Validity of Capsule Endoscopy in Monitoring Therapeutic Interventions in Patients with Crohn’s Disease
Abstract
1. Introduction
2. Patients & Methods
2.1. Patients
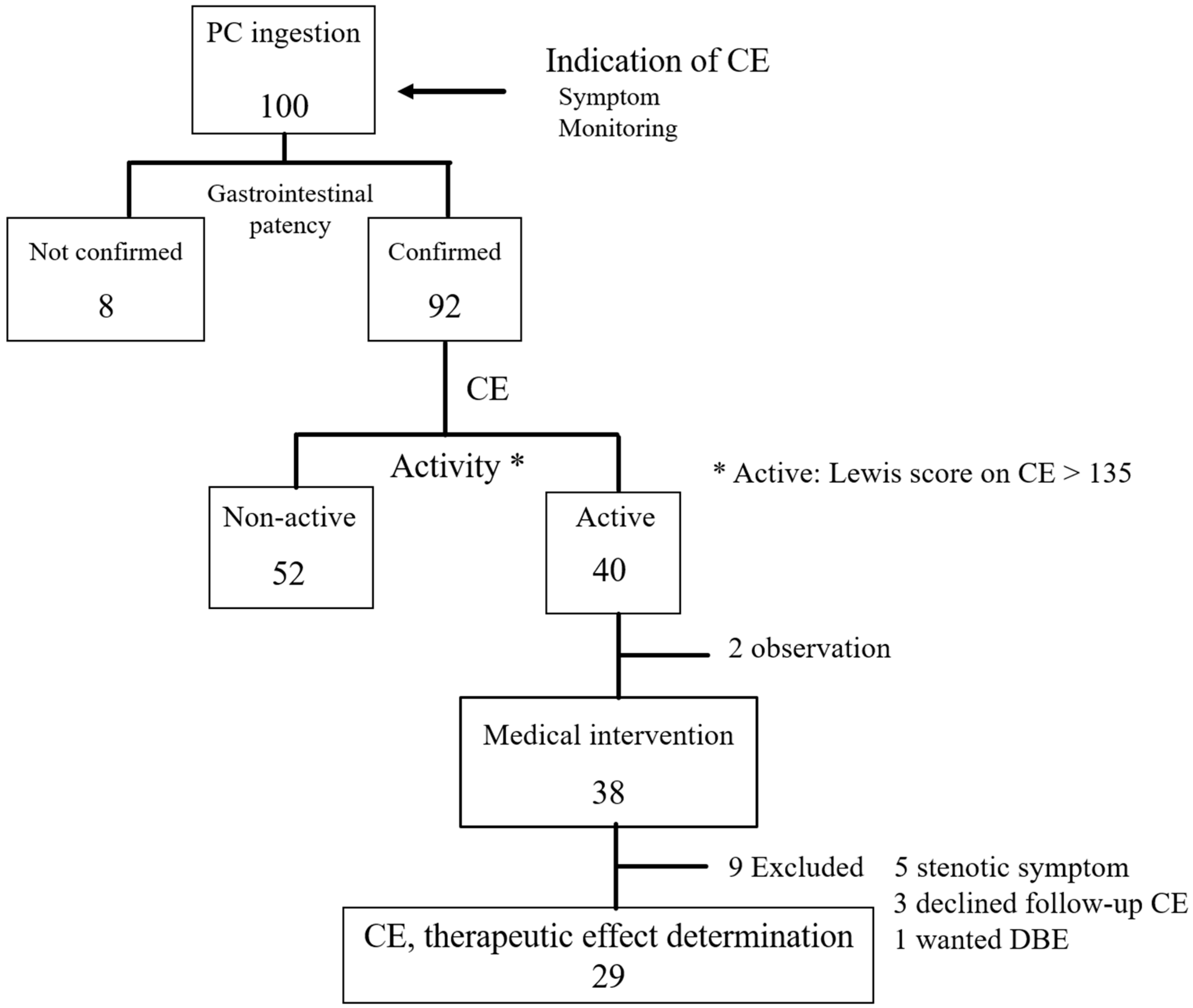
2.2. Study Protocol
2.3. Evaluations
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Baert, F.; Moortgat, L.; Van Assche, G.; Caenepeel, P.; Vergauwe, P.; De Vos, M.; Stokkers, P.; Hommes, D.; Rutgeerts, P.; Vermeire, S.; et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010, 138, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Ferrante, M.; Magro, F.; Campbell, S.; Franchimont, D.; Fidder, H.; Strid, H.; Ardizzone, S.; Veereman-Wauters, G.; Chevaux, J.B.; et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J. Crohns Colitis 2011, 5, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Frøslie, K.F.; Jahnsen, J.; Moum, B.A.; Vatn, M.H.; IBSEN Group. Mucosal healing in inflammatory bowel disease: Results from a Norwegian population-based cohort. Gastroenterology 2007, 133, 412–422. [Google Scholar]
- Sipponen, T.; Savilahti, E.; Kolho, K.L.; Nuutinen, H.; Turunen, U.; Farkkila, M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn’s disease activity index and endoscopic findings. Inflamm. Bowel Dis. 2008, 14, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Klang, E.; Yablecovitch, D.; Lahat, A.; Avidan, B.; Neuman, S.; Levhar, N.; Greener, T.; Rozendorn, N.; Beytelman, A.; et al. Magnetic resonance enterography versus capsule endoscopy activity indices for quantification of small bowel inflammation in Crohn’s disease. Ther. Adv. Gastroenterol. 2016, 9, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Moy, M.P.; Kaplan, J.L.; Moran, C.J.; Winter, H.S.; Gee, M.S. MR enterographic findings as biomarkers of mucosal healing in young patients with Crohn disease. Am. J. Roentgenol. 2016, 207, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Koulaouzidis, A.; Klang, E.; Carter, D.; Ben-Horin, S.; Eliakim, R. Monitoring of small bowel Crohn’s disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 1014–1058. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Matsumoto, T.; Watanabe, K.; Arakawa, T.; Naito, Y.; Matsuura, M.; Nakase, H.; Hibi, T.; Matsumoto, T.; Nouda, S.; et al. Use of capsule endoscopy in patients with Crohn’s disease in Japan: A multicenter survey. J. Gastroenterol. Hepatol. 