3.1. Clinical Characteristics
Clinical and laboratory data in the 60 elderly patients with AE are summarized in
Table 1. According to the serological atopic diathesis, these cases were classified into three types as IgE-allergic AE (
n = 38), indeterminate -allergic AE (
n = 9), and non-IgE-allergic AE (
n = 13), and we analyzed each characteristic. The male/female ratio was 41/19, and male predominance was seen in each type. Needless to say, the average of total IgE values of IgE-allergic AE showed a higher value with the significant difference than those of the other two types. However, the significant difference was not observed in the averages of peripheral blood eosinophils count and the Objective SCORAD score, which is a parameter of a clinical severity of AE [
10] in these three types. In regard to the personal history of associated diseases of AE, a significant difference was not observed in the incidence of BA, allergic rhino-conjunctivitis (AR), and urticaria. However, it became apparent that the incidence of ichthyosis is significantly lower in IgE-allergic AE as compared with indeterminate-allergic AE and non-IgE-allergic AE. There was no significant difference in family history in each type of elderly AE.
Table 1.
Clinical and laboratory data of the 60 elderly patients with atopic eczema (AE).
Table 1.
Clinical and laboratory data of the 60 elderly patients with atopic eczema (AE).
| | IgE-Allergic AE | Indeterminate-Allergic AE | Non-IgE-allergic AE | Total |
|---|
| n (%) | 38 (63.3) | 9 (15.0) | 13 (21.7) | 60 (100) |
| Gender, male/female | 27/11 ** | 7/2 ** | 7/6 | 41/19 ** |
| Age (years) ¶ | 76.2 ± 8.7 | 79.8 ± 7.4 | 77.9 ± 9.4 | 77.1 ± 8.6 |
| Total IgE (IU/mL) ¶ | 6975.3 ± 9908.4 *† | 339.4 ± 515.3 | 87.3 ± 96.0 | 4487.6 ± 8513.7 |
| Eosinophils (μL) ¶ | 1001.2 ± 1232.8 | 423.8 ± 189.2 | 476.9 ± 374.7 | 801.0 ± 1028.2 |
| Objective SCORAD¶ | 38.1 ± 17.4 | 23.3 ± 7.6 | 27.2 ± 16.2 | 33.5 ± 17.0 |
| Associated diseases | | | | |
| Personal history | | | | |
| BA | 14 (36.8) | 4 (44.4) | 2 (15.4) | 20 (33.3) |
| AR | 20 (52.6) | 4 (44.4) | 5 (38.5) | 27 (45.0) |
| BA + AR | 5 (13.2) | 2 (22.2) | 0 (-) | 7 (11.7) |
| Urticaria | 8 (21.1) | 2 (22.2) | 2 (15.4) | 12 (20.0) |
| Ichthyosis | 1 (2.6) *† | 3 (33.3) | 3 (23.1) | 7 (11.7) |
| Family history | | | | |
| Nonexistent | 19 (50.0) | 3 (33.3) | 6 (46.2) | 28 (46.7) |
| BA | 6 (15.8) | 1 (11.1) | 3 (23.1) | 10 (16.7) |
| AR | 6 (15.8) | 0 (-) | 1 (7.7) | 7 (11.7) |
| BA + AR | 2 (5.3) | 0 (-) | 0 (-) | 2 (3.3) |
| AE | 10 (26.3) | 2 (22.2) | 5 (38.5) | 17 (28.3) |
| AE + BA | 4 (10.5) | 1 (11.1) | 2 (15.4) | 7 (11.7) |
| AE + BA + AR | 2 (5.3) | 0 (-) | 0 (-) | 2 (3.3) |
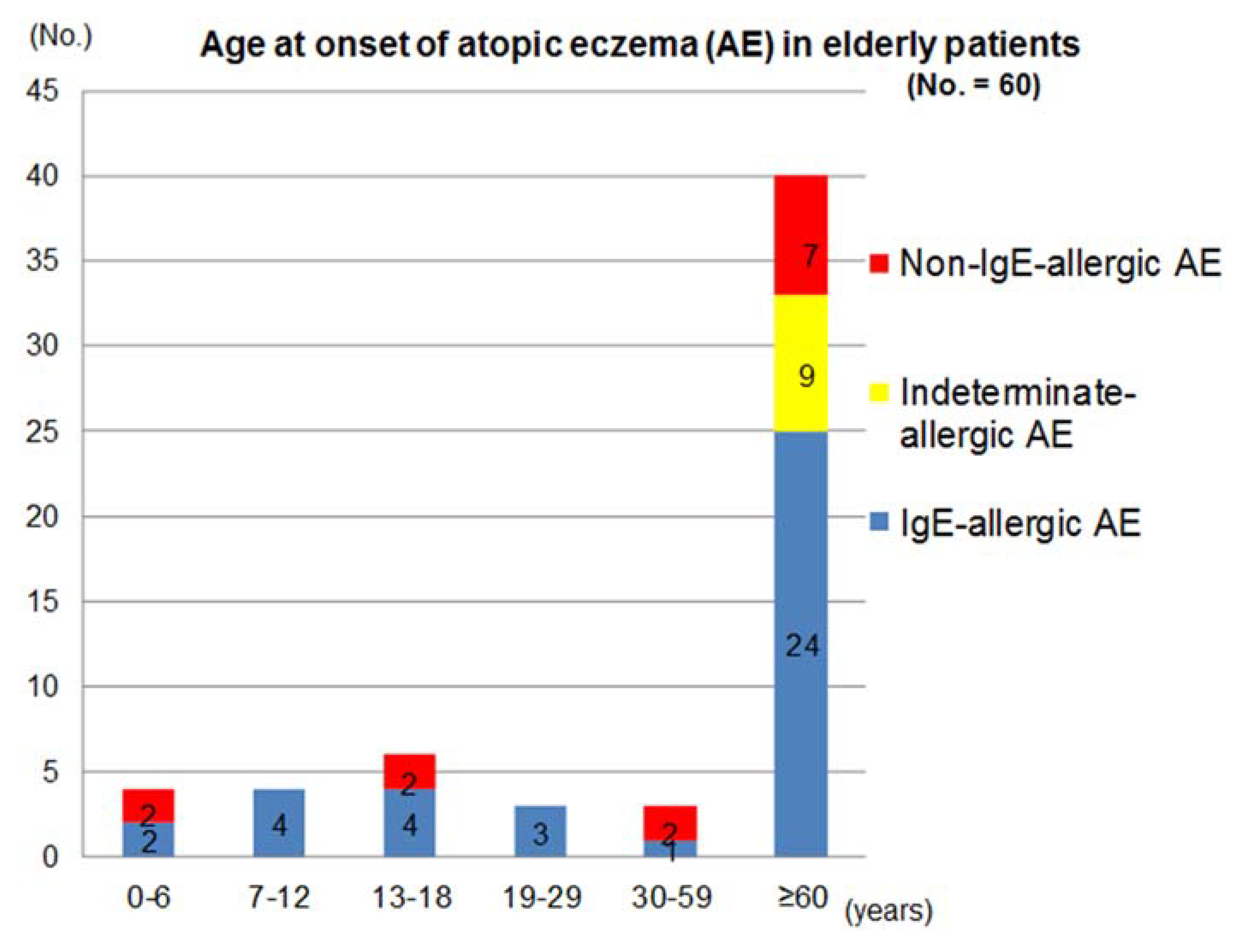
The distribution of age of AE-onset and the patterns of clinical courses after the onset in these patients are shown in
Figure 1 and
Table 2. Two thirds of the patients had senile onset of elderly AE. Since it was based upon the patient’s memory, a patient who reported an onset of AE in an infantile phase (age < 2 years) was the only one in the non-IgE-allergic AE. Onset of AE was seen in the phase of early childhood (0–6 years), late childhood (7–12 years), adolescence (13–18 years), young adulthood (19–29 years), late adulthood (30–59 years), and senile (≥60 years) in the IgE-allergic AE patients; and in the phase of early childhood, adolescence, late adulthood, and senile in the non-IgE-allergic AE patients. On the other hand, the onset of AE was significantly concentrated in the senile phase in the indeterminate-allergic AE patients. The incidence of AE in the elderly patients with IgE-allergic AE and non-IgE-allergic AE in the childhood phase (0–12 years), adolescence/young adulthood phase (13–29 years), late adulthood phase, and senile phase were 15.8% and 15.4%, 18.4% and 15.4%, 2.6% and 15.4%, and 63.2% and 53.8%, respectively. In the non-IgE-allergic AE, although the statistical-significance difference was not obtained, onsets of female patients were predominant in the senile phase. In the IgE-allergic AE and the non-IgE-allergic AE cases, two kinds of clinical course,
i.e., senile recurrence type with a history of outgrowing (outgrow-recurrence type) and continuous type were observed both in the cases with childhood onset and adolescence/young adulthood onset.
