy for chronic hepatitis c patients with renal dysfunction. J. Med. Virol. 2017, 89, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Puenpatom, A.; Hull, M.; McPheeters, J.; Schwebke, K. Disease burden, early discontinuation, and healthcare costs in hepatitis c patients with and without chronic kidney disease treated with interferon-free direct-acting antiviral regimens. Clin. Drug Investig. 2017, 37, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Koraishy, F.M.; Sise, M.E.; Lim, J.K.; Schmidt, M.; Chung, R.T.; Liapakis, A.; Nelson, D.R.; Fried, M.W.; Terrault, N.A. Safety and efficacy of sofosbuvir-containing regimens in hepatitis c-infected patients with impaired renal function. Liver Int. 2016, 36, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.P.; Park, J.A.; Burman, B.; Kozarek, R.A.; Siddique, A. Efficacy and safety of sofosbuvir-based regimens for treatment in chronic hepatitis c genotype 1 patients with moderately impaired renal function. Clin. Mol. Hepatol. 2017, 23, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Suda, G.; Nagasaka, A.; Yamamoto, Y.; Furuya, K.; Kumagai, K.; Uebayashi, M.; Terashita, K.; Kobayashi, T.; Tsunematsu, I.; et al. Safety and efficacy of sofosbuvir and ribavirin for genotype 2 hepatitis c japanese patients with renal dysfunction. Hepatol. Res. 2018, 48, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Sise, M.E.; Backman, E.; Ortiz, G.A.; Hundemer, G.L.; Ufere, N.N.; Chute, D.F.; Brancale, J.; Xu, D.H.; Wisocky, J.; Lin, M.V.; et al. Effect of sofosbuvir-based hepatitis c virus therapy on kidney function in patients with ckd. Clin. J. Am. Soc. Nephrol. 2017, 12, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Suda, G.; Nagasaka, A.; Yamamoto, Y.; Furuya, K.; Kumagai, K.; Kudo, M.; Terashita, K.; Kobayashi, T.; Tsunematsu, I.; Yoshida, J.; et al. Safety and efficacy of daclatasvir and asunaprevir in hepatitis c virus-infected patients with renal impairment. Hepatol. Res. 2017, 47, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qu, Y.; Guo, Y.; Wang, Y.; Wang, L. Efficacy and safety of direct-acting antivirals-based antiviral therapies for hepatitis c virus patients with stage 4–5 chronic kidney disease: A meta-analysis. Liver Int. 2017, 37, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.K.; Chai, B.; Roy, J.A.; Anderson, A.H.; Bansal, N.; Feldman, H.I.; Go, A.S.; He, J.; Horwitz, E.J.; Kusek, J.W. Abrupt decline in kidney function before initiating hemodialysis and all-cause mortality: The chronic renal insufficiency cohort (cric) study. Am. J. Kidney Dis. 2016, 68, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontán, F.; Azevedo, L.; Luna, E.; Caravaca, F. Patterns of progression of chronic kidney disease at later stages. Clin. Kidney J. 2018, 11, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Pockros, P.J.; Reddy, K.R.; Mantry, P.S.; Cohen, E.; Bennett, M.; Sulkowski, M.S.; Bernstein, D.E.; Cohen, D.E.; Shulman, N.S.; Wang, D.; et al. Efficacy of direct-acting antiviral combination for patients with hepatitis c virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology 2016, 150, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in ckd. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Desbois, A.C.; Isnard-Bagnis, C.; Rocatello, D.; Ferri, C. Hepatitis C virus infection and chronic kidney disease: Time for reappraisal. J. Hepatol. 2016, 65, S82–S94. [Google Scholar] [CrossRef] [PubMed]
- De Jager, D.J.; Grootendorst, D.C.; Jager, K.J.; van Dijk, P.C.; Tomas, L.M.; Ansell, D.; Collart, F.; Finne, P.; Heaf, J.G.; De Meester, J.; et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009, 302, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Maida, M.; Macaluso, F.S.; Barbara, M.; Licata, A.; Craxi, A.; Camma, C. Hepatitis C virus infection is associated with increased cardiovascular mortality: A meta-analysis of observational studies. Gastroenterology 2016, 150. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.; Jadoul, M.; Vallet-Pichard, A. An update on the management of hepatitis c virus-infected patients with stage 4–5 chronic kidney disease while awaiting the revised kdigo guidelines. Nephrol. Dial. Transplant. 2017, 32, 32–35. [Google Scholar] [CrossRef] [PubMed]
| Inclusion | Sample Size | |||||
|---|---|---|---|---|---|---|
| Location | Region | Period | NONE TO EARLY | Advanced | Dialysis | Treatment |
| Arai et al. | Japan | 10/2012 to | 181 | 54 | NA | Ombitasvir/Paritaprevir/ |
| (2018) [24] | 03/2017 | (eGFR ≥ 60 mL) | (eGFR < 60 mL) | Ritonavir | ||
