MicroRNAs in the Cholangiopathies: Pathogenesis, Diagnosis, and Treatment
Abstract
:1. Introduction
| Genetic |
| Alagille’s syndrome |
| Cystic fibrosis |
| Fibropolycystic diseases (i.e., Caroli’s syndrome, congenital hepatic fibrosis, ADPKD, ARPKD, ADPLD) |
| Immune-mediated |
| Primary biliary cirrhosis |
| Primary sclerosing cholangitis |
| Hepatic allograft rejection |
| Graft vs. host disease involving the liver |
| Autoimmune cholangitis |
| Infectious |
| Bacterial cholangitis |
| Parasitic cholangitis |
| Fungal cholangitis |
| Viral cholangitis (i.e., AIDS cholangiopathy) |
| Drug-induced (i.e., Floxuridine-induced cholangiopathy) |
| Vascular/Ischemic (i.e., postliver transplantation hepatic artery stenosis) |
| Idiopathic |
| Biliary atresia |
| Sarcoidosis Idiopathic childhood/adulthood ductopenia |
| Malignant |
| Cholangiocarcinoma (i.e., bile duct adenocarcinoma) |

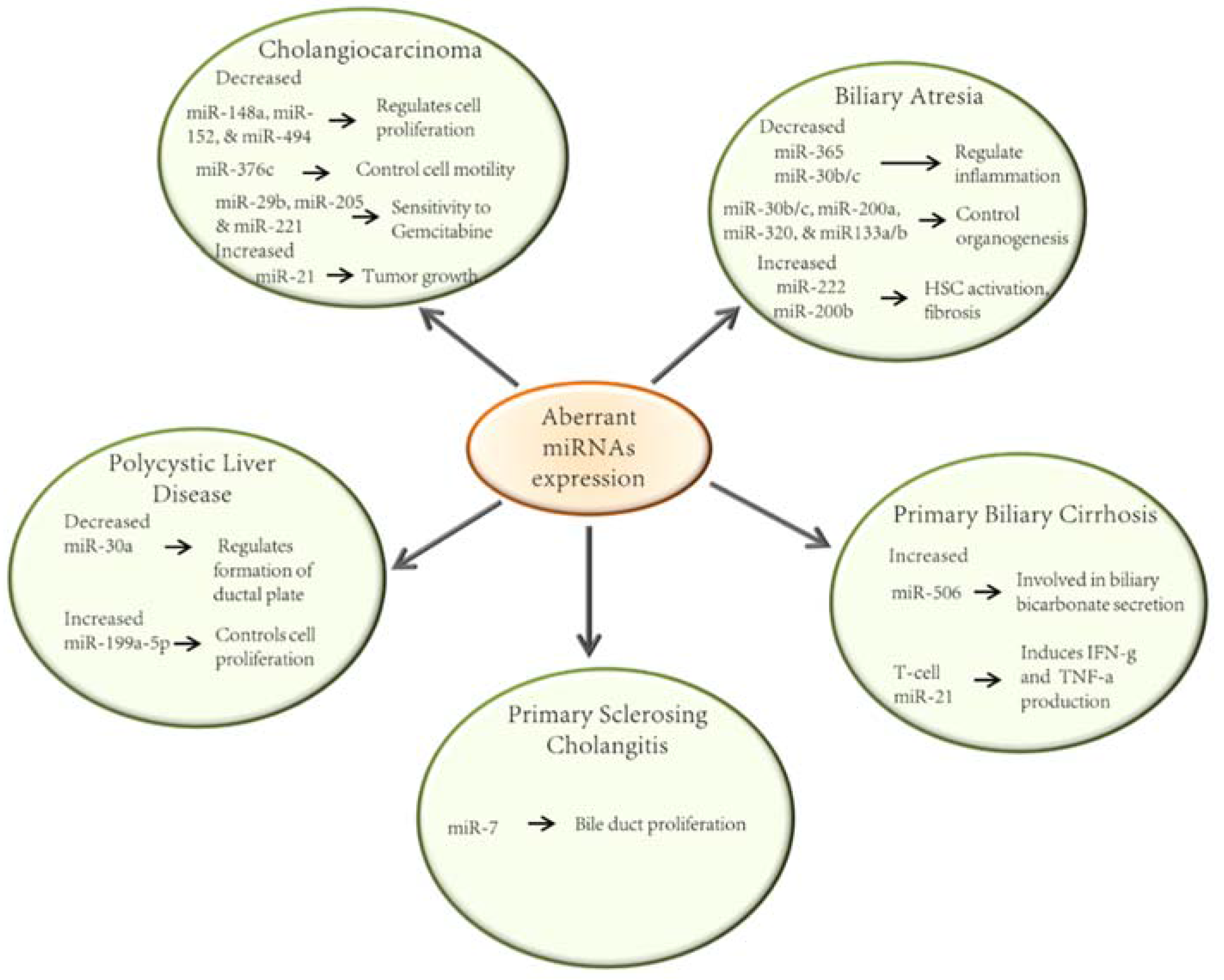
2. MicroRNAs in the Pathobiology of the Cholangiopathies
2.1. Biliary Atresia
2.2. Primary Biliary Cirrhosis
2.3. Primary Sclerosing Cholangitis
2.4. Polycystic Liver Disease
2.5. Cholangiocarcinoma
3. MiRNAs as Potential Biomarkers and Therapeutic Targets in the Cholangiopathies
3.1. Circulating MicroRNAs
3.2. MiRNAs as Biomarkers for the Cholangiopathies
3.2.1. Cholangiocarcinoma
| Cholangiopathy | Source | miRNA | Clinical Correlation | Human | Reference |
|---|---|---|---|---|---|
| CCA | |||||
| 1. Bile samples from patients who underwent prognostic and/or therapeutic bile drainage | Sensitivity level of 88.9% miR-9 miR-302c miR-199a-3p miR-222 Largest Area under ROC-curve: miR-9 and miR-145 Most promising miR: miR-9 | Diagnosis | Bile samples from patients with CCA (cholangiocarcinoma n = 7, gall bladder cancer n = 2) Bile samples from patients with choledocholithiasis without malignancy or inflammatory condition (age-matched) (n = 9) | [100] | |
| 2. Surgical specimens of ICC (chemo and radiotherapy naïve) | Overexpressed in ICC compared to control: miR-21 miR-31 miR-223 Down-regulated in ICC compared to control: miR-122 miR-145 miR-146a miR-200c miR-221 miR-222 | No correlation found with clinicopathological features but encouraged further prospective studies to explore the significance of findings | Surgical specimens of ICC (n = 21) Liver specimens of healthy controls (n = 98) | [102] | |
| 3. Surgical specimens of CCA (chemo and radiotherapy naïve) | Higher expression correlated with worse prognosis: miR-21 | Prognosis | Surgical specimens of CCA (n = 41) | [104] | |
| 4. Surgical specimens of CCA | Concordant dysregulation: miR-151-3p miR-126 | Prognosis and potential therapeutic targets | Surgical specimens of CCA with adjacent uninvolved bile duct epithelium (n = 32) | [103] | |
| 5. Bile samples obtained during ERCP or PTC | miR-species panel: miR-191 miR-486-3p miR-1274b miR-16 miR-484 | Diagnosis | Bile samples obtained during ERCP from CCA patients (n = 46) Controls (n = 50) | [49] | |
| 6. Tumor tissue from liver fluke (Opisthorchis Vierrini) (Ov)—associated cholangiocarcinoma and serum | miR significantly higher in the serum of CCA patients compared to healthy subjects: miR-192 | Prognosis and diagnosis | Tumor with adjacent non-tumor tissues (n = 30) Sera from patients with ICC (n=51) Sera from healthy subjects negative for Ov (n = 32) Sera from subjects infected with Ov (n = 10) Subjects with periductal fibrosis (n = 20) | [101] | |
| 7. Plasma from patients with Ov induced ICC | Detected in ICC but not control: miR-483-5p miR-505-3p miR-874 miR-885-5p miR-320b miR-92b-3p miR-1275 miR-1307-3p | Diagnosis | Plasma samples of Ov-induced ICC from patients: Well differentiated ICC (n = 4) Moderately differentiated ICC (n = 2) Papillary ICC (n = 6) Plasma controls (n = 5) | [105] | |
