Clinical Salvage Approaches for Surgical Site Infection After Autologous Microtia Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Ethical Compliance
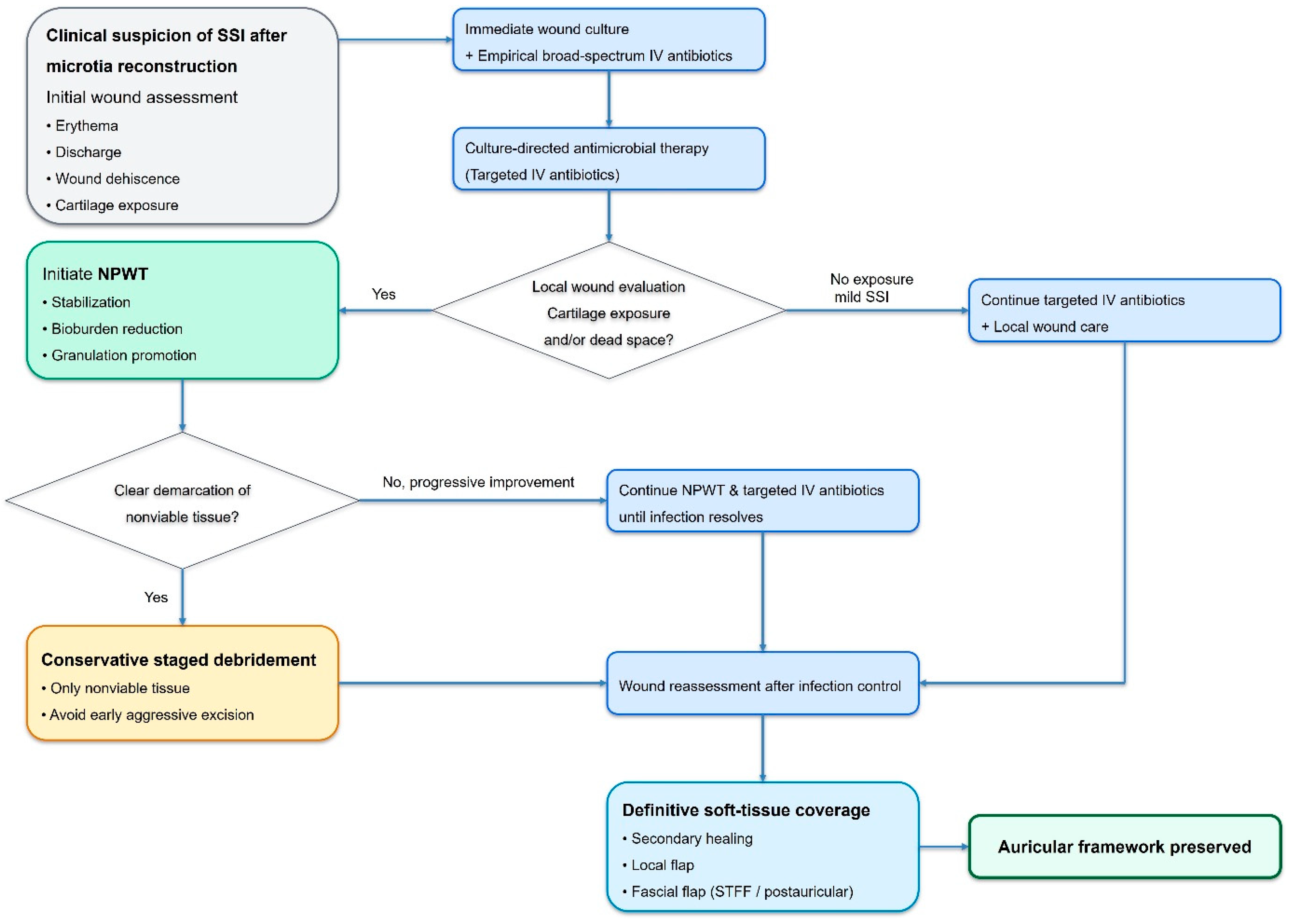
2.3. Infection Evaluation and Antibiotic Management
2.4. Negative-Pressure Wound Therapy
2.5. Conservative Staged Debridement Strategy
2.6. Outcome Assessment and Follow-Up
3. Results
3.1. Patient Demographics
3.2. Case Presentations
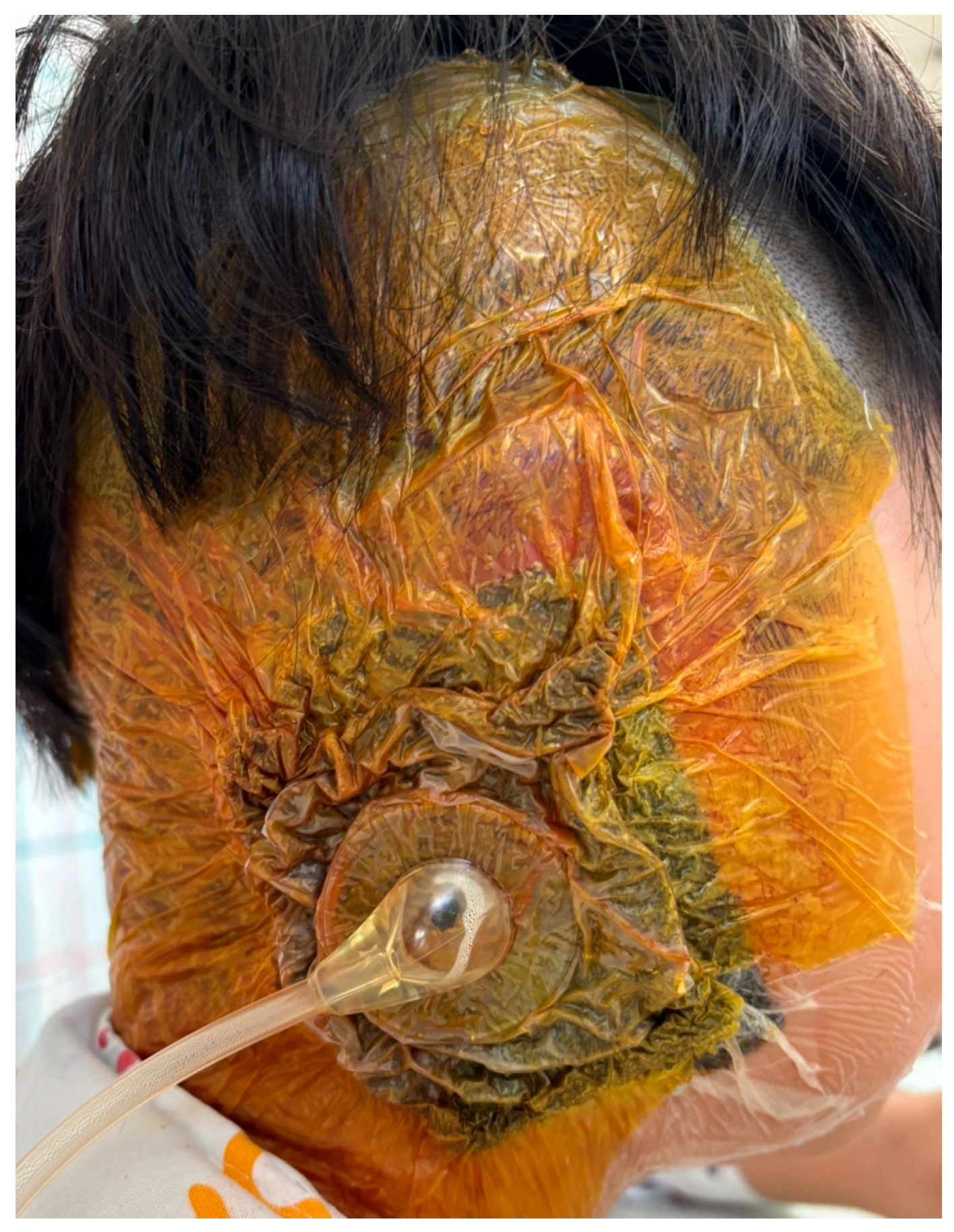
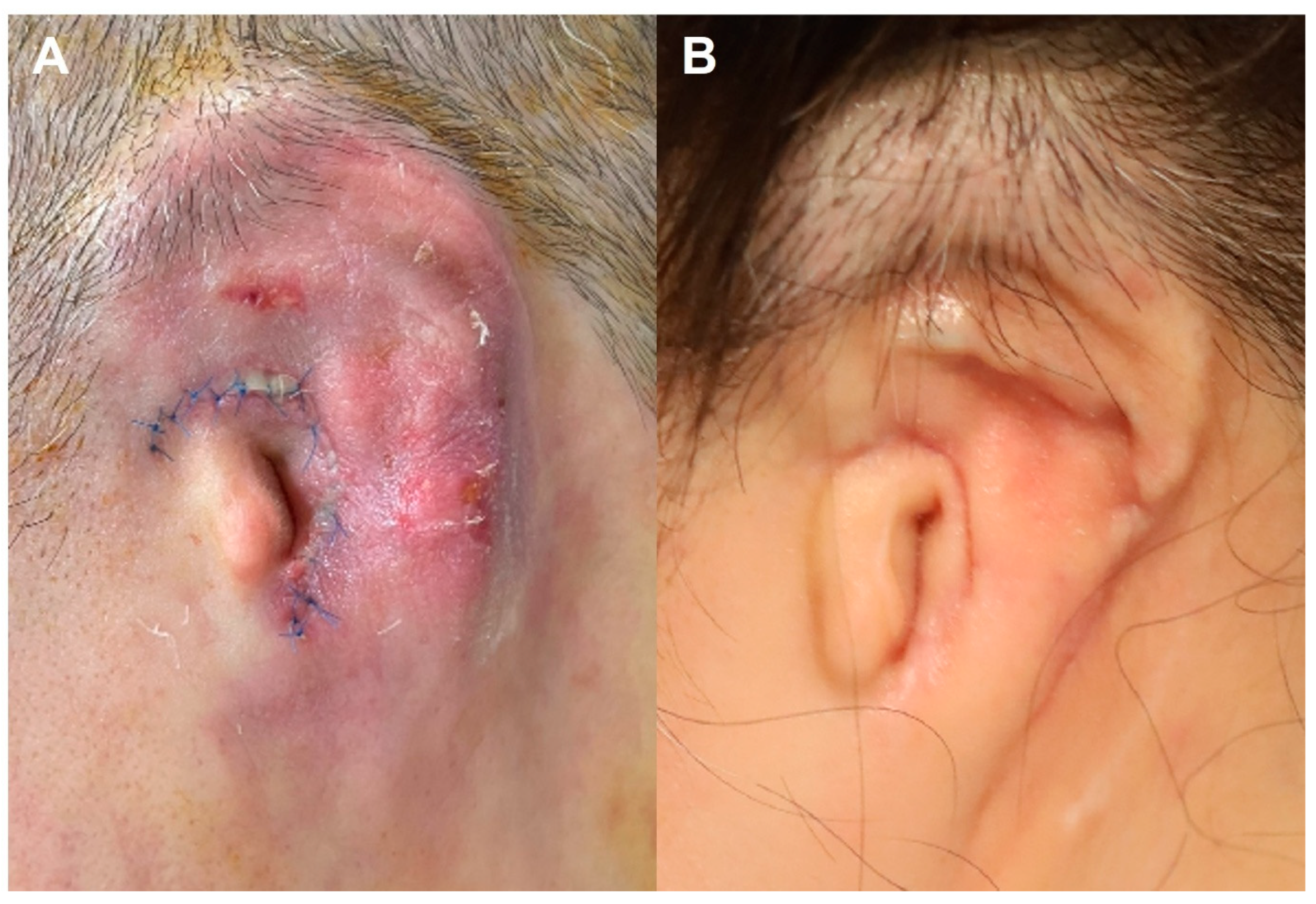