Figure 1.
Age at onset of atopic eczema in elderly patients.
Figure 1.
Age at onset of atopic eczema in elderly patients.
Table 2.
Age at onset and clinical course in elderly patients with atopic eczema (AE).
Table 2.
Age at onset and clinical course in elderly patients with atopic eczema (AE).
| | IgE-Allergic AE | Indeterminate-Allergic AE | Non-IgE-Allergic AE | Total |
|---|
| No. of patients (Male/Female) | 38 (27/11) | 9 (7/2) | 13 (7/6) | 60 (41/19) |
| Onset and course; | | | | |
| 0–12 years | | | | |
| outgrow and recurrence | 3 (2/1) | 0 (-) | 1 (1/0) | 4 (3/1) |
| continuous | 3 (2/1) | 0 (-) | 1 (1/0) | 4 (3/1) |
| 13–29 years | | | | |
| outgrow and recurrence | 2 (2/0) | 0 (-) | 1 (0/1) | 3 (2/1) |
| continuous | 5 (3/2) | 0 (-) | 1 (1/0) | 6 (4/2) |
| 30–59 years * | 1 (1/0) | 0 (-) | 2 (2/0) | 3 (3/0) |
| ≥60 years † | 24 (17/7) | 9 (7/2) | 7 (2/5) | 40 (26/14) |
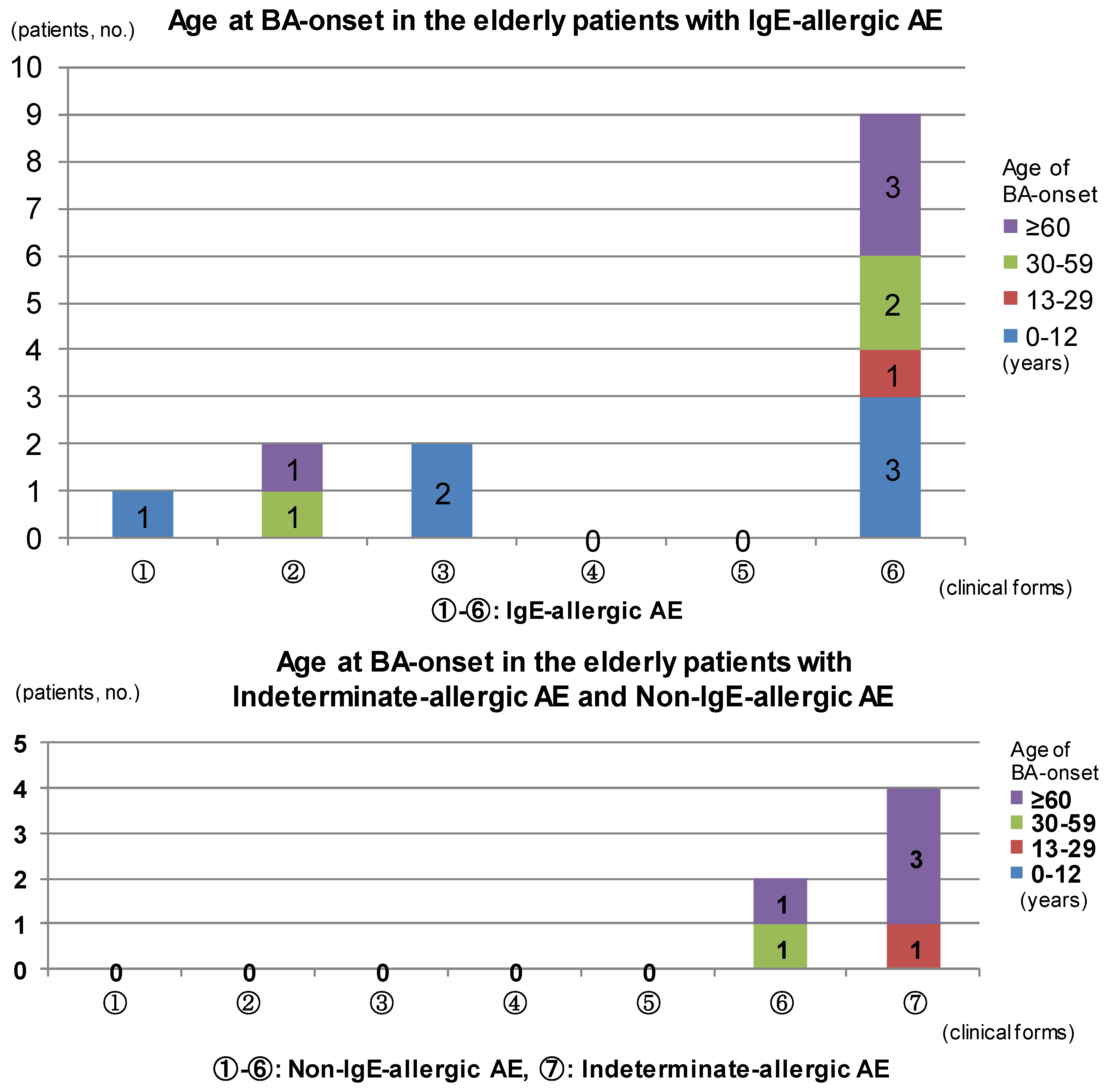
The results of analysis for complications of BA are summarized in
Figure 2. In IgE-allergic elderly AE patients, only childhood BA was seen in the outgrow-recurrence types of childhood-onset AE and adolescent/young adulthood-onset AE as an asthmatic complication. The complications of late adulthood-onset BA and senile-onset BA were seen in the continuous-type childhood-onset AE. Asthmatic complications were not recognized in continuous-type adolescent/young adulthood-onset AE. In senile-onset AE, complications of BA were observed in every phase,
i.e., childhood, adolescence/young adulthood, late adulthood, and senile phases (
Figure 2, upper bar chart). With indeterminate-allergic AE and non-IgE-allergic AE patients, no personal history of childhood BA was observed and asthmatic complications in which senile-onset BA predominated were recognized only in senile-onset AE (
Figure 2, lower bar chart). In cases of IgE-allergic and indeterminate-allergic AE, all asthmatic-merger patients except for two patients with IgE-allergic AE and a personal history of childhood BA showed positive results for serum-specific IgEs against
Dermatophagoides farinae (
Der f) In regard to the association between asthmatic complications and total IgE values, the mean total IgE values of elderly AE with a personal history of BA tended to be lower than those of elderly AE without a complication of BA, both in patients with IgE-allergic AE (4917.8 ± 6242.0 IU/mL
vs. 8175.6 ± 11481.9 IU/mL) and indeterminate-allergic AE (72.8 ± 62.5 IU/mL
vs. 552.8 ± 632.6 IU/mL), although no significant difference was identified. Complications of RA were recognized in adolescence, adulthood, or senile phases of the patients with elderly AE. As with BA, onset of AR was seen either before, simultaneous to, or after AE onset, but elderly AE patients tended to show an unclear memory of AR-onset as compared with BA-onset.