2014, 29, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, A.; Kopylov, U.; Koulaouzidis, A.; Wurm Johansson, G.; Thorlacius, H.; Amre, D.; Eliakim, R.; Seidman, E.G.; Toth, E. Use of patency capsule in patients with established Crohn’s disease. Endoscopy 2016, 48, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hirooka, Y.; Yamamura, T.; Miyahara, R.; Watanabe, O.; Ando, T.; Ohmiya, N.; Goto, H. Clinical usefulness of novel tag-less Agile patency capsule prior to capsule endoscopy for patients with suspected small bowel stenosis. Dig. Endosc. 2015, 27, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, M.; Spada, C.; Eliakim, R.; Keuchel, M.; May, A.; Mulder, C.J.; Rondonotti, E.; Adler, S.N.; Albert, J.; Baltes, P.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015, 47, 352–376. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Defranchis, R.; Seidman, E.; Leighton, J.A.; Legnani, P.; Lewis, B.S. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment. Pharmacol. Ther. 2008, 27, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, A.; Douglas, S.; Plevris, J.N. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn’s Disease Activity Index. Dig. Dis. Sci. 2012, 57, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Dias de Castro, F.; Boal Carvalho, P.; Monteiro, S.; Rosa, B.; Firmino-Machado, J.; Moreira, M.J.; Cotter, J. Lewis score—Prognostic value in patients with isolated small bowel Crohn’s disease. J. Crohns Colitis 2015, 9, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Niv, Y. Small-bowel mucosal healing assessment by capsule endoscopy as a predictor of long-term clinical remission in patients with Crohn’s disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Barnes, E.; Isaacs, K.; Morgan, D.; Herfarth, H.H. Impact of capsule endoscopy on management of inflammatory bowel disease: A single tertiary care center experience. Inflamm. Bowel Dis. 2011, 17, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Zúñiga, V.; de Vega, V.M.; Domènech, E.; Cabré, E.; Mañosa, M.; Boix, J. Impact of capsule endoscopy findings in the management of Crohn’s Disease. Dig. Dis. Sci. 2010, 55, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.; McAlindon, M.E.; Drew, K.; Hardcastle, S.; Cameron, I.C.; Sanders, D.S. Evaluating the role of small-bowel endoscopy in clinical practice: The largest single-centre experience. Eur. J. Gastroenterol. Hepatol. 2012, 24, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Daperno, M.; Rutter, M.D.; Amiot, A.; Bossuyt, P.; East, J.; Ferrante, M.; Götz, M.; Katsanos, K.H.; Kießlich, R.; et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohns Colitis 2013, 7, 982–1018. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Yablecovitch, D.; Lahat, A.; Neuman, S.; Levhar, N.; Greener, T.; Klang, E.; Rozendorn, N.; Amitai, M.M.; Ben-Horin, S.; et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am. J. Gastroenterol. 2015, 110, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Nakamura, M.; Ohmiya, N.; Hirooka, Y.; Itoh, A. Establishment of an interpretation system for video capsule endoscopy for obscure gastrointestinal bleeding. J. Gastroenterol. 2010, 45, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Nakamura, M.; Ohmiya, N. Advanced network system for reading capsule endoscopy images. Dig. Endosc. 2013, 25, 91. [Google Scholar] [CrossRef] [PubMed]
- Brotz, C.; Nandi, N.; Conn, M.; Daskalakis, C.; DiMarino, M.; Infantolino, A.; Katz, L.C.; Schroeder, T.; Kastenberg, D. A validation study of 3 grading systems to evaluate small-bowel cleansing for wireless capsule endoscopy: A quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest. Endosc. 2009, 69, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yung, D.E.; Rondonotti, E.; Sykes, C.; Pennazio, M.; Plevris, J.N.; Koulaouzidis, A. Systematic review and meta-analysis: Is bowel preparation still necessary in small bowel capsule endoscopy? Expert Rev. Gastroenterol. Hepatol. 2017, 11, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Cotter, J.; Dias de Castro, F.; Magalhães, J.; Moreira, M.J.; Rosa, B. Validation of the Lewis score for the evaluation of small-bowel Crohn’s disease activity. Endoscopy 2015, 47, 330–335. [Google Scholar] [CrossRef] [PubMed]
- De Cruz, P.; Kamm, M.A.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Gorelik, A.; Liew, D.; Prideaux, L.; Lawrance, I.C.; Andrews, J.M.; et al. Crohn’s disease management after intestinal resection: A randomised trial. Lancet 2015, 385, 1406–1417. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, S.J.; Park, J.J.; Yun, Y.H.; Hong, S.P.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Effect of follow-up endoscopy on the outcomes of patients with inflammatory bowel disease. Dig. Dis. Sci. 2014, 59, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Pineton de Chambrun, G.; Peyrin-Biroulet, L.; Lémann, M.; Colombel, J.F. Clinical implications of mucosal healing for the management of IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Ungar, B.; Levy, I.; Yavne, Y.; Yavzori, M.; Picard, O.; Fudim, E.; Loebstein, R.; Chowers, Y.; Eliakim, R.; Kopylov, U.; et al. Optimizing anti-TNF-α therapy: Serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2016, 14, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2017, 17, 32641–32647. [Google Scholar] [CrossRef]