| Gane et al. | multi-region a | 12/2015 to | NA | 19 | 85 | Glecaprevir-Pibrentasvir |
| (2017) [25] | 03/2016 | (eGFR < 45 mL) | ||||
| Kawakami et al. | Japan | 12/2014 to | 3 | NA | 18 | Daclatasvir (DCV) plus |
| (2016) [26] | 01/2016 | (eGFR ≥ 60 mL) | Asunaprevir (ASV) | |||
| Kondo et al. | Japan | 09/2014 to | 194 | 55 | NA | DCV and ASV |
| (2017) [27] | 09/2015 | (eGFR ≥ 60 mL) | (eGFR < 60 mL) | |||
| Morisawa et al. | Japan | 09/2014 to | 77 | 29 | NA | DCV plus ASV |
| (2016) [28] | 05/2015 | (eGFR ≥ 60 mL) | (eGFR < 60 mL) | |||
| Nakamura et al. | Japan | 09/2014 to | 126 | 21 | NA | DCV plus ASV |
| (2017) [29] | 08/2015 | (eGFR ≥ 50 mL) | (eGFR < 50 mL) | |||
| Puenpatom et al. | USA | 11/2013 to | 3202 | 236 | NA | Sofosbuvir-based |
| (2017) [30] | 06/2015 | (eGFR ≥ 90 mL) | (eGFR < 90 mL) | regimens (SOF) | ||
| Roth et al. | multi-region b | 03/2014 to | NA | 29 | 87 | Grazoprevir plus |
| (2015) [19] | 11/2014 | (eGFR < 45 mL) | Elbasvir | |||
| Saxena et al. | North America | 03/2015 | 1716 | 73 | NA | SOF-based regimens |
| (2016) [31] | and Europe | (eGFR ≥ 60 mL) | (eGFR < 60 mL) | |||
| Shin et al. | USA | 12/2013 to | 21 | 7 | NA | SOF-based regimens |
| (2017) [32] | 09/2015 | (eGFR ≥ 45 mL) | (eGFR < 45 mL) | |||
| Sho et al. | Japan | 07/2014 to | 191 (eGFR ≥ 60 mL) | 40 (eGFR < 60 mL) | NA | SOF and ribavirin |
| (2018) [33] | 05/2017 | 224 (eGFR ≥ 45 mL) | 7 (eGFR < 45 mL) | |||
| Sise et al. | USA | 11/2013 to | 74 | 24 | NA | SOF-based therapy |
| (2017) [34] | 12/2014 | (eGFR ≥ 60 mL) | (eGFR < 60 mL) | |||
| Suda et al. | Japan | 07/2014 to | 159 (eGFR ≥ 60 mL) | 95 (eGFR < 60 mL) | NA | DCV and ASV |
| (2017) [35] | 11/2016 | 298 (eGFR ≥ 45 mL) | 24 (eGFR < 45 mL) | |||
| Age | Sex (Male) | ||||||
|---|---|---|---|---|---|---|---|
| Location | None to Early | Advanced | Dialysis | None to Early | Advanced | Dialysis | Relevant Outcomes |
| Arai et al. | Overall: 67 | (27–89) | NA | Overall: 117 | (50%) | NA | RVR, SVR 12, VRET, rash (eruption), |
| (2018) [24] | ALT | ||||||
| Gane et al. | NA | Overall: 57 | (28–83) | NA | Overall: 79 | (76%) | SVR 12, adverse event |
| (2017) [25] | No comparison between groups | ||||||
| Kawakami et al. | 80 | NA | 68 | 0 | NA | 14 | ALT, diarrhea, fever, headache |