| PLD | |||||
| 1. Urine specimens of ADPKD patients | Higher in urine cells from ADPKD compared to other chronic kidney disease (CKD) patients: miR-143(2) Lower in urine cells from APKD compared to other CKD patients: miR-133b(2) miR-1(4) Increased abundance in ADPKD urine cells: miR-223(1) miR-199a(3) miR-199b(1) Less abundant in ADPKD urine microvesicles compared to other CKD patients miR-1(2) miR-133a(2) | Monitoring disease progression and treatment response | Urine specimens of ADPKD patients (n = 20) Urine specimens of patients with CKD of other etiologies (n = 20) | [106] | |
| PBC | |||||
| 1. Serum from patients with PBC | Downregulated in PBC: hsa-miR-505-3p miR-197-3p | Diagnosis | Sera of patients with PBC (n = 10) (treatment naïve), sera of patients with CH-B (n = 5), sera of patients with CH-C (n = 5), and sera of healthy controls (n = 5). | [107] | |
| 2. Peripheral blood mononuclear cells (PBMCs) from PBC patients | Upregulated in PBC: miR-15a-5p miR-20a-5p miR-140-3p miR-106b-5p Down-regulated in PBC: miR-3654 miR-181a-5p | Diagnosis or treatment | PBC patients (n = 9) and healthy controls (n = 9) matched by gender and age | [108] | |
| 3. Serum from patients with PBC | Upregulated in PBC: hsa-miR-122-5p hsa-miR-34a-5p hsa-miR-141-3p hsa-miR-27b-3p Downregulated in PBC: hsa-miR-26b-5p | Diagnosis | Sera of patients with PBC (n = 207) Sera of healthy controls (n = 173) | [109] | |
| BA | |||||
| 1. Serum | Upregulated in BA: miR-200b/429 | Diagnosis | [110] |
3.2.2. MiRNAs as Biomarkers in Other Cholangiopathies
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Glaser, S.; Meng, F.; Francis, H.; Marzioni, M.; McDaniel, K.; Alvaro, D.; Venter, J.; Carpino, G.; Onori, P.; et al. Recent advances in the morphological and functional heterogeneity of the biliary epithelium. Exp. Biol. Med. 2013, 238, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; M.T.; LaRusso, N.F. Physiology of Cholangiocytes. In Physiology of the Gastrointestinal Tract, 5th ed.; Johnson, L., Ghishan, F., Kaunitz, J., Merchant, J., Said, H., Wood, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1431–1459. [Google Scholar]
- Schaffner, F.; Popper, H. Electron. Microscopic Studies of Normal and Proliferated Bile Ductules. Am. J. Pathol. 1961, 38, 393–410. [Google Scholar] [PubMed]
- Sasaki, H.; Schaffner, F.; Popper, H. Bile ductules in cholestasis: Morphologic evidence for secretion and absorption in man. Lab. Investig. 1967, 16, 84–95. [Google Scholar] [PubMed]
- Alpini, G.P.J.; LaRusso, N.F. The biology of biliary epithelia. In The Liver: Biology and Pathobiology; Arias, J.B.I., Fausto, N., Jakoby, W., Schachter, D., Shafritz, D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 623–653. [Google Scholar]
- Masyuk, A.I.; Masyuk, T.V.; Splinter, P.L.; Huang, B.Q.; Stroope, A.J.; LaRusso, N.F. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 2006, 131, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, S.A.; Masyuk, A.I.; Splinter, P.L.; Banales, J.M.; Huang, B.Q.; Tietz, P.S.; Masyuk, T.V.; Larusso, N.F. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc. Natl. Acad. Sci. USA 2007, 104, 19138–19143. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Gradilone, S.A.; Banales, J.M.; Huang, B.Q.; Masyuk, T.V.; Lee, S.O.; Splinter, P.L.; Stroope, A.J.; Larusso, N.F. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G725–G734. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Huang, B.Q.; Ward, C.J.; Gradilone, S.A.; Banales, J.M.; Masyuk, T.V.; Radtke, B.; Splinter, P.L.; LaRusso, N.F. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G990–G999. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Cholangiocyte primary cilia in liver health and disease. Dev. Dyn. 2008, 237, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Bogert, P.T.; LaRusso, N.F. Cholangiocyte biology. Curr. Opin. Gastroenterol. 2007, 23, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.N.; Strazzabosco, M.; Larusso, N.F. The cholangiopathies: Disorders of biliary epithelia. Gastroenterology 2004, 127, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.P.; Tabibian, J.H.; Splinter, P.L.; LaRusso, N.F. The dynamic biliary epithelia: Molecules, pathways, and disease. J. Hepatol. 2013, 58, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. microRNA involvement in human cancer. Carcinogenesis 2012, 33, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell 2003, 113, 673–676. [Google Scholar] [CrossRef]
- Wang, N.; Bu, R.; Duan, Z.; Zhang, X.; Chen, P.; Li, Z.; Wu, J.; Cai, G.; Chen, X. Profiling and initial validation of urinary microRNAs as biomarkers in IgA nephropathy. Peer J. 2015, 3, e990. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, V.; Appierto, V.; Tiberio, P.; Fina, E.; Callari, M.; Daidone, M.G. Circulating Biomarkers for Prediction of Treatment Response. J. Natl. Cancer Inst. Monogr. 2015, 2015, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Fraile, E.; Ramos, E.; Conde, E.; Rodríguez, M.1.; Martín-Gómez, L.; Lietor, A.; Candela, Á.; Ponte, B.; Liaño, F.; García-Bermejo, M.L. A Pilot Study Identifying a Set of microRNAs As Precise Diagnostic Biomarkers of Acute Kidney Injury. PLoS ONE 2015, 10, e0127175. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Jeong, P.; Kang, H.W.; Kim, Y.H.; Kim, E.A.; Yan, C.; Choi, Y.K.; Kim, D.; Kim, J.M.; Kim, S.K.; et al. Urinary MicroRNAs of Prostate Cancer: Virus-Encoded hsv1-miRH18 and hsv2-miR-H9–5p Could Be Valuable Diagnostic Markers. Int. Neurourol. J. 2015, 19, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Nouraee, N.; Mowla, S.J. miRNA therapeutics in cardiovascular diseases: Promises and problems. Front. Genet. 2015, 6, 232. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Tonevitsky, A.G.; Cho, W.C.; Burwinkel, B. Check and mate to exosomal extracellular miRNA: New lesson from a new approach. Front. Mol. Biosci. 2015, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Lin, B.; Ye, Y.; Wen, D.; Chen, L.; Zhou, X. Differential expression of serum microRNAs in cirrhosis that evolve into hepatocellular carcinoma related to hepatitis B virus. Oncol. Rep. 2015, 33, 2863–2870. [Google Scholar] [PubMed]
- Li, G.; Shen, Q.; Li, C.; Li, D.; Chen, J.; He, M. Identification of circulating MicroRNAs as novel potential biomarkers for hepatocellular carcinoma detection: A systematic review and meta-analysis. Clin. Transl. Oncol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, V.V.; Rykova, E.; Ponomareva, A.A.; Zaporozhchenko, I.A.; Morozkin, E.S.; Cherdyntseva, N.V.; Laktionov, P.P. Circulating microRNAs in lung cancer: Prospects for diagnostics, prognosis and prediction of antitumor treatment efficiency. Mol. Biol. 2015, 49, 55–66. [Google Scholar] [CrossRef]
- Lan, H.; Lu, H.; Wang, X.; Jin, H. MicroRNAs as potential biomarkers in cancer: Opportunities and challenges. Biomed. Res. Int. 2015, 2015, 125094. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.P.; Sallustio, F.; Serino, G. microRNAs in glomerular diseases from pathophysiology to potential treatment target. Clin. Sci. 2015, 128, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, S.A.; O’Hara, S.P.; Masyuk, T.V.; Pisarello, M.J.; LaRusso, N.F. MicroRNAs and benign biliary tract diseases. Semin. Liver Dis. 2015, 35, 26–35. [Google Scholar] [PubMed]
