Feasibility of Serial 6-min Walk Tests in Patients with Acute Heart Failure
Abstract
:1. Background
2. Methods
3. Results
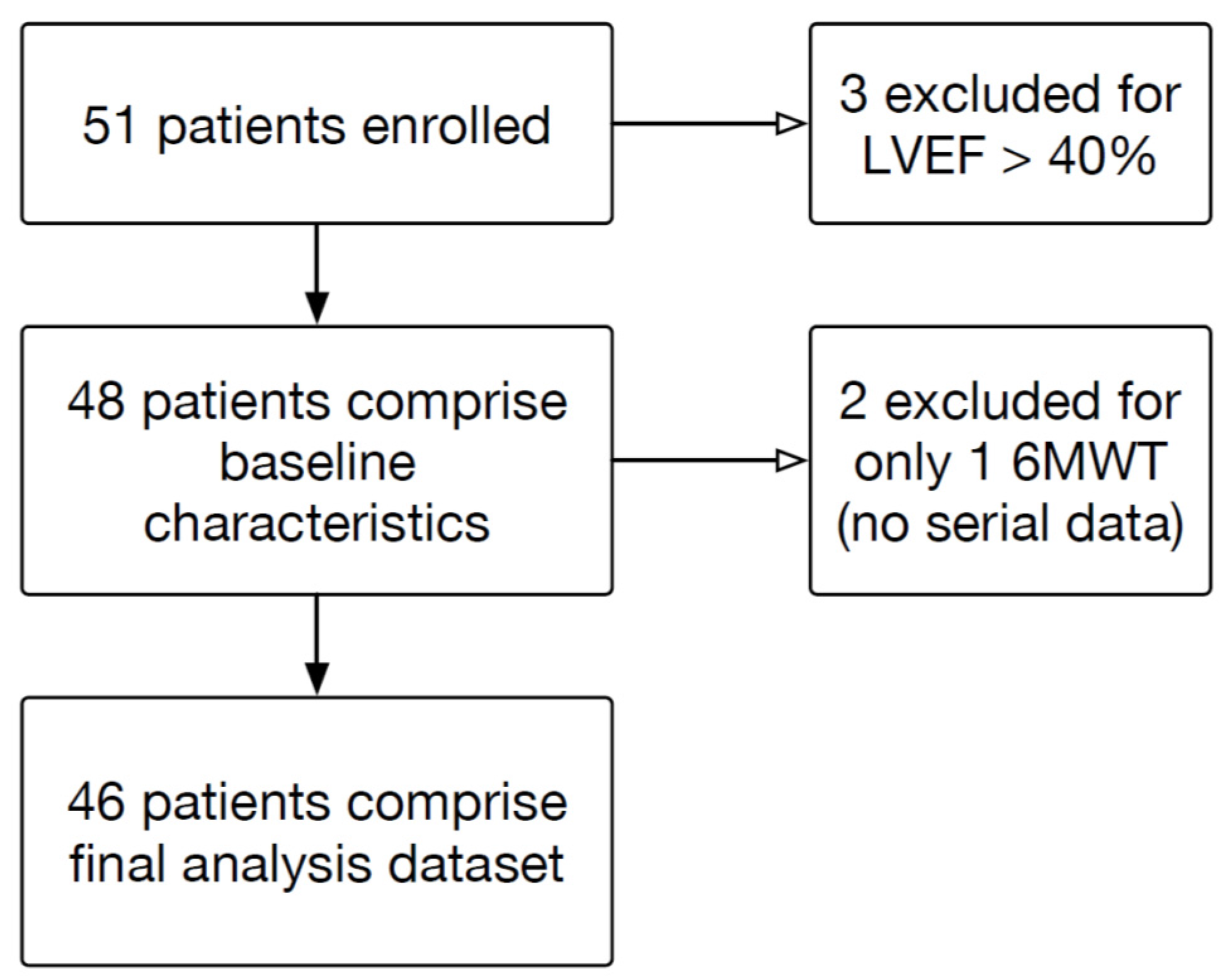
3.1. Participant Characteristics
3.2. Safety and Feasibility of 6MWT
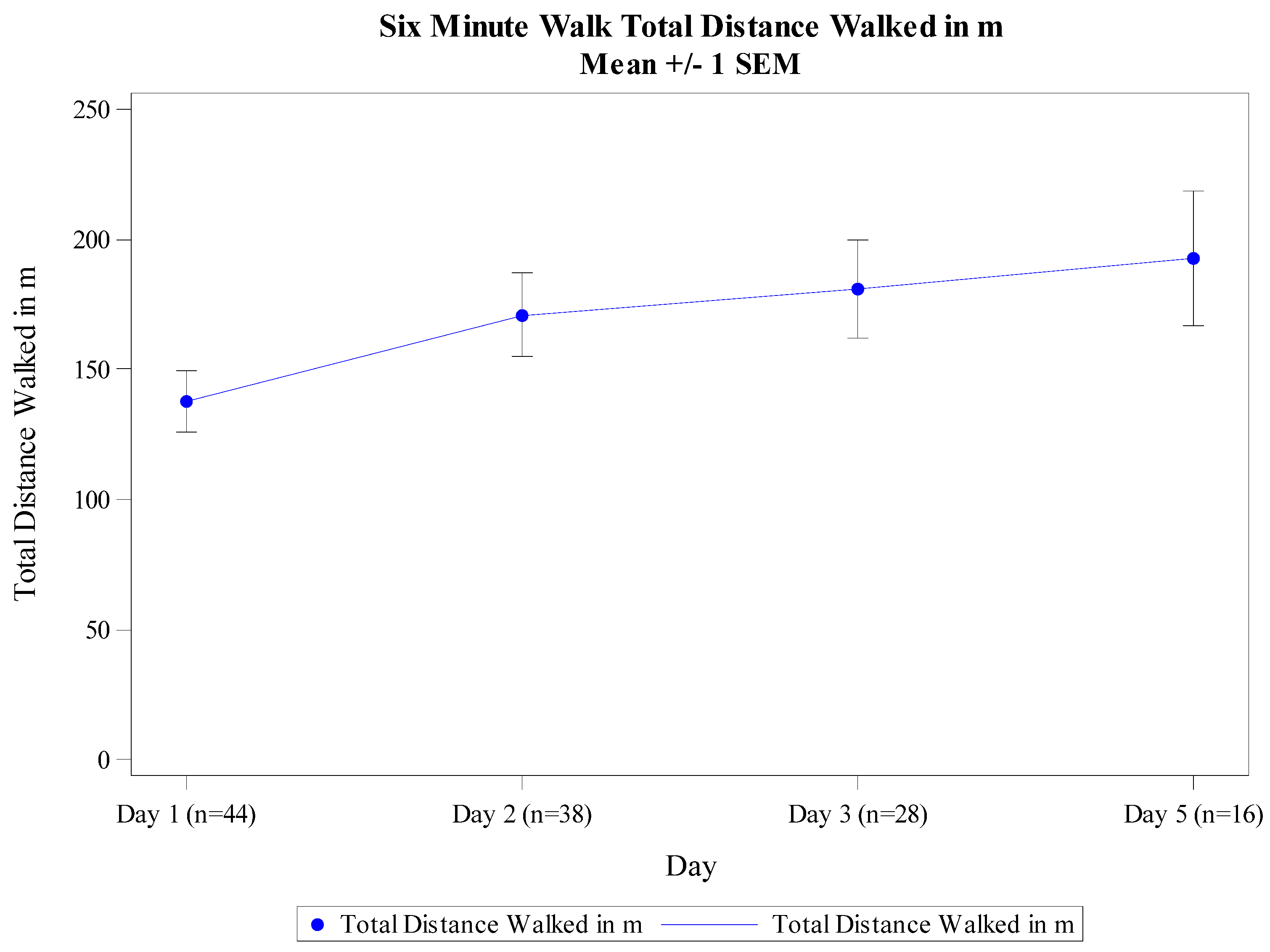
3.3. 6MWT Performance
3.4. 6MWT Correlation with Biomarkers and Outcomes
4. Discussion
Author Contributions
Conflicts of Interest and disclosures
References
- Stevenson, L.W.; Hellkamp, A.S.; Leier, C.V. Changing preferences for survival after hospitalization with advanced heart failure. J. Am. Coll. Cardiol. 2008, 52, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: A randomized clinical trial. JAMA 2016, 316, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, J.R.; Cotter, G.; Davison, B.A. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 2013, 381, 29–39. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Starling, R.C.; Hernandez, A.F. Effect of nesiritide in patients with acute decompensated heart failure. New Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.E.; Lin, L.; Ellis, S.J. Outcomes, health policy, and managed care: Relationships between patient-reported outcome measures and clinical measures in outpatients with heart failure. Am Heart J. 2009, 158, S64–S71. [Google Scholar] [CrossRef] [PubMed]
- Hasin, T.; Topilsky, Y.; Kremers, W.K. Usefulness of the six-minute walk test after continuous axial flow left ventricular device implantation to predict survival. Am. J. Cardiol. 2012, 110, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Dardas, T.; Li, Y.; Reed, S.D. Incremental and independent value of cardiopulmonary exercise test measures and the Seattle Heart Failure Model for prediction of risk in patients with heart failure. J. Heart Lung Transplant. 