- Gal, E.; Geller, A.; Fraser, G.; Levi, Z.; Niv, Y. Assessment and validation of the new capsule endoscopy Crohn’s disease activity index (CECDAI). Dig. Dis. Sci. 2008, 53, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Niv, Y.; Ilani, S.; Levi, Z.; Hershkowitz, M.; Niv, E.; Fireman, Z.; O’Donnel, S.; O’Morain, C.; Eliakim, R.; Scapa, E.; et al. Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): A multicenter prospective study. Endoscopy 2012, 44, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, A.; Sipponen, T.; Nemeth, A.; Makins, R.; Kopylov, U.; Nadler, M.; Giannakou, A.; Yung, D.E.; Johansson, G.W.; Bartzis, L.; et al. Association between fecal calprotectin levels and small-bowel inflammation score in capsule endoscopy: A multicenter retrospective study. Dig. Dis. Sci. 2016, 61, 2033–2040. [Google Scholar] [CrossRef] [PubMed]



| Total | Active Group | Non Active Group | p Value | |
|---|---|---|---|---|
| N | 92 | 40 | 52 | |
| Age, mean ± SD, years old | 37.2 ± 12.3 | 37.5 ± 12.3 | 37.1 ± 12.9 | 0.8624 |
| Gender, M/F | 68/24 | 29/11 | 39/13 | 0.9751 |
| BMI | 21.3 ± 3.2 | 21.5 ± 3.6 | 21.1 ± 2.9 | 0.603 |
| Duration of disease, mean ± SD, months | 117.1 ± 96.7 | 93.9 ± 73.3 | 135 ± 108.8 | 0.093 |
| Montreal classification | ||||
| Age at diagnosis | ||||
| <17 years | 7 | 3 | 4 | |
| 17–40 years | 70 | 30 | 40 | |
| >40 years | 15 | 7 | 8 | |
| Location | ||||
| L1—ileal | 38 | 17 | 21 | |
| L2—colonic | 0 | |||
| L3—ileocolonic | 54 | 23 | 31 | |
| Behavior | ||||
| B1—Non-stricturing, non-penetrating | 72 | 28 | 44 | |
| B2—Stricturing | 15 | 9 | 6 | |
| B3—Penetrating | 5 | 3 | 2 | |
| p-perianal disease | 9 | 6 | 3 | |
| History of GI surgery | 53/92 | 25/40 | 28/52 | 0.5353 |
| Ileo-colonic resection | 26 | 12 | 14 | |
| Ileal resection | 21 | 9 | 12 | |
| Ileo-colonic resection plus Ileal resection | 4 | 3 | 1 | |
| Colonic resection | 2 | 1 | 1 | |
| Any symptom | 28/92 | 19/40 | 9/52 | 0.0038 |
| CDAI | 104 ± 56 | 116 ± 72 | 95 ± 39 | 0.277 |
| Laboratory data | ||||
| CRP (mg/dL), mean ± SD | 0.36 ± 0.62 | 0.53 ± 0.75 | 0.24 ± 0.47 | 0.0761 |
| Hb (g/dL), mean ± SD | 13.3 ± 2.1 | 12.7 ± 2.5 | 13.8 ± 1.6 | 0.0201 |
| Albumin (g/dL), mean ± SD | 4.0 ± 0.5 | 3.9 ± 0.5 | 4.2 ± 0.4 | 0.0014 |
| Indication of CE | ||||
| Symptom(s) | 28 | 19 | 9 | |
| diarrhea | 20 | 15 | 5 | |
| abdominal pain | 4 | 2 | 2 | |
| bloody stools | 3 | 2 | 1 | |
| abdominal fullness | 1 | 0 | 1 | |