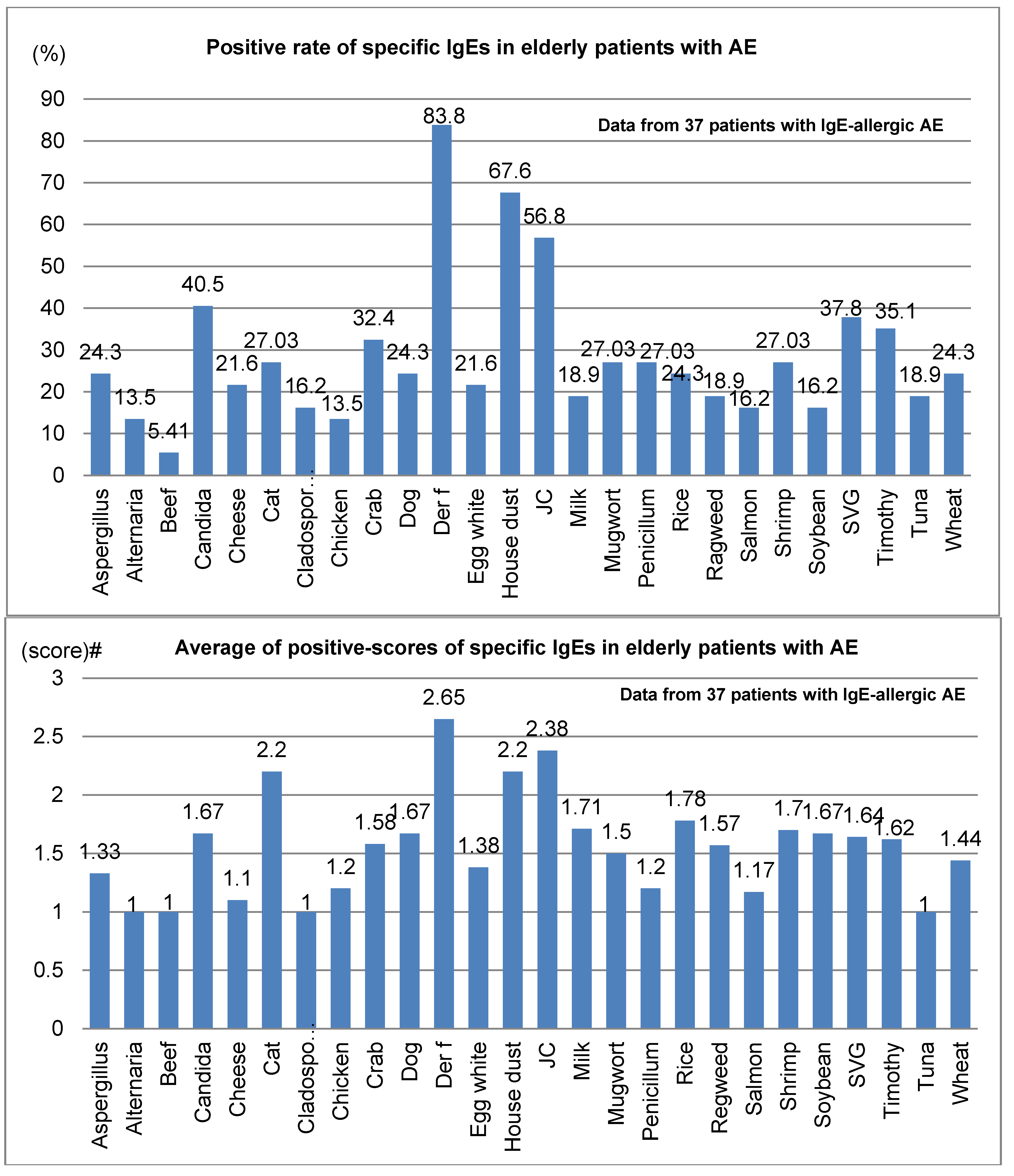
Positive rates and average positive scores for serum-specific IgE for 26 common environmental allergens among 37 elderly patients with IgE-allergic AE are shown in
Figure 3. In the positive rates of serum-specific IgE, the positive rate for
Der f peaked at 83.8%, compared to 67.6% for house dust and 56.8% for Japanese cedar (
Figure 3; upper bar chart). Also, the mean positive scores for serum-specific IgE were highest for
Der f, at 2.65, followed by Japanese cedar, 2.38; house dust, 2.2; and cat dander, 2.2 (lower bar chart,
Figure 3). Besides the 26 common allergens, positive rates and mean positive scores for serum-specific IgEs for notable allergens in 18 elderly patients with IgE-allergic AE were as follows: birch, 22.2% and 1.3; peanuts, 16.7% and 1.7; and latex, 11.1% and 1.5, respectively.