| (2016) [26] | (62–81) | (47–82) | (0%) | (78%) | |||
| Kondo et al. | Overall: 71 | (25–87) | NA | Overall: 105 | (42%) | NA | RVR, SVR 12, VRET, rash (eruption), |
| (2017) [27] | ALT, renal disorder, discontinuation | ||||||
| Morisawa et al. | 72.3 ± 7 | 74.9 ± 8 | NA | 28 | 13 | NA | RVR, SVR 12, VRET, ALT, |
| (2016) [28] | (36%) | (45%) | discontinuation | ||||
| Nakamura et al. | 73 Me | 78 Me | NA | 52 | 4 | NA | SVR 12, VRET, itching or rash (eruption), |
| (2017) [29] | (43–88) | (57–88) | (41%) | (21%) | ALT, discontinuation | ||
| Puenpatom et al. | 58.76 ± 9.50 | 61.96 ± 7.74 | NA | 2013 | 167 | NA | Rash (eruption), anemia, discontinuation |
| (2017) [30] | (62.87%) | (70.76%) | |||||
| Roth et al. | NA | NA | NA | NA | NA | NA | SVR 12 |
| (2015) [19] | |||||||
| Saxena et al. | n = 271(16%) | n = 17 | NA | 1107 | 33 | NA | SVR 12, renal disorder, anemia |
| (2016) [31] | age ≥ 65 | age ≥ 65 | (65%) | (45%) | discontinuation | ||
| Shin et al. | 61 | 62.9 | NA | 19 | 5 | NA | SVR 12, rash (eruption), anemia, |
| (2017) [32] | (27–78) | (56–72) | (48%) | (71%) | discontinuation | ||
| Sho et al. | Overall: 62 | (22–88) | NA | Overall: 106 | (46%) | NA | RVR, SVR 12, VRET, ALT, renal disorder, |
| (2018) [33] | anemia, discontinuation | ||||||
| Sise et al. | 61 ± 8 | 65 ± 10 | NA | 61 | 15 | NA | SVR, adverse event |
| (2017) [34] | (82%) | (63%) | Without raw data. | ||||
| Suda et al. | 70.5 Me | 70 Me | NA | 103 | 10 | NA | RVR, SVR 12, VRET, ALT, anemia, |
| (2017) [35] | (48–85) | (30–92) | (35%) | (42%) | renal disorder, discontinuation | ||
| Comparisons | Events/Patients | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Studies | Group 1 | Group 2 | RR | 95% CI | I-Square | p |
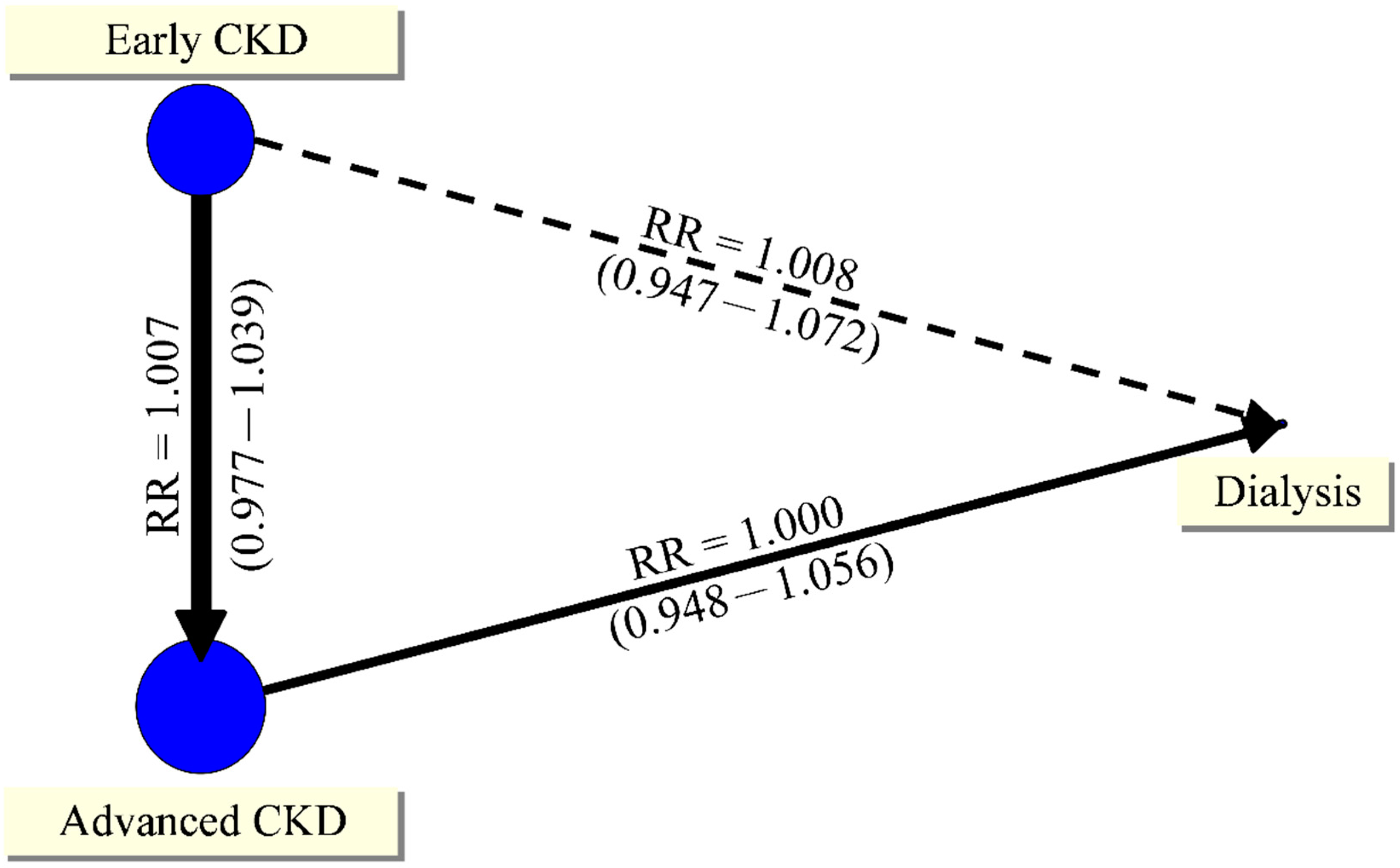
| Early CKD | Advanced CKD | 4 | 10/162 | 30/750 | 0.73 | (0.29–1.81) | 29% | 0.24 |
| Early CKD | Dialysis | 1 | 3/18 | 1/3 | 1.46 | (0.18−12.03) | NA | NA |
| Advanced CKD | Dialysis | Indirect | NA | NA | 2.00 | (0.30−13.44) | NA | NA |
| Secondary | Events/Patients | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|
| outcomes | Studies | None to Early | Advanced CKD | RR | 95% CI | I-Square | p |
| Renal disorder | 6 | 24/2585 | 14/246 | 0.14 | (0.04−0.43) | 35% | 0.20 |