| Monitoring | 64 | 21 | 43 | |
| PPC and CE | ||||
| PPC intact body excretion | 62/92 | 26/40 | 36/52 | 0.8377 |
| Gastric transit time (min.) | 45.5 ± 42.4 | 45.6 ± 40.3 | 47.2 ± 44.3 | 0.9904 |
| SBTT (min.) | 248.8 ± 128.8 | 270.7 ± 149.5 | 231.7 ± 108.5 | 0.3042 |
| Lewis score, mean ± SD | 396 ± 706 | 844 ± 892 | 52.1 ± 66.1 | <0.0001 |
| Treatment | ||||
| Anti TNF-α agent | 54/92 | 23/40 | 31/52 | |
| 5-ASA | 78/92 | 35/40 | 43/52 | |
| Immunomodulator | 14/92 | 5/40 | 9/52 | |
| Elemental diet | 57/92 | 23/40 | 34/52 | |
| Case | Medicine | Intervention | Lewis Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Score | Villous Edema | Ulcer | Stenosis | |||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||
| 1 | Infliximab | dose up 5 ⇒ 10 mg/kg | 2914 | 2824 | 112 | 112 | 450 | 360 | 2352 | 2352 |
| 2 | Infliximab | introduction 5 mg/kg | 2585 | 280 | 8 | 0 | 225 | 0 | 2352 | 280 |
| 3 | Adalimumab | introduction 160 mg | 2140 | 600 | 340 | 0 | 1800 | 600 | 0 | 0 |
| 4 | Azathiopurin | introduction 50 mg | 1012 | 337 | 112 | 112 | 900 | 225 | 0 | 0 |
| 5 | mercaptopurine | introduction 20 mg | 900 | 1368 | 0 | 168 | 900 | 1200 | 0 | 0 |
| 6 | 5-ASA | dose up 3000 ⇒ 4000 mg | 712 | 712 | 112 | 112 | 600 | 600 | 0 | 0 |
| 7 | Infliximab | introduction 5 mg/kg | 712 | 421 | 112 | 0 | 600 | 225 | 0 | 196 |
| 8 | Adalimumab | introduction 160 mg | 654 | 0 | 204 | 0 | 450 | 0 | 0 | 0 |
| 9 | Adalimumab | introduction 160 mg | 600 | 0 | 0 | 0 | 600 | 0 | 0 | 0 |
| 10 | Infliximab | dose up 5 ⇒ 10 mg/kg | 562 | 196 | 112 | 0 | 450 | 0 | 0 | 196 |
| 11 | 5-ASA | dose up 1500 ⇒ 2000 mg | 562 | 225 | 112 | 0 | 450 | 225 | 0 | 0 |
| 12 | 5-ASA | introduction 3000 mg | 562 | 337 | 112 | 112 | 450 | 225 | 0 | 0 |
| 13 | Infliximab | introduction 5 mg/kg | 562 | 233 | 112 | 8 | 450 | 225 | 0 | 0 |
| 14 | Azathiopurin | introduction 50 mg | 504 | 180 | 204 | 0 | 300 | 180 | 0 | 0 |
| 15 | Prednisolone | introduction 20 mg | 458 | 450 | 8 | 0 | 450 | 450 | 0 | 0 |
| 16 | 5-ASA | dose up 2000 ⇒ 3000 mg | 458 | 147 | 8 | 12 | 450 | 135 | 0 | 0 |
| 17 | Azathiopurin | introduction 50 mg | 450 | 458 | 0 | 8 | 450 | 450 | 0 | 0 |
| 18 | Adalimumab | introduction 160 mg | 436 | 225 | 136 | 0 | 300 | 225 | 0 | 0 |
| 19 | Elemental diet | dose up | 429 | 225 | 204 | 0 | 225 | 225 | 0 | 0 |
| 20 | Elemental diet | dose up | 412 | 225 | 112 | 0 | 300 | 225 | 0 | 0 |
| 21 | Infliximab | dose up 5 ⇒ 10 mg/kg | 412 | 225 | 112 | 0 | 300 | 225 | 0 | 0 |
