The Presentation and Treatment of Myointimoma: A Systematic Review and the First Case Report of Penile Myointimoma as a Cause of Urethral Obstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Sources
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Data Synthesis
3. Results
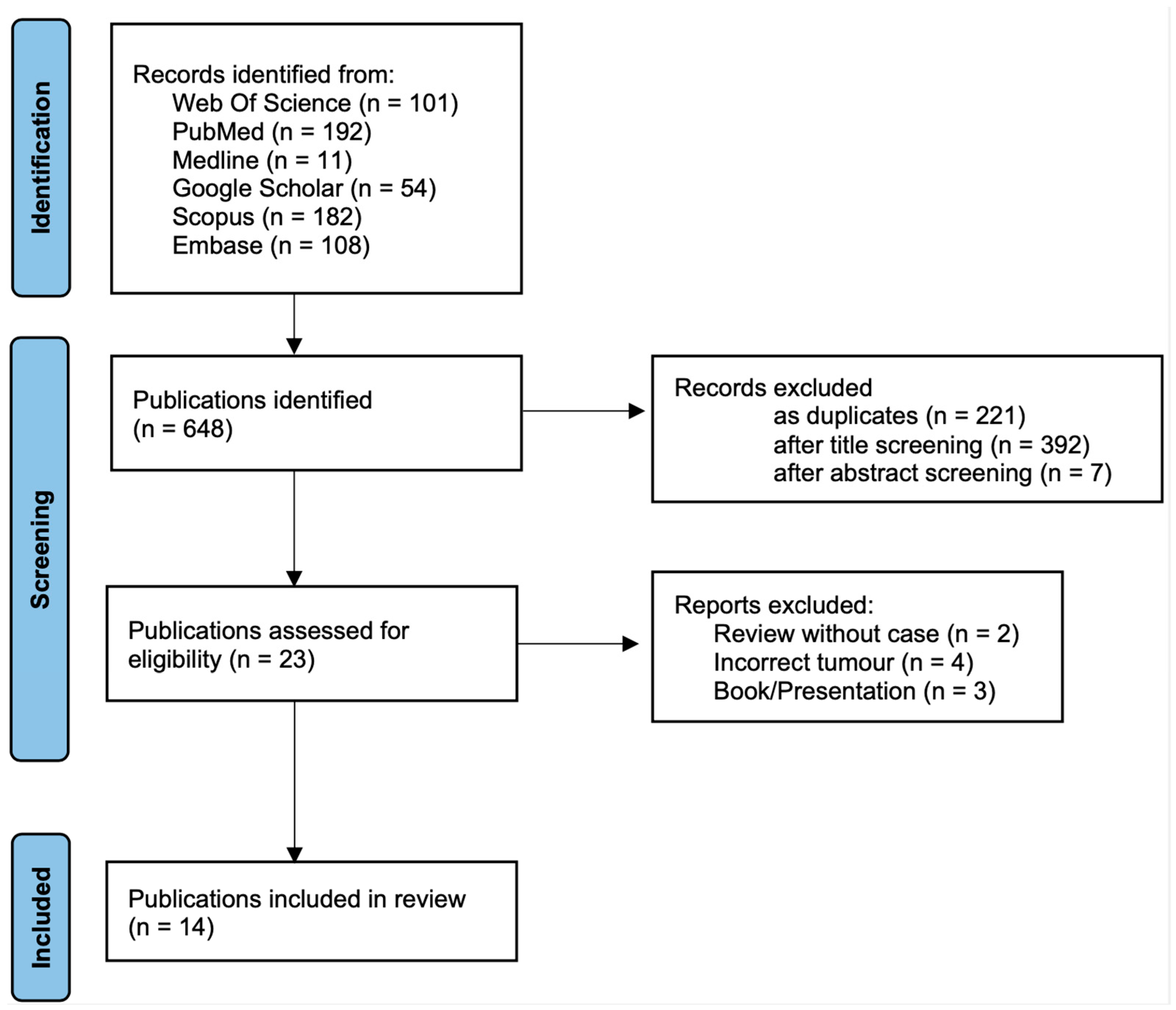
3.1. Study Selection
3.2. Study Characteristics
3.3. Clinical Presentation and Diagnostic Workup
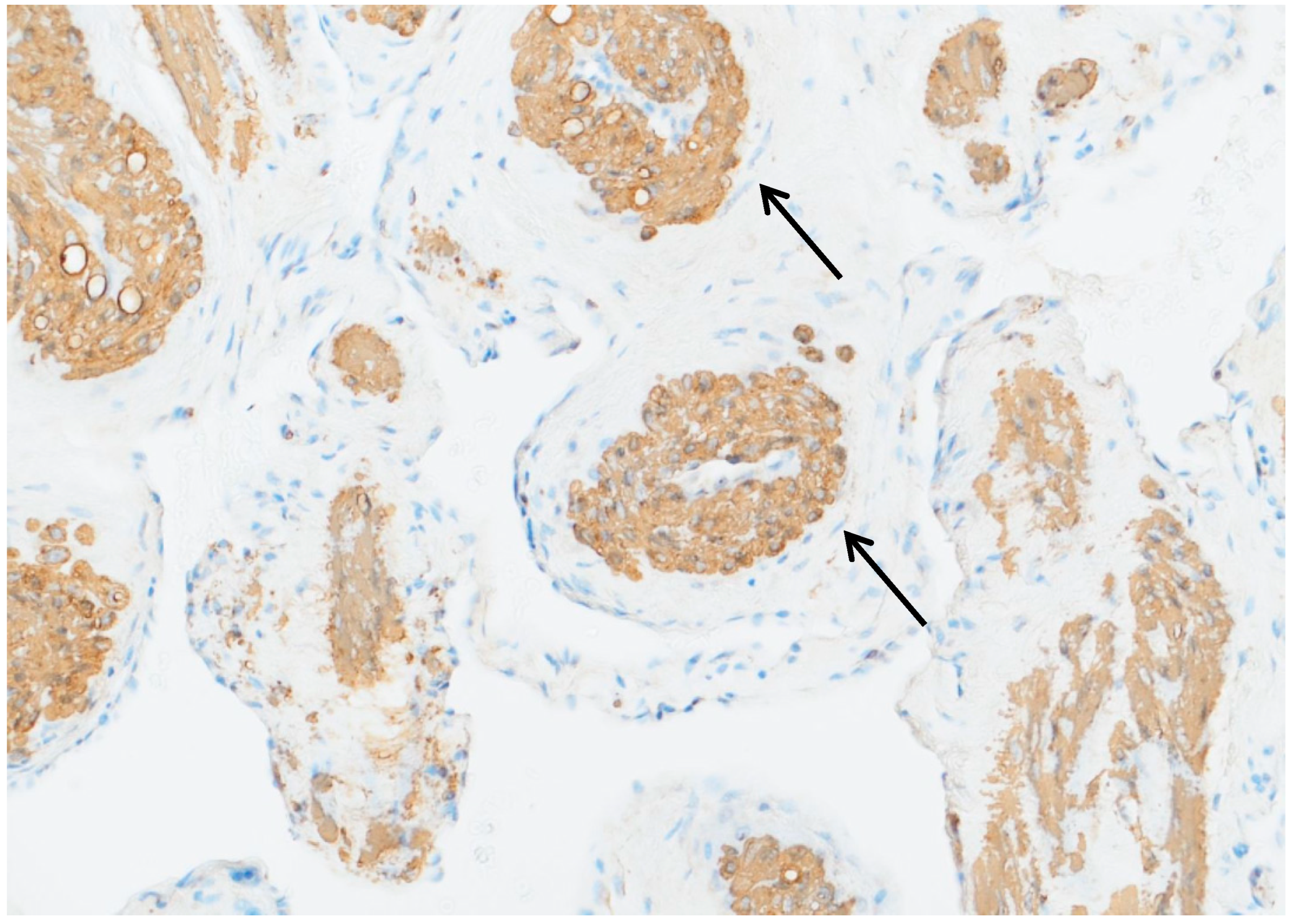
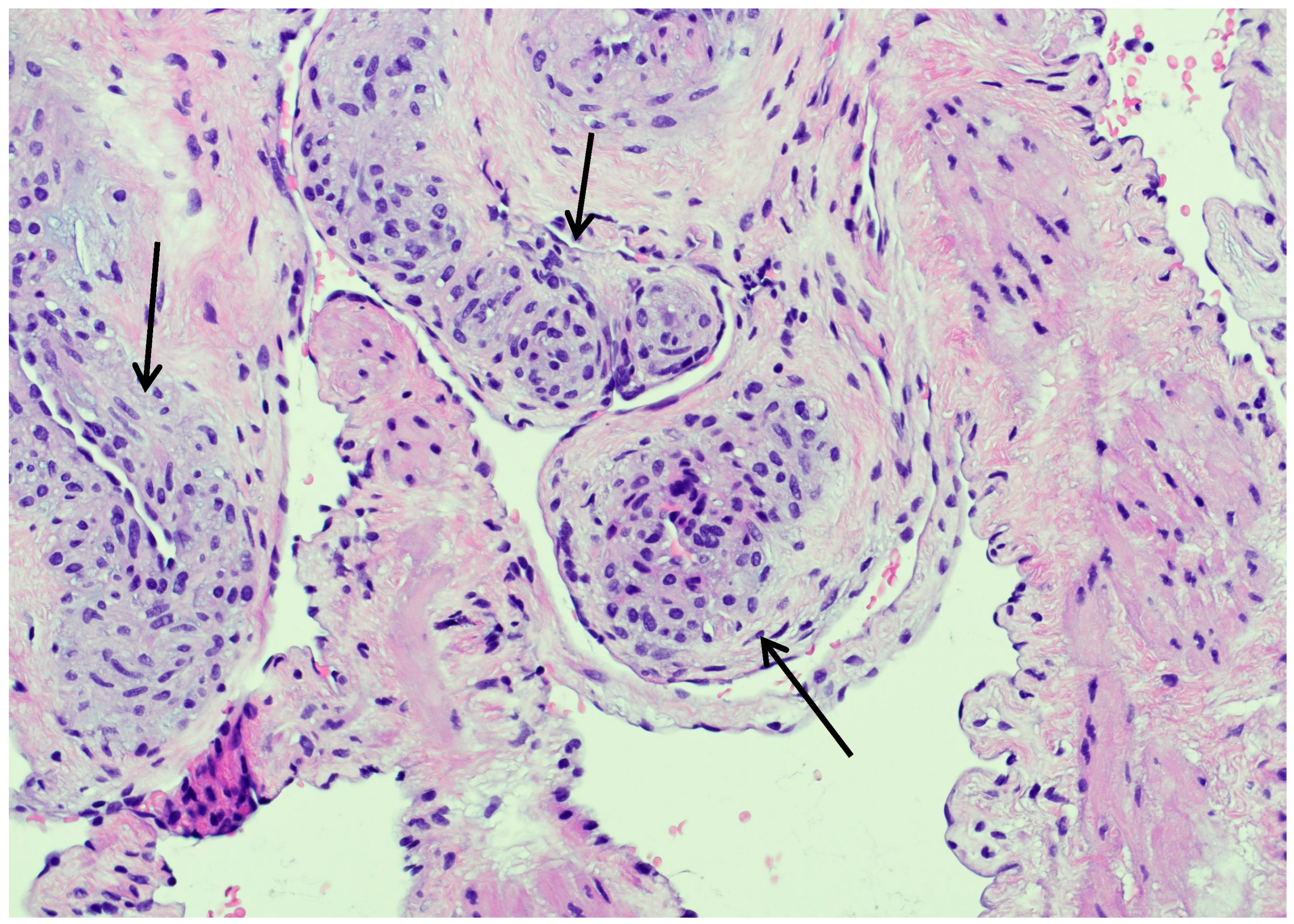
3.4. Histopathological and Immunohistochemical Findings
3.5. Treatment and Outcomes
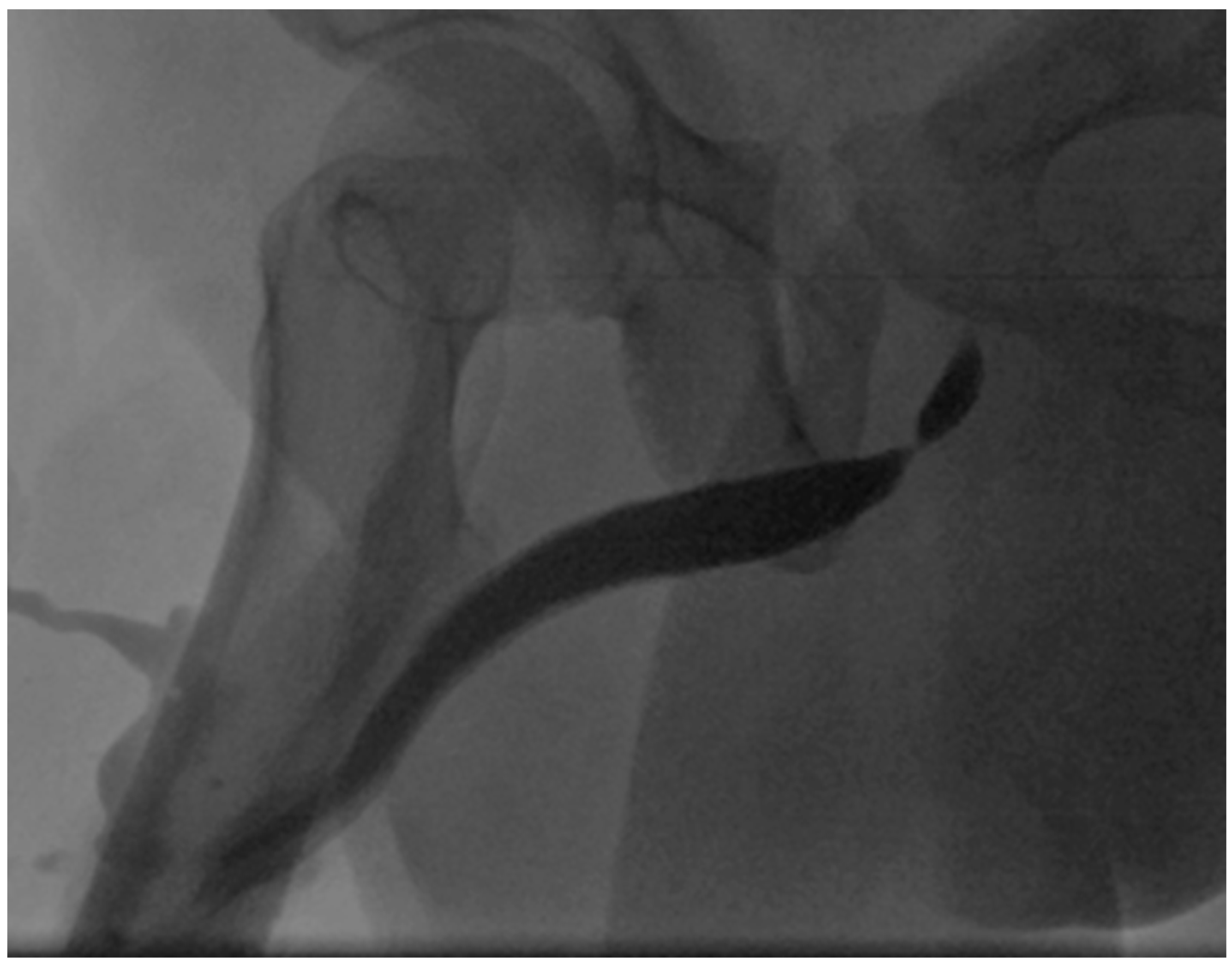
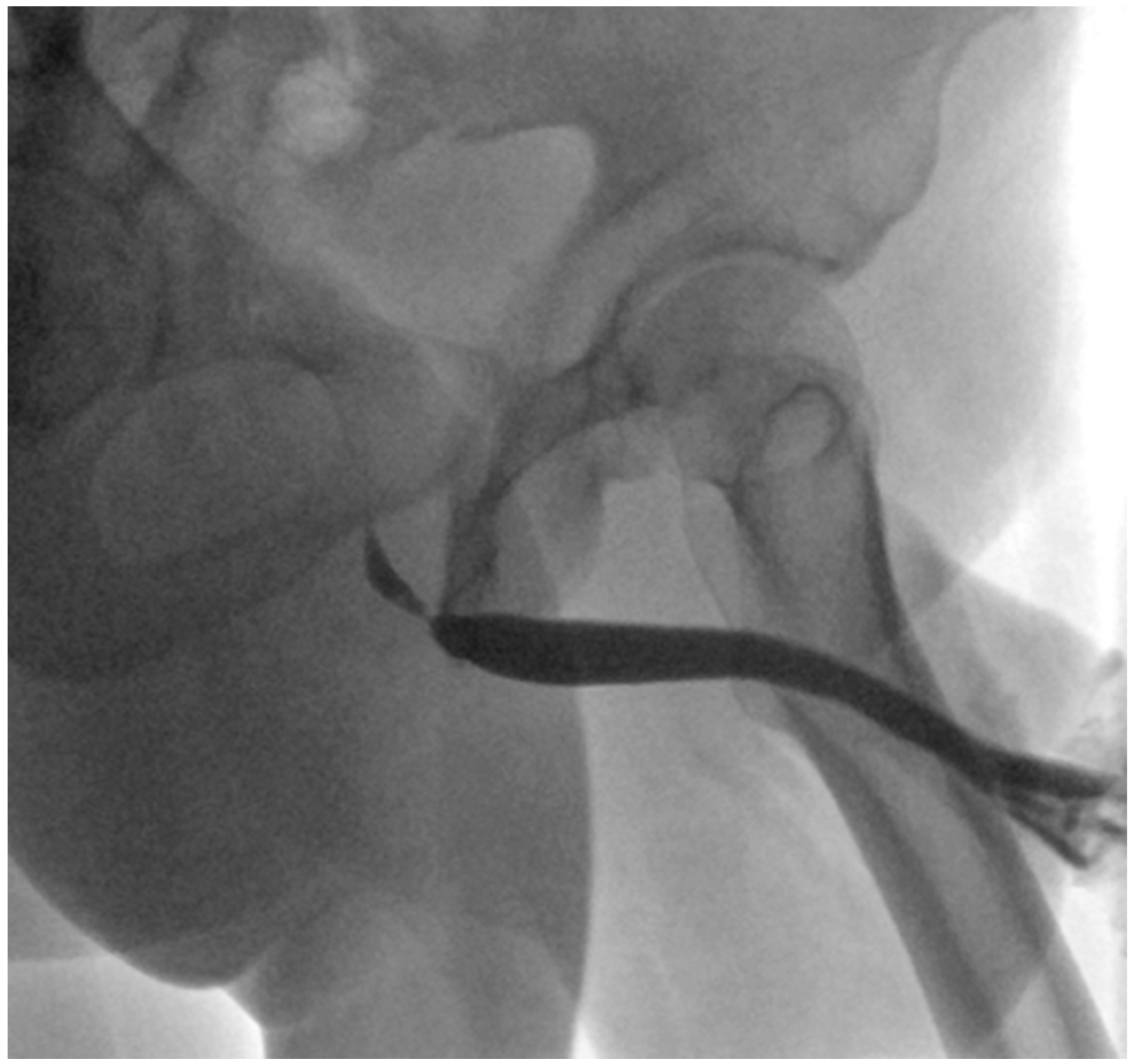
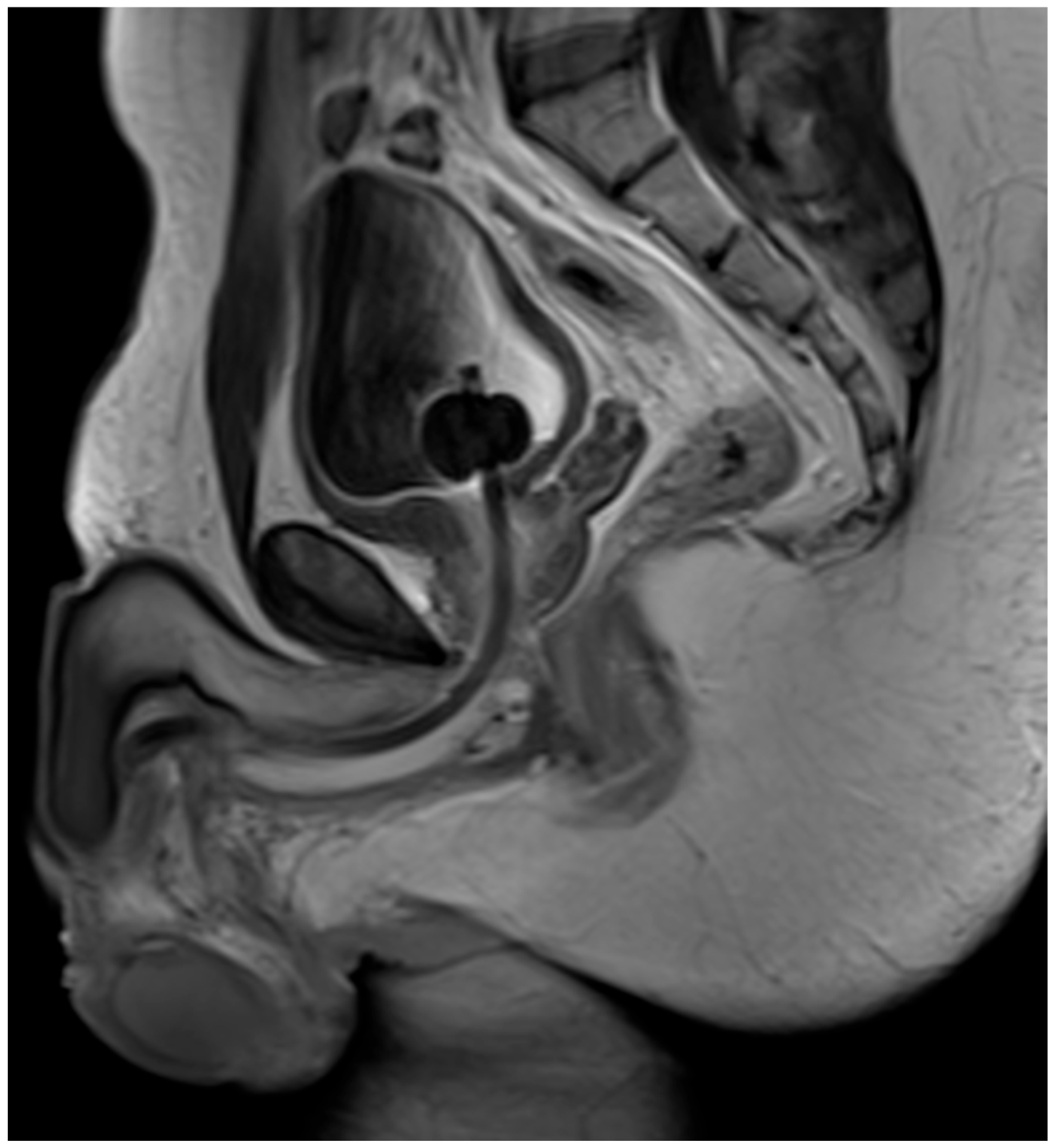
4. Case Report
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSA | prostate-specific antigen |
| UCG | urethrocystography |
| DVUI | direct visual internal urethrotomy |
| TWOC | trial without a catheter |
| ntEPA | non-transecting excision and primary anastomosis |
| uroflow | urinary flow measurement |
| q/max | maximal urinary flow |
| NA | not available |
| NED | no evidence of disease |
| Yr(s) | year(s) |
| MO | months |
| Wks | weeks |
| D | day(s) |
| DM | diabetes mellitus |