2015, 34, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.M.; Stiell, I.G.; Clement, C.M.; Acheson, J.; Aaron, S.D. Feasibility of a structured 3-minute walk test as a clinical decision tool for patients presenting to the emergency department with acute dyspnoea. Emerg. Med. J. 2009, 26, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Rogers, R.K.; Brass, E.P. The treadmill is a better functional test than the 6-minute walk test in therapeutic trials of patients with peripheral artery disease. Circulation 2014, 130, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.J.; Curtis, A.B.; Vangsnes, E.; Dickinson, M.G. Triangulating Clinically Meaningful Change in the Six-minute Walk Test in Individuals with Chronic Heart Failure: A Systematic Review. Cardiopulm. Phys. Ther. J. 2012, 23, 5–15. [Google Scholar] [PubMed]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A. Advanced heart failure treated with continuous-flow left ventricular assist device. New Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- AbouEzzeddine, O.F.; Lala, A.; Khazanie, P.P. Evaluation of a provocative dyspnea severity score in acute heart failure. Am. Heart J. 2016, 172, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S.; Shah, K.S.; Barnard, D. How B-Type Natriuretic Peptide (BNP) and Body Weight Changes Vary in Heart Failure With Preserved Ejection Fraction Compared With Reduced Ejection Fraction: Secondary Results of the HABIT (HF Assessment With BNP in the Home) Trial. J. Card. Fail. 2016, 22, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Birkhahn, R.H.; Guss, D. Rapid Emergency Department Heart Failure Outpatients Trial (REDHOT II): A randomized controlled trial of the effect of serial B-Type natriuretic peptide testing on patient management. Circ. Heart Fail. 2009, 2, 287–293. [Google Scholar] [CrossRef] [PubMed]


| Parameter | N * (%) | Mean (SD) |
|---|---|---|
| Age | 48 | 55.6 (13.0) |
| Gender | ||
| Male | 32 (67) | |
| Female | 16 (33) | |
| Race | ||
| White | 13 (27) | |
| Black/African-American | 35 (73) | |
| Primary HF Etiology | ||