- Hand, N.J.; Horner, A.M.; Zankhana, R.M.; Boateng, L.A.; LeGuen, C.; Uvaydova, M.; Friedman, J.R. MicroRNA profiling identifies miR-29 as a regulator of disease-associated pathways in experimental biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Bessho, K.; Shanmukhappa, K.; Sheridan, R.; Shivakumar, P.; Mourya, R.; Walters, S.; Kaimal, V.; Dilbone, E.; Jegga, A.G.; Bezerra, J.A. Integrative genomics identifies candidate microRNAs for pathogenesis of experimental biliary atresia. BMC Syst. Biol. 2013, 7, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hand, N.J.; Master, Z.R.; Eauclaire, S.F.; Weinblatt, D.E.; Matthews, R.P.; Friedman, J.R. The microRNA-30 family is required for vertebrate hepatobiliary development. Gastroenterology 2009, 136, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, G.; Dong, R.; Zhao, R.; Zheng, S. MicroRNA-21/PTEN/Akt axis in the fibrogenesis of biliary atresia. J. Pediatr. Surg. 2014, 49, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zheng, Y.; Chen, G.; Zhao, R.; Zhou, Z.; Zheng, S. miR-222 Overexpression May Contribute to Liver Fibrosis in Biliary Atresia by Targeting PPP2R2A. Hepatology 2015, 60, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, J.; Chen, Y.; Zhou, K.; Wen, J.; Wang, Y.; Zhou, Y.; Pan, W.; Cai, W. Up-regulation of miR-200b in biliary atresia patients accelerates proliferation and migration of hepatic stellate cells by activating PI3K/Akt signaling. Cell. Signal. 2014, 26, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.U.; Urban, G.W.; Bettermann, K.; Vucur, M.; Zimmermann, H.; Schmidt, S.; Janssen, J.; Koppe, C.; Knolle, P.; Castoldi, M.; et al. Micro-RNA Profiling Reveals a Role for miR-29 in Human and Murine Liver Fibrosis. Hepatology 2011, 53, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Saez, E.; Uriz, M.; Sarvide, S.; Urribari, A.D.; Splinter, P.; Tietz Bogert, P.S.; Bujanda, L.; Prieto, J.; Medina, J.F.; et al. Upregulation of mir-506 Leads to Decreased AE2 Expression in Biiary Epithelium of Patients with Primary Biliary Cirrhosis. Hepatology 2012, 56, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Yang, G.X.; Penny, T.P.; Kawata, K.; Zhang, W.; Huang, W.; Leung, P.S.C.; Lian, Z.X.; Okazaki, K.; Ansari, A.A.; et al. Overexpression of microRNA-21 is associated with elevated pro-inflammatory cytokines in dominant-negative TGF-β receptor type II mouse. J. Autoimmun. 2013, 41, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Perugorria, M.J.; Masyuk, T.V.; Marin, J.J.; Marzioni, M.; Bujanda, L.; LaRusso, N.F.; Banales, J.M. Polycystic liver diseases: Advanced insights into the molecular mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, T.; Masyuk, A.; LaRusso, N. MicroRNAs in cholangiociliopathies. Cell Cycle 2009, 8, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, J.; Wu, M.; Sun, H.; Zhou, C.; Fu, L.; Xu, C.; Mei, C. Inhibition of MiR-199a-5p reduced cell proliferation in autosomal dominant polycystic kidney disease through targeting CDKN1C. Med. Sci. Monit. 2015, 21, 195–200. [Google Scholar] [PubMed]