| 22 | Infliximab | introduction 5 mg/kg | 337 | 135 | 112 | 0 | 225 | 135 | 0 | 0 |
| 23 | Azathiopurin | introduction 50 mg | 300 | 278 | 0 | 8 | 300 | 270 | 0 | 0 |
| 24 | Azathiopurin | dose up 50 ⇒ 75 mg | 300 | 180 | 0 | 0 | 300 | 180 | 0 | 0 |
| 25 | Azathiopurin | introduction 50 mg | 233 | 143 | 8 | 8 | 225 | 135 | 0 | 0 |
| 26 | Infliximab | dose up 5 ⇒ 10 mg/kg | 225 | 233 | 0 | 8 | 225 | 225 | 0 | 0 |
| 27 | 5-ASA | dose up 2000 ⇒ 3000 mg | 225 | 225 | 0 | 0 | 225 | 225 | 0 | 0 |
| 28 | 5-ASA | dose up 1500 ⇒ 3000 mg | 225 | 450 | 0 | 0 | 225 | 450 | 0 | 0 |
| 29 | 5-ASA | dose up 2000 ⇒ 3000 mg | 180 | 135 | 0 | 0 | 180 | 135 | 0 | 0 |
| Pre-Treatment | Post-Treatment | p Value | |
|---|---|---|---|
| CDAI | 102 (5–253) | 68 (0–231) | 0.0057 |
| Lewis score | 458 (180–2914) | 233 (0–2824) | 0.0004 |
| CRP level (mg/dL) | 0.55 ± 0.80 | 0.30 ± 0.51 | 0.0652 |
| WBC count (/µL) | 6822 ± 2602 | 5920 ± 1807 | 0.1663 |
| Hb level (g/dL) | 12.7 ± 2.5 | 13.2 ± 1.9 | 0.7843 |
| Plt count(×1000/mm3) | 26.6 ± 6.1 | 26.7 ± 7.0 | 0.5014 |
| Albumin level (g/dL) | 3.9 ± 0.6 | 4.1 ± 0.5 | 0.1297 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Yamamura, T.; Maeda, K.; Sawada, T.; Mizutani, Y.; Ishikawa, T.; Furukawa, K.; Ohno, E.; Kawashima, H.; Miyahara, R.; et al. Validity of Capsule Endoscopy in Monitoring Therapeutic Interventions in Patients with Crohn’s Disease. J. Clin. Med. 2018, 7, 311. https://doi.org/10.3390/jcm7100311
Nakamura M, Yamamura T, Maeda K, Sawada T, Mizutani Y, Ishikawa T, Furukawa K, Ohno E, Kawashima H, Miyahara R, et al. Validity of Capsule Endoscopy in Monitoring Therapeutic Interventions in Patients with Crohn’s Disease. Journal of Clinical Medicine. 2018; 7(10):311. https://doi.org/10.3390/jcm7100311
Chicago/Turabian StyleNakamura, Masanao, Takeshi Yamamura, Keiko Maeda, Tsunaki Sawada, Yasuyuki Mizutani, Takuya Ishikawa, Kazuhiro Furukawa, Eizaburo Ohno, Hiroki Kawashima, Ryoji Miyahara, and et al. 2018. "Validity of Capsule Endoscopy in Monitoring Therapeutic Interventions in Patients with Crohn’s Disease" Journal of Clinical Medicine 7, no. 10: 311. https://doi.org/10.3390/jcm7100311
APA StyleNakamura, M., Yamamura, T., Maeda, K., Sawada, T., Mizutani, Y., Ishikawa, T., Furukawa, K., Ohno, E., Kawashima, H., Miyahara, R., Koulaouzidis, A., Hirooka, Y., & The Nagoya University Crohn’s Disease Study Group. (2018). Validity of Capsule Endoscopy in Monitoring Therapeutic Interventions in Patients with Crohn’s Disease. Journal of Clinical Medicine, 7(10), 311. https://doi.org/10.3390/jcm7100311