References
- Bleeker, M.C.; Heideman, D.A.; Snijders, P.J.; Horenblas, S.; Dillner, J.; Meijer, C.J. Penile cancer: Epidemiology, pathogenesis and prevention. World J. Urol. 2009, 27, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, J.F.; Brinsko, R.W.; Davis, C.J., Jr.; Mostofi, F.K.; Sesterhenn, I.A. A distinctive myointimal proliferation (‘myointimoma’) involving the corpus spongiosum of the glans penis: A clinicopathologic and immunohistochemical analysis of 10 cases. Am. J. Surg. Pathol. 2000, 24, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.; Wang, L.; Tretiakova, M.; Cibull, T.; Pease, G. Penile Myointimoma: A Clinicopathologic Study of 4 Tumors. Int. J. Surg. Pathol. 2023, 31, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Cito, G.; Santi, R.; Gemma, L.; Galli, I.C.; Cocci, A.; Carini, M.; Minervini, A.; Nesi, G. Myointimoma of the penis. Int. J. Impot. Res. 2021, 33, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, E.Z.; Zequi, S.d.C.; Pinto, C.A.; Santos, G.C.; Lopes, A. A rare case of insidious Myointimoma–Case report. Appl. Cancer Res. 2007, 27, 30–32. [Google Scholar]