- Olaru, A.V.; Ghiaur, G.; Yamanaka, S.; Luvsanjav, D.; An, F.; Popescu, I.; Alexandrescu, S.; Allen, S.; Pawlik, T.M.; Torbenson, M.; et al. MicroRNA down-regulated in human cholangiocarcinoma control cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology 2011, 54, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Selaru, F.M.; Olaru, A.V.; Kan, T.; David, S.; Cheng, Y.; Mori, Y.; Yang, J.; Paun, B.; Jin, Z.; Agarwal, R.; et al. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology 2009, 49, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Byrnes, K.; Han, C.; Wang, Y.; Wu, T. miR-21 targets 15-PGDH and promotes cholangiocarcinoma growth. Mol. Cancer Res. 2014, 12, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Braconi, C.; Huang, N.; Patel, T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology 2010, 51, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Padgett, K.A.; Lan, R.Y.; Leung, P.C.; Lleo, A.; Dawson, K.; Pfeiff, J.; Mao, T.K.; Coppel, R.L.; Ansari, A.A.; Gershwin, M.E. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J. Autoimmun. 2009, 32, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Katsushima, F.; Takahashi, A.; Sakamoto, N.; Kanno, Y.; Abe, K.; Ohira, H. Expression of micro-RNAs in peripheral blood mononuclear cells from primary biliary cirrhosis patients. Hepatol. Res. 2014, 44, E189–E197. [Google Scholar] [PubMed]
- Tabibian, J.H.; O’Hara, S.P.; Splinter, P.L.; Trussoni, C.E.; LaRusso, N.F. Cholangiocyte Senescence by Way of N-Ras Activation Is a Characteristic of Primary Sclerosing Cholangitis. Hepatology 2014, 59, 2263–2275. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Masica, D.; Ishida, M.; Tomuleasa, C.; Umegaki, S.; Kalloo, A.N.; Georgiades, C.; Singh, V.K.; Khashab, M.; Amateu, S.; et al. Human Bile Contains MicroRNA-Laden Extracellular Vesicles That Can. Be Used for Cholangiocarcinoma Diagnosis. Hepatology 2014, 60, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, T.; Masyuk, A.; LaRusso, N. MicroRNAs in cholangiociliopathies. Cell Cycle 2009, 8, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Gabow, P.A. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1993, 329, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J.; Chapman, A.B.; Torres, V.E. Volume progression in autosomal dominant polycystic kidney disease: The major factor determining clinical outcomes. Clin. J. Am. Soc. Nephrol. 2006, 1, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Onori, P.; Franchitto, A.; Mancinelli, R.; Carpino, G.; Alvaro, D.; Francis, H.; Alpini, G.; Gaudio, E. Polycystic liver diseases. Dig. Liver Dis. 2010, 42, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Mi, Q.S.; Dong, Z. microRNAs in kidneys: Biogenesis, regulation, and pathophysiological roles. Am. J. Physiol. Renal Physiol. 2011, 300, F602–F610. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Masyuk, T.; Splinter, P.; Banales, J.M.; Masyuk, A.; Stroope, A.; Larusso, N. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J. Clin. Investig. 2008, 118, 3714–3724. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Brors, B.; Srivastava, P.K.; Bott, A.; Boehn, S.N.; Groene, H.J.; Gretz, N. Microarray-based approach identifies microRNAs and their target functional patterns in polycystic kidney disease. BMC Genom. 2008, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Williams, D.; Hajarnis, S.; Hunter, R.; Pontoglio, M.; Somlo, S.; Igarashi, P. miR-17~92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2013, 110, 10765–10770. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Hajarnis, S.; Williams, D.; Hunter, R.; Huynh, D.; Igarashi, P. MicroRNAs regulate renal tubule maturation through modulation of Pkd1. J. Am. Soc. Nephrol. 2012, 23, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Dweep, H.; Sticht, C.; Kharkar, A.; Pandey, P.; Gretz, N. Parallel analysis of mRNA and microRNA microarray profiles to explore functional regulatory patterns in polycystic kidney disease: Using PKD/Mhm rat model. PLoS ONE 2013, 8, e53780. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Qin, S.; Ho, J.; Zhou, J.; Kreidberg, J.A. Systems biology approach to identify transcriptome reprogramming and candidate microRNA targets during the progression of polycystic kidney disease. BMC Syst. Biol. 2011, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Wills, E.S.; Roepman, R.; Drenth, J.P. Polycystic liver disease: Ductal plate malformation and the primary cilium. Trends Mol. Med. 2014, 20, 261–270. [Google Scholar] [CrossRef] [PubMed]
- De Groen, P.C.; Gores, G.J.; LaRusso, N.F.; Gunderson, L.L.; Nagorney, D.M. Biliary tract cancers. N. Engl. J. Med. 1999, 341, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Nikitina, E.G.; Urazova, L.N.; Stegny, V.N. MicroRNAs and human cancer. Exp. Oncol. 2012, 34, 2–8. [Google Scholar] [PubMed]
- Mendell, J.T.; Olson, E.N. MicroRNAs in stress signaling and human disease. Cell 2012, 148, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Garrido, P.; García-Fernández de Barrena, M.; Hijona, E.; Carracedo, M.; Marín, J.J.; Bujanda, L.; Banales, J.M. MicroRNAs in biliary diseases. World J. Gastroenterol. 2012, 18, 6189–6196. [Google Scholar] [CrossRef] [PubMed]
- Haga, H.; Yan, I.; Takahashi, K.; Wood, J.; Patel, T. Emerging insights into the role of microRNAs in the pathogenesis of cholangiocarcinoma. Gene Expr. 2014, 16, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, H.X.; Yang, W.; Hu, L.; Yu, L.X.; Liu, Q.; Li, L.; Huang, D.D.; Ding, J.; Shen, F.; et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J. Hepatol. 2009, 50, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Campbell, N.R.; An, F.; Kuo, S.C.; Potter, J.J.; Mezey, E.; Maitra, A.; Selaru, F.M. Coordinated effects of microRNA-494 induce G(2)/M arrest in human cholangiocarcinoma. Cell Cycle 2012, 11, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Huang, F.; Deng, G.; Nie, W.; Huang, W.; Zeng, X. miR-31 promotes oncogenesis in intrahepatic cholangiocarcinoma cells via the direct suppression of RASA1. Exp. Ther. Med. 2013, 6, 1265–1270. [Google Scholar] [PubMed]
- Razumilava, N.; Bronk, S.F.; Smoot, R.L.; Fingas, C.D.; Werneburg, N.W.; Roberts, L.R.; Mott, J.L. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology 2012, 55, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, Y.; Tang, C.; Li, H.; Wang, B.; Yan, Q.; Hu, J.; Zou, S. MicroRNA-144 suppresses cholangiocarcinoma cell proliferation and invasion through targeting platelet activating factor acetylhydrolase isoform 1b. BMC Cancer 2014, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Smith, H.; Ueno, Y.; Patel, T. The MicroRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J. Biol. Chem. 2007, 282, 8256–8264. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, J.; Kikuchi, K.; Mizuguchi, Y.; Kawahigashi, Y.; Yoshida, H.; Uchida, E.; Takizawa, T. MiR-376c down-regulation accelerates EGF-dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line. PLoS ONE 2013, 8, e69496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, C.; Zhu, H.; Song, K.; Wu, T. miR-101 inhibits cholangiocarcinoma angiogenesis through targeting vascular endothelial growth factor (VEGF). Am. J. Pathol. 2013, 182, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Miyoshi, K.; Murawaki, Y. miR-29b, miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells. PLoS ONE 2013, 8, e77623. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Henson, R.; Lang, M.; Wehbe, H.; Maheshwari, S.; Mendell, J.T.; Jiang, J.; Schmittgen, T.D.; Patel, T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 2006, 130, 2113–2129. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Greening, D.W.; Barnes, T.W.; Lim, J.W.; Tauro, B.J.; Rai, A.; Xu, R.; Adda, C.; Mathivanan, S.; Zhao, W.; et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics 2013, 13, 1672–1686. [Google Scholar] [CrossRef] [PubMed]