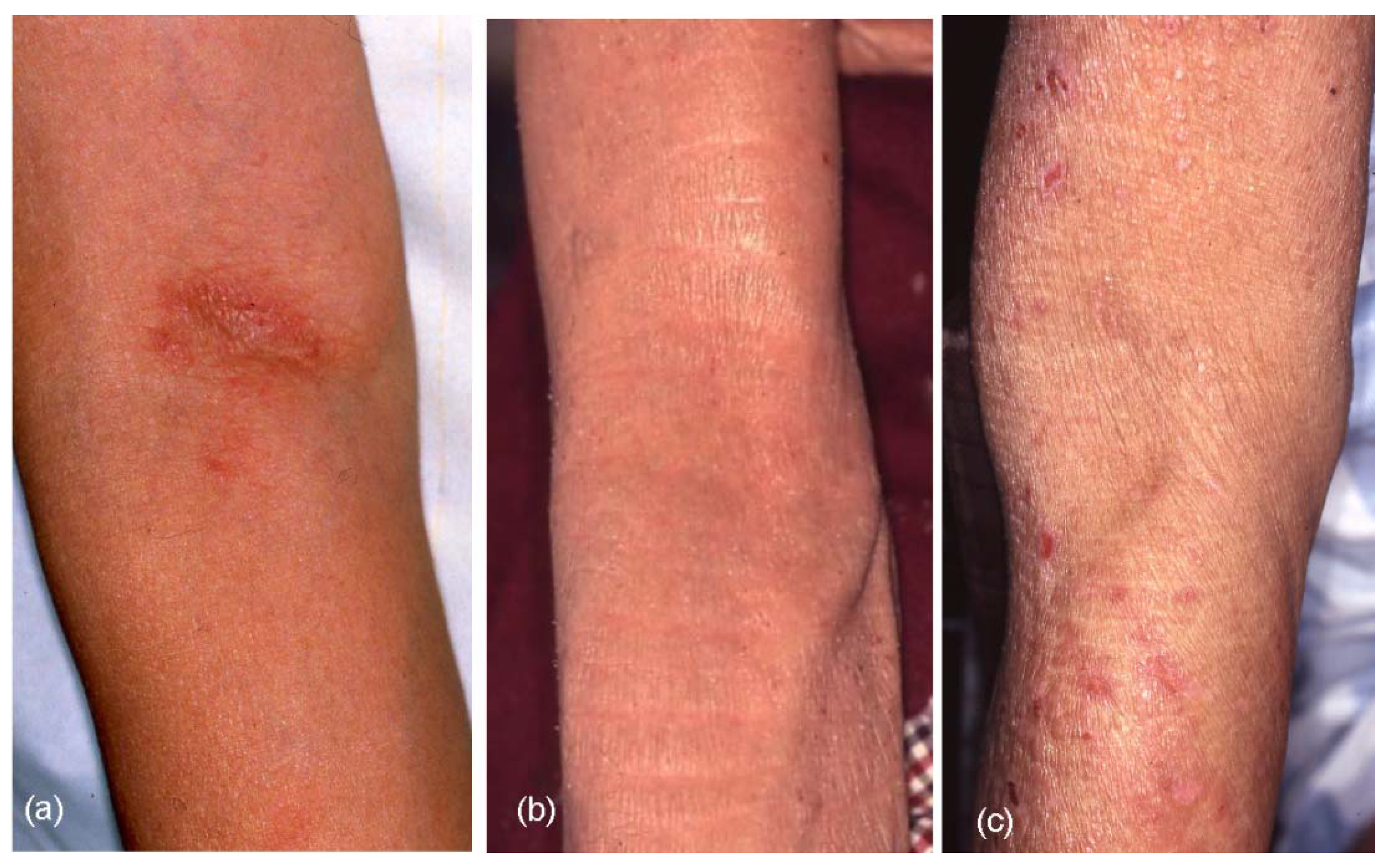
The results of clinical-picture analyses are summarized in
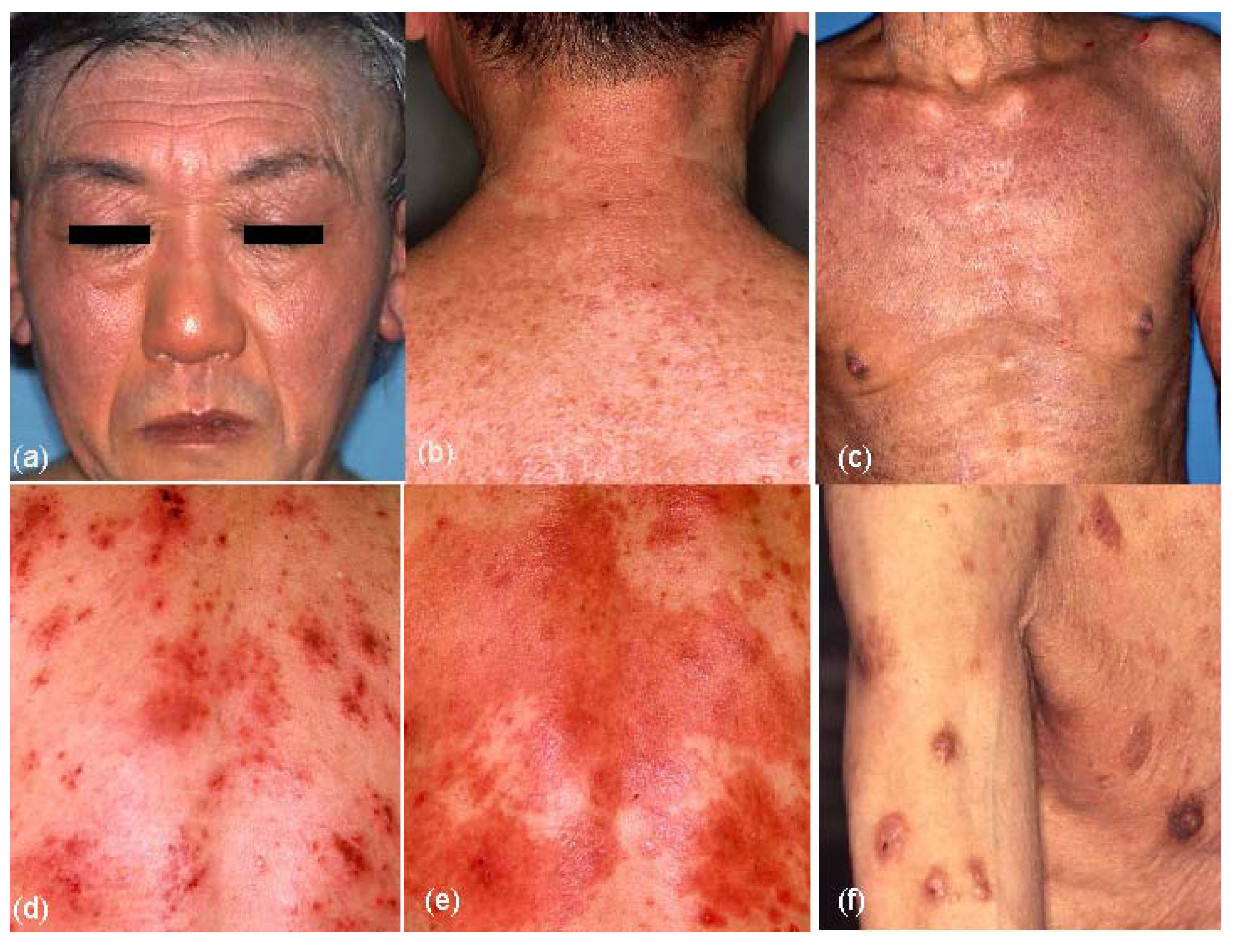
Table 3. Although various forms of skin manifestations were observed in elderly patients with AE (
Figure 4a–f), no significant difference was observed in the three types of elderly AE. On aggregate, eczematous dermatitis presented in 65.0% of cases involving the face and neck, 91.7% involving the trunk, 95.0% involving the upper extremities, and 83.3% involving the lower extremities in elderly AE patients. Hertoghe’s sign was recognized in 30% of elderly AE patients. Prurigo-forming papules and/or nodules on the trunk and extremities were observed in approximately 27% of patients. Exudative inflammatory erythema on the trunk and eczematous erythroderma were seen in 21.7% and 23.3% of patients, respectively. In regard to lichenified eczema (lichenification) in the extremities, lichenification was observed in 73.3% of extensor sites of the upper extremities and/or wrist, and 51.7% of extensor sites of the lower extremities. Lichenification in the elbow and knee folds showed relatively low positive rates as 23.3% (localized form, 10.0%; diffuse form, 13.3%) of the elbow folds and 18.3% (localized form, 8.3%; diffuse form, 10.0%) of the knee folds. Although 76.7% and 81.7% of elderly AE patients showed no lichenification in the elbow or knee folds, respectively, the reverse sign of lichenification around the folds was observed in 40.0% of patients in antecubital areas and 11.7% of patients in popliteal areas. Furthermore, 10% (IgE-allergic AE, 2.6%; non-IgE-allergic AE, 38.5%) of elderly AE patients reported a history of lichenified chronic eczema in the elbow and knee folds when they lacked lichenification in those folds at the time of medical examination. Lichenification in the antecubital areas (
Figure 5a–c) in the three types of the elderly AE patients was as follows: localized, 5.3%; diffuse, 18.4%; around the folds, 39.5% in IgE-allergic AE, localized, 11.1%; diffuse,11.1%; around the folds, 33.3% in indeterminate-allergic AE, and localized, 23.1%; diffuse, none; and around the folds, 46.2% in non-IgE-allergic AE.