- Drlík, M.; Gregová, M.; Sedláček, J.; Kočvara, R. Myointimoma (angiocentric myofibroblastic tumor) of the glans penis in an adolescent: A case report and review of the literature. BMC Urol. 2022, 22, 186. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.K.; Collins, M.H.; Carretero, A.P.; Boyd, T.K.; Redman, J.F.; Parham, D.M. Penile myointimoma in children and adolescents: A clinicopathologic study of 5 cases supporting a distinct entity. Am. J. Surg. Pathol. 2007, 31, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Monsalvez, V.; Rodriguez-Peralto, J.L.; Fuertes, L.; Garrido, C.; Lopez-Gomez, S. Myointimoma: A rare tumor of the penis. Actas Dermo-Sifiliográficas 2009, 100, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.B.; Kohler, S. Penile nodule in a 54-year-old man: A case of a myointimoma. J. Am. Acad. Dermatol. 2005, 53, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, H.I.; Yilmaz, O.; Nese, N.; Taneli, C.; Genc, A. Myointimoma of the glans penis. J. Pediatr. Surg. Case Rep. 2019, 44, 101189. [Google Scholar] [CrossRef]
- Thurber, S.; Robbins, J.; Kohler, S. Myointimoma of the Glans Penis. J. Cutan. Pathol. 2006, 33, 68. [Google Scholar] [CrossRef]