- Chiam, K.; Wang, T.; Watson, D.I.; Mayne, G.C.; Irvine, T.S.; Bright, T.; Smith, L.; White, I.A.; Bowen, J.M.; Keefe, D.; et al. Circulating Serum Exosomal miRNAs As Potential Biomarkers for Esophageal Adenocarcinoma. J. Gastrointest. Surg. 2015, 19, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Brunella, T.; Emiliani, C. Signaling pathways in exosomes biogenesis, secretion and fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Odorizzi, G. Get on the exosome bus with ALIX. Nat. Cell Biol. 2012, 14, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Babst, M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr. Opin. Cell Biol. 2011, 23, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Juarez, G.; Gupta, S.K.; Weight, R.M.; Polo-Parada, L.; Papagiorgio, C.; Bunch, J.D.; Viator, J.A. Optical Photoacoustic Detection of Circulating Melanoma Cells In Vitro. Int. J. Thermophys. 2010, 31, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.P.; Ismail, N.; Zhang, X.; Aguda, B.D.; Lee, E.J.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.L.; Schmittgen, T.D.; et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE 2008, 3, e3694. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, R.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Bang, C.; Thum, T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Pressman, S.; Andress, A.P.; Kim, K.; White, J.L.; Cassidy, J.J.; Li, X.; Lubell, K.; do Lim, H.; Cho, I.S.; et al. Silencing by small RNAs is linked to endosomal trafficking. Nat. Cell Biol. 2009, 11, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Gibbings, D.J.; Ciaudo, C.; Erhardt, M.; Voinnet, O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 2009, 11, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Farber, E.L.; Rapoport, A.L.; Tejada, D.; Deniskin, R.; Akhmedov, N.B.; Farber, D.B. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE 2009, 4, e4722. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- De Krijger, I.; Mekenkamp, L.J.; Punt, C.J.; Nagtegaal, I.D. MicroRNAs in colorectal cancer metastasis. J. Pathol. 2011, 224, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Kimura, M.; Asari, S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol. Rep. 2012, 28, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hou, L.; Li, A.; Duan, Y.; Gao, H.; Song, X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Janowska-Wieczorek, A.; Majka, M.; Kijowski, J.; Baj-Krzyworzeka, M.; Reca, R.; Turner, A.R.; Ratajczak, J.; Emerson, S.G.; Kowalska, M.A.; Ratajczak, M.Z. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood 2001, 98, 3143–3149. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Toti, F.; Hugel, B.; Freyssinet, J.M. Cellular microparticles: A disseminated storage pool of bioactive vascular effectors. Curr. Opin. Hematol. 2004, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Shigehara, K.; Yokomuro, S.; Ishibashi, O.; Mizuguchi, Y.; Arima, Y.; Kawahigashi, Y.; Kanda, T.; Akagi, I.; Tajiri, T.; Yoshida, H.; et al. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLoS ONE 2011, 6, e23584. [Google Scholar] [CrossRef] [PubMed]