Figure 2.
Age at bronchial asthma (BA) onset in elderly patients with atopic eczema. Abbreviations: AE, atopic eczema; BA, bronchial asthma; ① childhood-onset AE, outgrow-recurrence type; ② childhood-onset AE, continuous type; ③ adolescent and young adult-onset AE, outgrow-recurrence type; ④ adolescent and young adult-onset AE, continuous type; ⑤ late adult-onset AE; ⑥ senile-onset AE; ⑦ senile-onset AE.
Figure 2.
Age at bronchial asthma (BA) onset in elderly patients with atopic eczema. Abbreviations: AE, atopic eczema; BA, bronchial asthma; ① childhood-onset AE, outgrow-recurrence type; ② childhood-onset AE, continuous type; ③ adolescent and young adult-onset AE, outgrow-recurrence type; ④ adolescent and young adult-onset AE, continuous type; ⑤ late adult-onset AE; ⑥ senile-onset AE; ⑦ senile-onset AE.
Figure 3.
Positive rate (top) and average of positive scores (bottom) of specific IgEs in elderly patients with atopic eczema. Abbreviations: AE, atopic eczema; Der f, Dermatophagoides farinae; JC, Japanese cedar; SVG, sweet vernal grass. # Average of class intensity (score) of the positive allergens for multiple antigen simulations test (MAST)-26 or MAST-33; the adjust class intensity for the common 26 allergens are decided as follows: score 1 (mild) = class 1 for MAST26 or class 2 for MAST33; score 2 (moderate) = class 2 for MAST26 or class3 for MAST33; score 3 (strong) = class 3 for MAST26 or class 4, 5 and 6 for MAST33; score 4 (very strong) = over class 3 for MAST26 or over class 6 for MAST33.
Figure 3.
Positive rate (top) and average of positive scores (bottom) of specific IgEs in elderly patients with atopic eczema. Abbreviations: AE, atopic eczema; Der f, Dermatophagoides farinae; JC, Japanese cedar; SVG, sweet vernal grass. # Average of class intensity (score) of the positive allergens for multiple antigen simulations test (MAST)-26 or MAST-33; the adjust class intensity for the common 26 allergens are decided as follows: score 1 (mild) = class 1 for MAST26 or class 2 for MAST33; score 2 (moderate) = class 2 for MAST26 or class3 for MAST33; score 3 (strong) = class 3 for MAST26 or class 4, 5 and 6 for MAST33; score 4 (very strong) = over class 3 for MAST26 or over class 6 for MAST33.
Table 3.
Skin manifestations in elderly patients with atopic eczema (AE).
Table 3.
Skin manifestations in elderly patients with atopic eczema (AE).
| Skin Manifestations ¶ | IgE-allergic AE (n = 38) n (%) | Indeterminate-Allergic AE (n = 9) n (%) | Non-IgE-Allergic AE (n = 13) n (%) | Total (n = 60) n (%) |
|---|
| Face and neck | | | | |
| Eczema | 26 (68.4) | 4 (44.4) | 9 (69.2) | 39 (65.0) |
| Hertoghe’s sign | 12 (31.6) | 1 (11.1) | 5 (55.6) | 18 (30) |
| Trunk | | | | |
| Eczema | 36 (94.7) | 8 (88.9) | 11 (84.6) | 55 (91.7) |
| Prurigo | 11 (28.9) | 2 (22.2) | 3 (23.1) | 16 (26.7) |
| Exudative inflamm. | 10 (26.3) | 2 (22.2) | 1 (7.7) | 13 (21.7) |
| Elbow folds | | | | |
| Lichenification | | | | |
| Localized | 2 (5.3) | 1 (11.1) | 3 (23.1) | 6 (10.0) |
| Diffuse | 7 (18.4) | 1 (11.1) | 0 (-) | 8 (13.3) |
| None | 29 (76.3) | 7 (77.8) | 10 (76.9) | 46 (76.7) |
| Around the folds | 15 (39.5) | 3 (33.3) | 6 (46.2) | 24 (40.0) |
| Upper extremities | | | | |
| Lichenification | 30 (78.9) | 6 (66.7) | 8 (61.5) | 44 (73.3) |
| Eczema | 37 (97.4) | 9 (100) | 11 (84.6) | 57 (95.0) |
| Prurigo | 13 (34.2) | 2 (22.2) | 2 (15.4) | 17 (28.3) |
| Knee folds | | | | |
| Lichenification | | | | |
| Localized | 4 (10.5) | 0 (-) | 1 (7.7) | 5 (8.3) |
| Diffuse | 6 (15.8) | 0 (-) | 0 (-) | 6 (10.0) |
| None | 28 (71.8) | 9 (100) | 12 (92.3) | 49 (81.7) |
| Around the folds | 5 (12.8) | 0 (-) | 2 (15.4) | 7 (11.7) |
| Lower extremities | | | | |
| Lichenification | 22 (57.9) | 2 (22.2) | 7 (53.8) | 31 (51.7) |
| Eczema | 32 (84.2) | 7 (77.8) | 12 (92.3) | 50 (83.3) |
| Prurigo | 11 (28.9) | 2 (22.2) | 3 (23.1) | 16 (26.7) |
| Erythroderma | 10 (26.3) | 1 (11.1) | 3 (23.1) | 14 (23.3) |
Figure 4.