- Tolinger, P.; Fiala, M.; Petrik, A.; Lukacova, K. Myointimoma of penis-case report and literature overview. Eur. Urol. Suppl. 2014, 13, e1246. [Google Scholar]
- Turner, B.M.; Reith, J.D.; Al-Quran, S.Z. Penile myointimoma. J. Cutan. Pathol. 2009, 36, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Vardar, E.; Gunlusoy, B.; Arslan, M.; Kececi, S. Myointimoma of the glans penis. Pathol. Int. 2007, 57, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Wyner, L.M.; Sebo, T.J. Myointimoma-Like Lymphoedematous Fibroepithelial Polyp Associated with Prostatic Adenocarcinoma Metastatic to the Penis. AJSP Rev. Rep. 2016, 21, 301–303. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mundy, A.R.; Andrich, D.E. Urethral strictures. BJU Int. 2011, 107, 6–26. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | Location | Size (cm) | Durtion | Therapy | Follow-Up | Out-Come | Country | Author | Publication (Year) | DM | Trauma | Collagen-Vascular Disease | Autoimmune Disease | Genitourinary Infection |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Glans | 0.5 | NA | complete resection | NA | NA | USA | Fetsch | 2000 | non | non | non | non | non |
| 2 | Glans | 1.0 | NA | punch biopsy | 10 yrs | Remission | USA | Fetsch | 2000 | non | non | non | non | non |