- Silakit, R.; Loilome, W.; Yongvanit, P.; Chusorn, P.; Techasen, A.; Boonmars, T.; Khuntikeo, N.; Chamadol, N.; Pairojkul, C.; Namwat, N. Circulating miR-192 in liver fluke-associated cholangiocarcinoma patients: A prospective prognostic indicator. J. Hepatobiliary Pancreat. Sci. 2014, 21, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Papaconstantinou, I.; Gazouli, M.; Lyberopoulou, A.; Polymeneas, G.; Voros, D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol. Carcinog. 2013, 52, 297–303. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.E.; Collins, A.; Wojcik, S.E.; Liu, J.; Henry, J.C.; Jiang, J.; Schmittgen, T.; Bloomston, M. Concomitant dysregulation of microRNAs miR-151–3p and miR-126 correlates with improved survival in resected cholangiocarcinoma. HPB 2013, 15, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, L.; Liu, C.H.; You, H.; Shao, F.; Xie, F.; Lin, X.S.; Hu, S.Y.; Zhang, C.H. MicroRNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potential biomarker for cancer prognosis. Asian Pac. J. Cancer Prev. 2013, 14, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Plieskatt, J.; Rinaldi, G.; Feng, Y.; Peng, J.; Easley, S.; Jia, X.; Potriquet, J.; Pairojkul, C.; Bhudhisawasdi, V.; Sripa, B.; et al. A microRNA profile associated with Opisthorchis viverrini-induced cholangiocarcinoma in tissue and plasma. BMC Cancer 2015, 15, 309. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, I.Z.; Tan, Y.C.; Morozov, P.; Wilson, P.D.; Rennert, H.; Blumenfeld, J.D.; Tuschl, T. Urine microRNA as potential biomarkers of autosomal dominant polycystic kidney disease progression: Description of miRNA profiles at baseline. PLoS ONE 2014, 9, e86856. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, M.; Kondo, Y.; Funayama, R.; Nagashima, T.; Kogure, T.; Kakazu, E.; Kimura, O.; Ueno, Y.; Nakayama, K.; Shimosegawa, T. Distinct microRNAs expression profile in primary biliary cirrhosis and evaluation of miR 505–3p and miR197–3p as novel biomarkers. PLoS ONE 2013, 8, e66086. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Huang, F.; Liang, Y.; Yang, Z.; Zhong, R. Analysis of altered microRNA expression profiles in peripheral blood mononuclear cells from patients with primary biliary cirrhosis. J. Gastroenterol. Hepatol. 2013, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Pan, T.; Ye, Y.; Ge, G.; Chen, L.; Wen, D.; Zou, S. Serum microRNAs as potential biomarkers of primary biliary cirrhosis. PLoS ONE 2014, 9, e111424. [Google Scholar] [CrossRef] [PubMed]
- Zahm, A.M.; Hand, N.J.; Boateng, L.A.; Friedman, J.R. Circulating MicroRNA is a Biomarker of Biliary Atresia. J. Pediatr. Gastroenterol Nutr. 2012, 55, 366–369. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisarello, M.J.L.; Loarca, L.; Ivanics, T.; Morton, L.; LaRusso, N. MicroRNAs in the Cholangiopathies: Pathogenesis, Diagnosis, and Treatment. J. Clin. Med. 2015, 4, 1688-1712. https://doi.org/10.3390/jcm4091688
Pisarello MJL, Loarca L, Ivanics T, Morton L, LaRusso N. MicroRNAs in the Cholangiopathies: Pathogenesis, Diagnosis, and Treatment. Journal of Clinical Medicine. 2015; 4(9):1688-1712. https://doi.org/10.3390/jcm4091688
Chicago/Turabian StylePisarello, Maria Jose Lorenzo, Lorena Loarca, Tommy Ivanics, Leslie Morton, and Nicholas LaRusso. 2015. "MicroRNAs in the Cholangiopathies: Pathogenesis, Diagnosis, and Treatment" Journal of Clinical Medicine 4, no. 9: 1688-1712. https://doi.org/10.3390/jcm4091688
APA StylePisarello, M. J. L., Loarca, L., Ivanics, T., Morton, L., & LaRusso, N. (2015). MicroRNAs in the Cholangiopathies: Pathogenesis, Diagnosis, and Treatment. Journal of Clinical Medicine, 4(9), 1688-1712. https://doi.org/10.3390/jcm4091688