3.2.1. Case 1—Early Postoperative Coagulase-Negative Staphylococcus Infection with Partial Framework Necrosis
3.2.2. Case 2—Early Postoperative Serratia marcescens Infection
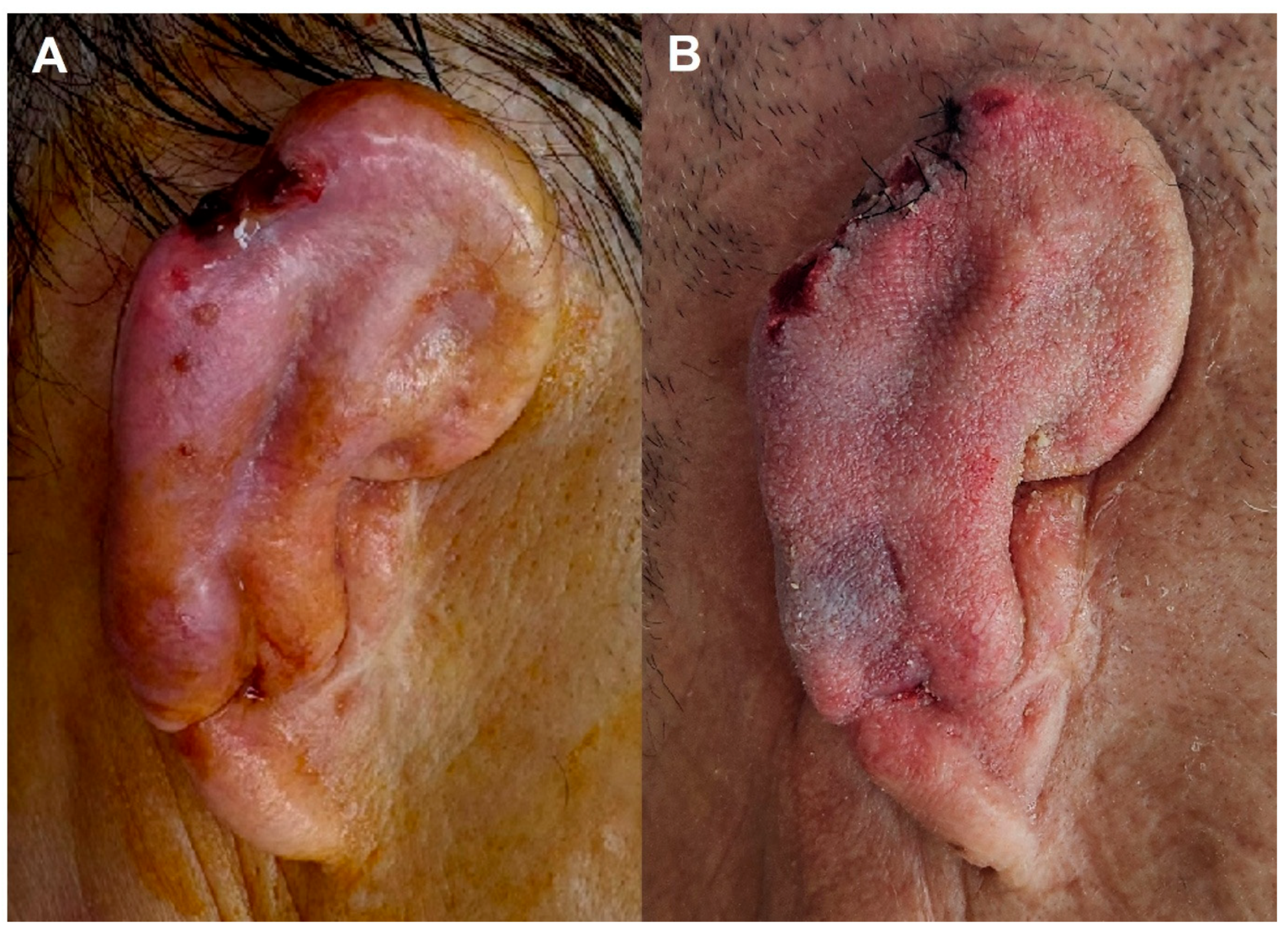
3.2.3. Case 3—Late Traumatic Pseudomonas aeruginosa Infection
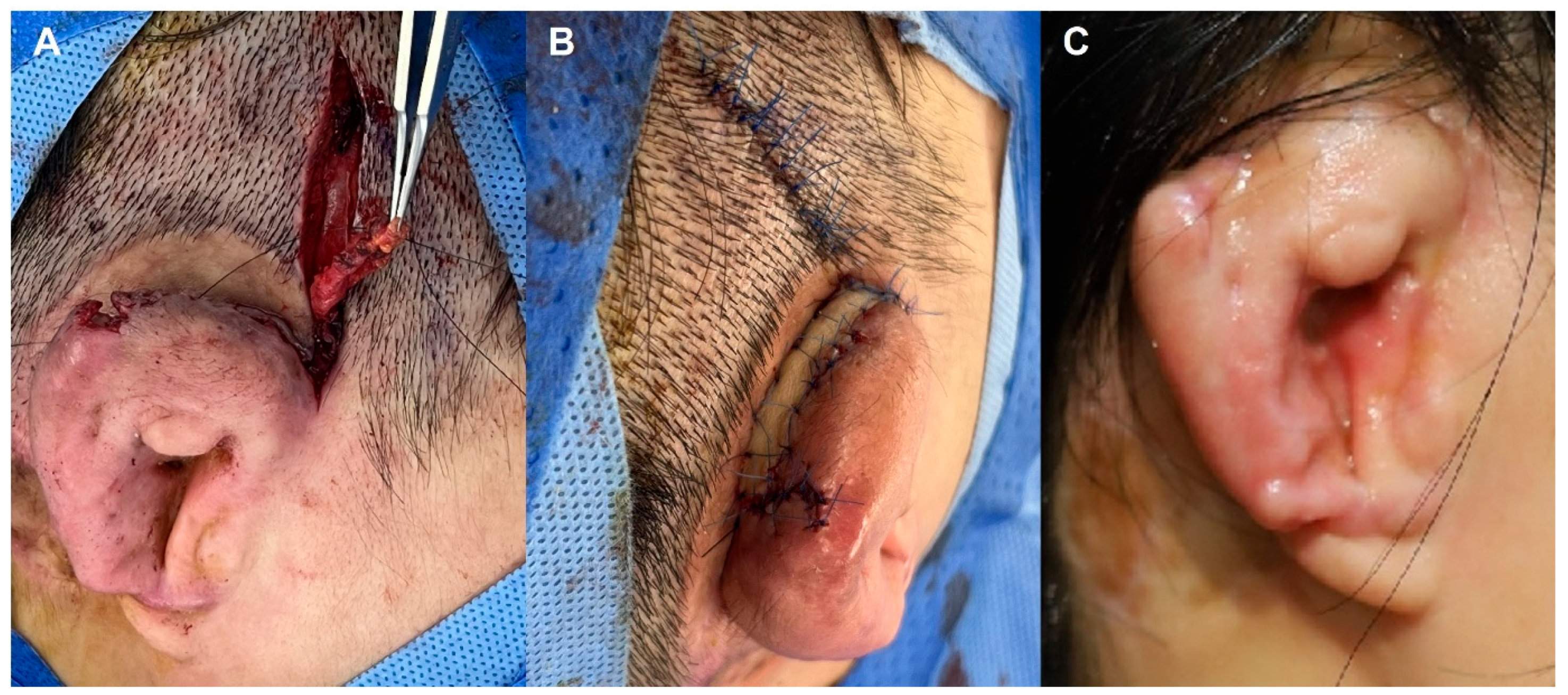
3.2.4. Case 4—Post-Elevation Multidrug-Resistant Enterobacter Infection
3.3. Comparative Summary
4. Discussion
4.1. Microbiologic Considerations and Implications for Management
4.2. Antimicrobial Strategy as a Foundation for Salvage