Skin manifestations of elderly patients with atopic eczema (AE). (a) Facial eczematous erythema (atopic red face) with Hertoghe’s sign (loss of lateral eyebrows); (b) Diffuse eczematous erythema and papules on the neck and upper back; (c) Lichenified eczema of erythroderma on the trunk; (d) Nummular-form eczema with acute inflammation on the back; (e) Nummular-form eczema changed to exudative inflammatory erythema after 1 week; (f) Prurigo-forming papules and nodules on the upper extremities and trunk.
Figure 4.
Skin manifestations of elderly patients with atopic eczema (AE). (a) Facial eczematous erythema (atopic red face) with Hertoghe’s sign (loss of lateral eyebrows); (b) Diffuse eczematous erythema and papules on the neck and upper back; (c) Lichenified eczema of erythroderma on the trunk; (d) Nummular-form eczema with acute inflammation on the back; (e) Nummular-form eczema changed to exudative inflammatory erythema after 1 week; (f) Prurigo-forming papules and nodules on the upper extremities and trunk.
Figure 5.
Lichenification (lichenified eczema) in the antecubital areas of elderly patients with atopic eczema (AE). (a) Localized lichenified eczema in the elbow fold; (b) Diffuse lichenified eczema in the elbow fold and flexure site of the arm; (c) Lichenified eczema around the scarcely involved elbow fold (reverse sign).
Figure 5.
Lichenification (lichenified eczema) in the antecubital areas of elderly patients with atopic eczema (AE). (a) Localized lichenified eczema in the elbow fold; (b) Diffuse lichenified eczema in the elbow fold and flexure site of the arm; (c) Lichenified eczema around the scarcely involved elbow fold (reverse sign).
3.2. Treatments and Outcomes
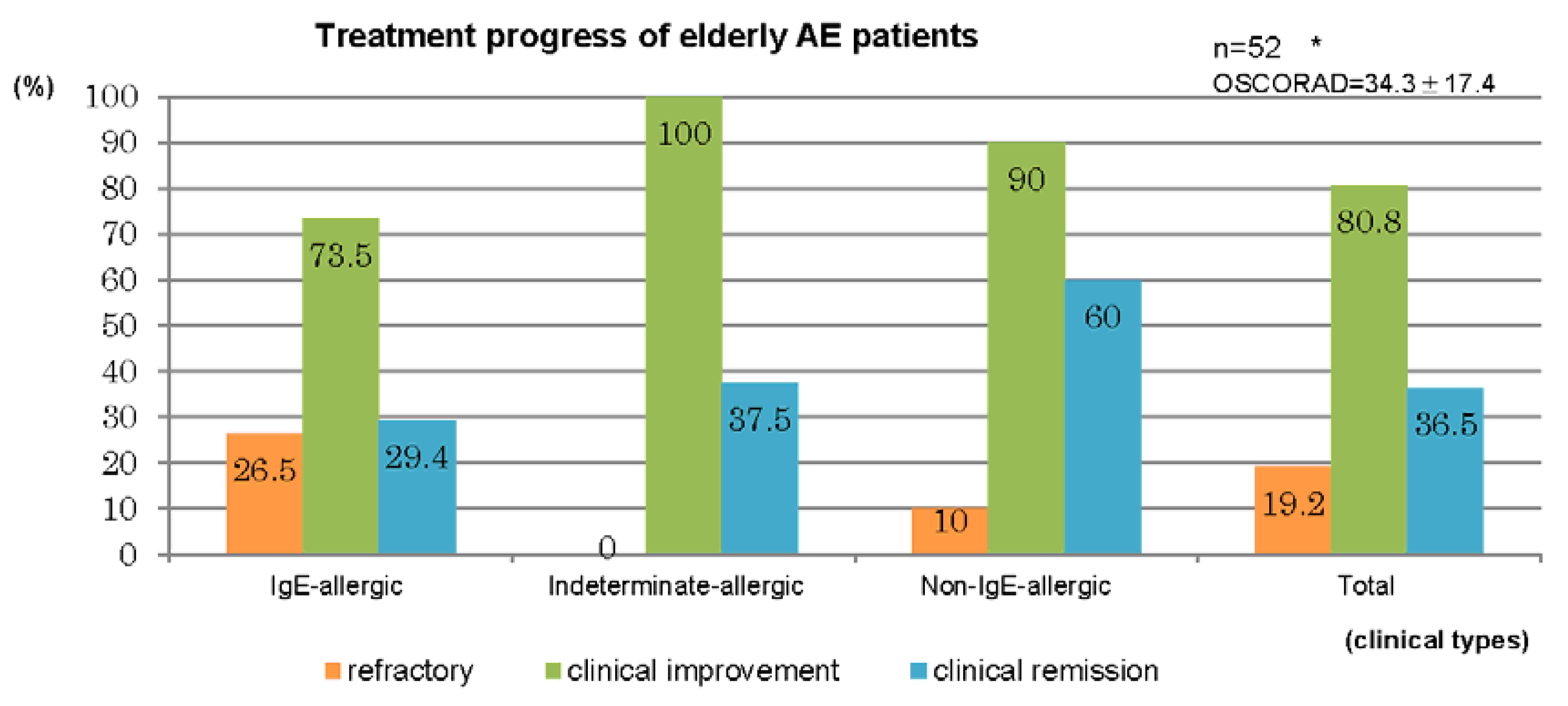
Therapeutic results for the 52 cases in which treatment of AE was performed in our department for a duration of ≥6 months are summarized in
Figure 6. Treatment was performed by the dermatologists in accordance with the atopic dermatitis guidelines of the Japanese Dermatological Association [
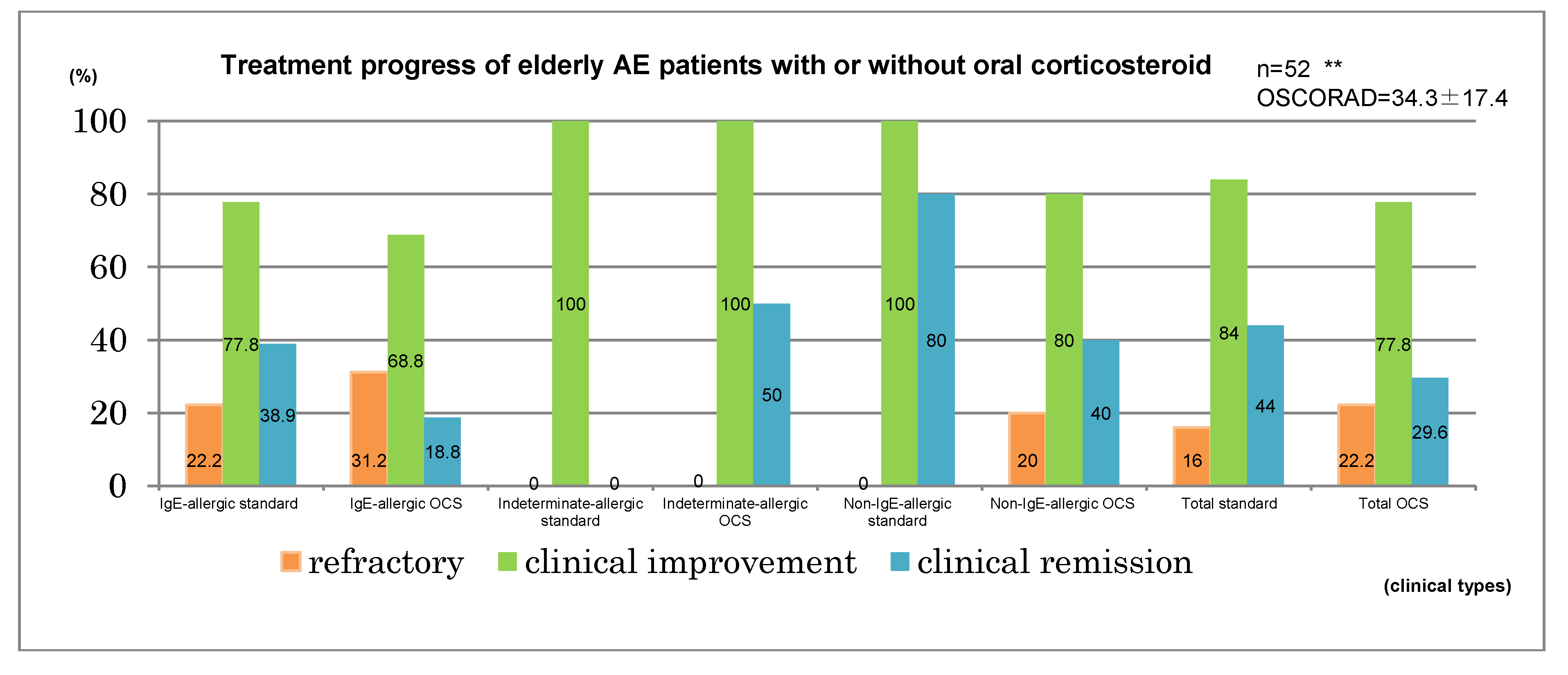
12]. A clinical improvement was observed in elderly cases comprising 73.5% of IgE-allergic AE, 100% of indeterminate-allergic AE, 90% of non-IgE-allergic AE, and 80.8% of the total. Clinical remission was obtained in elderly cases for 29.4% of IgE-allergic AE, 37.5% of indeterminate-allergic AE, 60% of non-IgE-allergic AE, and 36.5% of the total (
Figure 6, upper bar chart). Standard treatments, which included topical corticosteroid and moisturizer, topical tacrolimus, oral antihistamines, and oral anti-allergic drugs [
1,
12], had been applied for 25 cases. By the standard treatments, clinical improvement was observed in the elderly cases of 77.8% of IgE-allergic AE, 100% of indeterminate-allergic AE, 100% of non-IgE-allergic AE, and 84% of the total, and clinical remission was obtained in elderly cases of 38.9% of IgE-allergic AE, 0% of indeterminate-allergic AE, 80% of non-IgE-allergic AE, and 44% of the total. Oral corticosteroid therapy with the standard treatments had been carried out for 27 cases. In three of the 27 cases, the oral corticosteroid had originally been started as therapy for complicated BA. With oral corticosteroid and standard treatments, clinical improvement was observed in elderly cases of 68.8% of IgE-allergic AE, 100% of indeterminate-allergic AE, 80% of non-IgE-allergic AE, and 77.8% of the total, and clinical remission was obtained in the elderly cases of 18.8% of IgE-allergic AE, 50% of indeterminate-allergic AE, 40% of non-IgE-allergic AE, and 29.6% of the total (