| Ischemic | 15 (31) | |
| Dilated | 18 (28) | |
| Non-ischemic | 11 (23) | |
| Chemotherapy | 2 (4.2) | |
| Idiopathic | 1 (2.1) | |
| Combined Ischemic(MI) & Non-ischemic(HTN) | 1 (2.1) | |
| NYHA Class | ||
| III | 43 (90) | |
| IV | 3 (6.3) | |
| Unknown | 2 (4.2) | |
| History of Angina | ||
| Yes | 8 (17) | |
| No | 40 (83) | |
| LVEF by echocardiogram | 48 | 24 (9.1) |
| eGFR | 46 | 71 (22.0) |
| Baseline SBP (mmHg) | 48 | 127 (19) |
| Baseline DBP (mmHg) | 48 | 78 (15) |
| Baseline HR (bpm) | 48 | 86 (16) |
| Weight (kg) | 47 | 92.8 (30.1) |
| IV Diuretics | 41 (65) | |
| Topical/Sublingual Nitroglycerin | 7 (15) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collins, S.P.; Thorn, M.; Nowak, R.M.; Levy, P.D.; Fermann, G.J.; Hiestand, B.C.; Cowart, T.D.; Venuti, R.P.; Hiatt, W.R.; Foo, S.; et al. Feasibility of Serial 6-min Walk Tests in Patients with Acute Heart Failure. J. Clin. Med. 2017, 6, 84. https://doi.org/10.3390/jcm6090084
Collins SP, Thorn M, Nowak RM, Levy PD, Fermann GJ, Hiestand BC, Cowart TD, Venuti RP, Hiatt WR, Foo S, et al. Feasibility of Serial 6-min Walk Tests in Patients with Acute Heart Failure. Journal of Clinical Medicine. 2017; 6(9):84. https://doi.org/10.3390/jcm6090084
Chicago/Turabian StyleCollins, Sean P., Michael Thorn, Richard M. Nowak, Phillip D. Levy, Gregory J. Fermann, Brian C. Hiestand, Tillman Douglas Cowart, Robert P. Venuti, William R. Hiatt, ShiYin Foo, and et al. 2017. "Feasibility of Serial 6-min Walk Tests in Patients with Acute Heart Failure" Journal of Clinical Medicine 6, no. 9: 84. https://doi.org/10.3390/jcm6090084
APA StyleCollins, S. P., Thorn, M., Nowak, R. M., Levy, P. D., Fermann, G. J., Hiestand, B. C., Cowart, T. D., Venuti, R. P., Hiatt, W. R., Foo, S., & Pang, P. S. (2017). Feasibility of Serial 6-min Walk Tests in Patients with Acute Heart Failure. Journal of Clinical Medicine, 6(9), 84. https://doi.org/10.3390/jcm6090084