| 4 | Corona of glans | 0.7 | 4-5 mos | complete resection | 1 yr 7 mos | NED | USA | Fetsch | 2000 | non | non | non | non | non |
| 19 | Glans | 1.1 | >6 mos | complete resection | NA | NA | USA | Fetsch | 2000 | non | non | non | non | non |
| 30 | Glans | 0.6 | 3 wks | complete resection | 6 yrs 5 mos | NED | USA | Fetsch | 2000 | non | non | non | non | non |
| 32 | Glans | 0.7 | 4 wks | Incomplete excision | 6 mos | Persistent/stable disease | USA | Fetsch | 2000 | non | non | non | non | non |
| 37 | Corona of glans | 1.1 | NA | complete resection | 13 yrs | NED | USA | Fetsch | 2000 | non | non | non | non | non |
| 53 | Corona of glans | 1.9 | 4 wks | complete resection | 5 yrs | NED | USA | Fetsch | 2000 | non | non | non | non | non |
| 54 | Glans | 0.9 | 4 days | complete resection | 9 yrs 9 mos | NED | USA | Fetsch | 2000 | non | non | non | non | non |
| 61 | Corona of Glans | 0.8 | 5 mos | complete resection | 3 mos | NED, tenderness | USA | Fetsch | 2000 | non | non | non | non | non |
| 54 | Corona of glans | 0.4 | 2 mos | Incisional biopsy | 1 mo | NED | USA | Robbins | 2005 | non | non | non | non | non |