Figure 6, lower bar chart). Mean initial dosage of oral corticosteroid was 7.7 ± 4.1 mg/day (IgE-allergic AE, 8.6 ± 4.7 mg/day; indeterminate-allergic AE, 5.0 ± 3.1 mg/day; non-IgE-allergic AE, 7.5 ± 1.8 mg/day) in prednisolone-equivalents. As other supplementary treatments, oral cyclosporine therapy (100 mg/day) and narrowband ultraviolet b (UVB) therapy were performed in two cases (IgE-allergic AE,
n = 1; indeterminate-allergic AE,
n = 1) and four cases (IgE-allergic AE only), respectively.
Figure 6.
Treatment progress of elderly patients with atopic eczema. * IgE-allergic: n = 34, OSCORAD = 38.5 ± 17.6; Indeterminate-allergic: n = 8, OSCORAD = 24.1 ± 7.8; Non-IgE-allergic: n = 10, OSCORAD = 28.5 ± 18.2. ** IgE-allergic standard: n = 18, OSCORAD = 35.7 ± 16.3; IgE-allergic OCS: n = 16, OSCORAD = 41.6 ± 19.1; Indeterminate-allergic standard: n = 2, OSCORAD = 28.7 ± 6.4; Indeterminate-allergic OCS: n = 6, OSCORAD = 22.5 ± 8.0; Non-IgE-allergic standard: n = 5, OSCORAD = 19.3 ± 7.9; Non-IgE-allergic OCS: n = 5, OSCORAD = 37.7 ± 21.7; Total standard: n = 25, OSCORAD= 31.8 ± 15.6; Total OCS: n = 27, OSCORAD = 36. 7± 18.9. Abbreviations: AE, atopic eczema; standard, standard treatments (including topical corticosteroids and moisturizer, oral antihistamines, oral anti-allergic drugs, and topical tacrolimus); OCS, standard treatment with oral corticosteroids; OSCORAD, Objective Severity Scoring of Atopic Dermatitis (mean ± SD).
Figure 6.
Treatment progress of elderly patients with atopic eczema. * IgE-allergic: n = 34, OSCORAD = 38.5 ± 17.6; Indeterminate-allergic: n = 8, OSCORAD = 24.1 ± 7.8; Non-IgE-allergic: n = 10, OSCORAD = 28.5 ± 18.2. ** IgE-allergic standard: n = 18, OSCORAD = 35.7 ± 16.3; IgE-allergic OCS: n = 16, OSCORAD = 41.6 ± 19.1; Indeterminate-allergic standard: n = 2, OSCORAD = 28.7 ± 6.4; Indeterminate-allergic OCS: n = 6, OSCORAD = 22.5 ± 8.0; Non-IgE-allergic standard: n = 5, OSCORAD = 19.3 ± 7.9; Non-IgE-allergic OCS: n = 5, OSCORAD = 37.7 ± 21.7; Total standard: n = 25, OSCORAD= 31.8 ± 15.6; Total OCS: n = 27, OSCORAD = 36. 7± 18.9. Abbreviations: AE, atopic eczema; standard, standard treatments (including topical corticosteroids and moisturizer, oral antihistamines, oral anti-allergic drugs, and topical tacrolimus); OCS, standard treatment with oral corticosteroids; OSCORAD, Objective Severity Scoring of Atopic Dermatitis (mean ± SD).
3.3. Complications and Final Prognosis
Coexisting/underlying disorders in the 60 cases of elderly AE were as follows. Incidence of hypertension was highest, at 53.3%, followed by heart disease (26.7%), spinal disease (25.0%), and cerebrovascular disease (21.7%). Complications of diabetes mellitus and chronic renal disease were observed at frequencies of 16.7% and 8.3%, respectively.