| eGFR ≥ 60 vs. <60 | 5 | 20/2282 | 13/222 | 0.12 | (0.04−0.43) | 35% | 0.22 |
| eGFR ≥ 45 vs. <45 | 1 | 4/298 | 1/24 | 0.32 | (0.04–2.77) | NA | NA |
| Anemia a | 5 | 328/5428 | 42/257 | 0.34 | (0.20−0.57) | 47% | 0.11 |
| eGFR ≥ 90 vs. <90 | 1 | 25/3202 | 6/113 | 0.15 | (0.06–0.35) | NA | NA |
| eGFR ≥ 60 vs. <60 | 2 | 290/1907 | 32/113 | 0.49 | (0.35–0.67) | 0% | 0.33 |
| eGFR ≥ 45 vs. <45 | 2 | 13/319 | 4/31 | 0.32 | (0.11–0.96) | 0% | 0.98 |
| Eruption | 5 | 71/3724 | 10/250 | 0.74 | (0.14–3.28) | 75% | >0.01 |
| eGFR ≥ 90 vs. <90 | 1 | 36/3202 | 7/113 | 0.18 | (0.08–0.40) | NA | NA |
| eGFR ≥ 60 vs. <60 | 2 | 5/375 | 1/109 | 0.84 | (0.09–8.18) | 23% | 0.25 |
| eGFR ≥ 50 vs. <50 | 1 | 29/126 | 2/21 | 2.42 | (0.62–9.38) | NA | NA |
| eGFR ≥ 45 vs. <45 | 1 | 1/21 | 0/7 | 1.09 | (0.05–24.13) | NA | NA |
| Discontinuation | 8 | 439/5858 | 48/329 | 0.41 | (0.30–0.56) | 3% | 0.40 |
| eGFR ≥ 90 vs. <90 | 1 | 324/3202 | 31/113 | 0.37 | (0.27–0.51) | NA | NA |
| eGFR ≥ 60 vs. <60 | 4 | 88/2178 | 15/197 | 0.50 | (0.28–0.89) | 0% | 0.60 |
| eGFR ≥ 50 vs. <50 | 1 | 1/126 | 1/21 | 0.17 | (0.01–2.56) | NA | NA |
| eGFR ≥ 45 vs. <45 | 2 | 26/319 | 1/31 | 2.09 | (0.30−14.77) | NA | NA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, C.-C.; Lin, Y.-S.; Chu, H.-C.; Fang, T.-C.; Wu, M.-S.; Kang, Y.-N. Association of Renal Function and Direct-Acting Antiviral Agents for HCV: A Network Meta-Analysis. J. Clin. Med. 2018, 7, 314. https://doi.org/10.3390/jcm7100314
Kao C-C, Lin Y-S, Chu H-C, Fang T-C, Wu M-S, Kang Y-N. Association of Renal Function and Direct-Acting Antiviral Agents for HCV: A Network Meta-Analysis. Journal of Clinical Medicine. 2018; 7(10):314. https://doi.org/10.3390/jcm7100314
Chicago/Turabian StyleKao, Chih-Chin, Yu-Shiuan Lin, Heng-Cheng Chu, Te-Chao Fang, Mai-Szu Wu, and Yi-No Kang. 2018. "Association of Renal Function and Direct-Acting Antiviral Agents for HCV: A Network Meta-Analysis" Journal of Clinical Medicine 7, no. 10: 314. https://doi.org/10.3390/jcm7100314
APA StyleKao, C.-C., Lin, Y.-S., Chu, H.-C., Fang, T.-C., Wu, M.-S., & Kang, Y.-N. (2018). Association of Renal Function and Direct-Acting Antiviral Agents for HCV: A Network Meta-Analysis. Journal of Clinical Medicine, 7(10), 314. https://doi.org/10.3390/jcm7100314