| 12 | Glans | 0.4 | NA | marginal excision | 7 mos | NED | USA | McKenney | 2007 | non | non | non | non | non |
| 4 | Glans | 0.7 | NA | marginal excision | 3 yrs 9 mos | NED | USA | McKenney | 2007 | non | non | non | non | non |
| 9 | Glans | 0.5 | NA | incomplete excision | 1 yr 9 mos | NED | USA | McKenney | 2007 | non | non | non | non | non |
| 15 | Glans | 1.8 | NA | incomplete excision | 2 mos | NED | USA | McKenney | 2007 | non | non | non | non | non |
| 9 | Glans | 1.0 | NA | incomplete excision | 1 yr 6 mos | NED | USA | McKenney | 2007 | non | non | non | non | non |
| 50 | ventral portion of the penis; next to the glans | 1.5 | 30 yrs | complete excision | 6 mos | NED | Brazil | Cordeiro | 2007 | non | non | non | non | non |
| 50 | Glans | 1.0 | 2 mos | excisional biopsy | 9 mos | NED | Turkey | Vardar | 2007 | non | non | non | non | non |
| 54 | Corona of glans | 0.4 | 3 mos | incisional biopsy | 1 mo | NED | USA | Thurber | 2008 | NA | non | NA | NA | NA |
| 14 | Glans | 1.0 | 1 mo | excisional biopsy | NA | NA | USA | Turner | 2009 | non | non | non | non | non |
| 74 | Glans | 1.0 | 4 mos | NA | 10 mos | persistent/stable disease | Spain | Mossálvez | 2009 | non | non | non | non | non |