In cases of IgE-allergic AE, indeterminate-allergic AE and non-IgE-allergic AE, incidences were as follows: hypertension—58.3%, 44.4%, and 53.8%; heart disease—23.7%, 33.3%, and 30.8%; spinal disease—23.7%, 11.1%, and 38.5%; cerebrovascular disease—26.3%, 7.7%, and 22.2%; diabetes mellitus—18.4%, 22.2%, and 7.7%; and chronic renal disease—10.5%, 0%, and 7.7%, respectively. In each disorder, no significant differences in incidence were recognized among the three types of elderly AE. Steroid-induced diabetes mellitus with mild symptoms relevant to oral corticosteroid therapy was found in one patient with IgE-allergic AE and a low compliance to oral therapy.
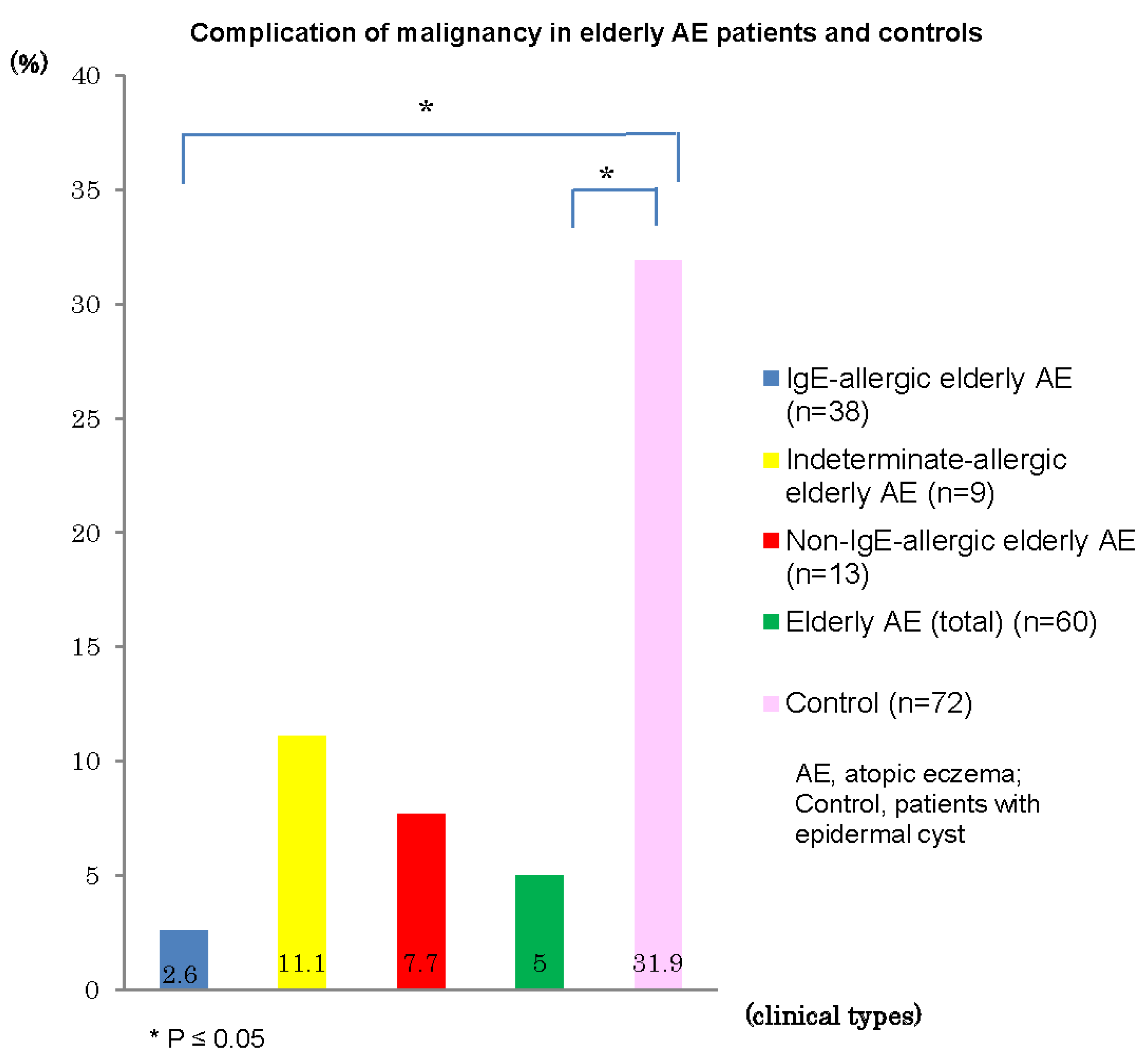
Complications of malignancy in personal histories and/or in coexisting/underlying disorders were recognized in three (5%) of the 60 elderly AE patients: rectal cancer and prostatic carcinoma in one patient with IgE-allergic AE, prostate carcinoma in one patient with indeterminate-allergic AE, and stomach cancer in one patient with non-IgE-allergic AE. The incidences of malignancy in elderly patients with IgE-allergic AE (2.6%) and with AE total (5%) were significantly lower than that (31.9%) of control patients with epidermal cysts (
Figure 7). In control subjects, 23 (4 with double cancer) of the 72 patients showed complications of malignancy, as follows: pulmonary carcinoma,
n = 5; stomach cancer,
n = 4; colon cancer,
n = 4; bladder cancer,
n = 3; mammary carcinoma,
n = 2; malignant lymphoma,
n = 2; gallbladder and bile duct carcinoma,
n = 2; basal cell carcinoma,
n = 1; and other internal malignancy,
n = 5. In addition, precancerous skin lesions, e.g., solar keratosis, were observed in two control subjects, but not in elderly AE patients.
Figure 7.
Complication of malignancy in elderly patients with atopic eczema and controls.
Figure 7.
Complication of malignancy in elderly patients with atopic eczema and controls.
The number of patients in whom death was confirmed by our hospital as of the end of 2014 was nine (IgE-allergic AE, n = 6; indeterminate-allergic AE, n = 1; non-IgE-allergic AE, n = 2). Mean age at death was 86.3 ± 6.8 years (IgE-allergic AE, 87.5 ± 7.9 years; indeterminate-allergic AE, 86 ± 0 years; and non-IgE-allergic AE, 83 ± 5.7 years). Causes of death were pneumonia, n = 3; respiratory failure, n = 1; fulminant hepatitis, n = 1; renal failure, n = 1; and natural death, n = 3. Among the identified patients, no deaths were attributable to malignancy. On the other hand, the mean age at death was 81.1 ± 4.2 years in the nine control subjects in whom death was confirmed by our hospital, and the cause of death for four of these nine subjects (44.4%) was malignancy. The incidence of death by malignancy among elderly patients with IgE-allergic AE (0%) and with AE total (0%) was lower than that (44.4%) in control subjects, but no significant difference was identified due to the small number of cases involved. In addition, most patients who died had been continuing treatment for AE until just before death.