| 53 | Glans | 0.8 | NA | complete excision | 6 mos | NED | Czech Repuplic | Tolinger | 2014 | non | non | non | non | non |
| 69 | Glans | 1.0 | 22 mos | excision | 3 mos | NED | USA | Wyner | 2016 | yes | yes | non | non | NA |
| 11 | Glans | 1.0 | 2 wks | complete excision | 1 yr | NED | Turkey | Tanriverdi | 2019 | NA | non | NA | NA | non |
| 49 | Glans | 1.1 | 1 yr | complete excision | 1 yr 6 mos | NED | Italy | Cito | 2020 | non | non | non | non | non |
| 15 | Glans | 1.0 | 6 mos | excisional biopsy | 3 yrs | NED | Czech Republic | Drlík | 2022 | non | non | non | non | non |
| 20 | Glans | 0.9 | 2 wks | NA | 9 mos | NED | USA | Casa | 2023 | non | non | non | non | non |
| 42 | Glans | NA | NA | NA | 11 yrs | NED | USA | Casa | 2023 | non | non | non | non | non |
| 32 | Glasn | NA | NA | NA | 4 yrs 8 mos | NED | USA | Casa | 2023 | non | non | non | non | non |
| 68 | Glans | NA | NA | NA | 15 yrs | NED | USA | Casa | 2023 | non | non | non | non | non |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
König-Castillo, D.M.; Henning, A.; Wasicky, R.; Kinsky, C.; Klingler, H.C.; Compérat, E.M. The Presentation and Treatment of Myointimoma: A Systematic Review and the First Case Report of Penile Myointimoma as a Cause of Urethral Obstruction. J. Clin. Med. 2026, 15, 1130. https://doi.org/10.3390/jcm15031130
König-Castillo DM, Henning A, Wasicky R, Kinsky C, Klingler HC, Compérat EM. The Presentation and Treatment of Myointimoma: A Systematic Review and the First Case Report of Penile Myointimoma as a Cause of Urethral Obstruction. Journal of Clinical Medicine. 2026; 15(3):1130. https://doi.org/10.3390/jcm15031130
Chicago/Turabian StyleKönig-Castillo, Deirdre Maria, Armin Henning, Richard Wasicky, Clemens Kinsky, H. Christoph Klingler, and Eva M. Compérat. 2026. "The Presentation and Treatment of Myointimoma: A Systematic Review and the First Case Report of Penile Myointimoma as a Cause of Urethral Obstruction" Journal of Clinical Medicine 15, no. 3: 1130. https://doi.org/10.3390/jcm15031130
APA StyleKönig-Castillo, D. M., Henning, A., Wasicky, R., Kinsky, C., Klingler, H. C., & Compérat, E. M. (2026). The Presentation and Treatment of Myointimoma: A Systematic Review and the First Case Report of Penile Myointimoma as a Cause of Urethral Obstruction. Journal of Clinical Medicine, 15(3), 1130. https://doi.org/10.3390/jcm15031130