4.3. Central Role of Negative-Pressure Wound Therapy
4.4. Conservative Staged Debridement over Radical Excision
4.5. Soft-Tissue Envelope Restoration and Definitive Coverage
4.6. Salvage as the Default Objective
4.7. Proposed Salvage-Oriented Management Protocol
- Early recognition of SSI with prompt clinical diagnosis.
- Immediate wound culture acquisition and initiation of broad-spectrum intravenous antibiotics.
- Rapid transition to culture-directed antimicrobial therapy.
- Application of NPWT for wound stabilization, bioburden reduction, and cartilage protection.
- Conservative staged debridement limited to clearly nonviable tissues.
- Definitive soft-tissue coverage once infection has resolved and healthy granulation tissue is established.
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagata, S. A new method of total reconstruction of the auricle for microtia. Plast. Reconstr. Surg. 1993, 92, 187–201. [Google Scholar] [CrossRef]
- Cho, B.C.; Kim, J.Y.; Byun, J.S. Two-stage reconstruction of the auricle in congenital microtia using autogenous costal cartilage. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 998–1006. [Google Scholar] [CrossRef]
- Hellies, F.; Fracaro, S.; Marioni, G.; Trotta, A.; Todesco, M.; Casarin, M.; Bagno, A.; Zanoletti, E.; Albertin, G.; Astolfi, L. Systematic Review on Microtia: Current Knowledge and Future Directions. Children 2025, 12, 411. [Google Scholar] [CrossRef]
- Eley, K.A.; Gault, D.T. The bacteriology of children prior to 1st stage autologous ear reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.N.; Walker, M.E.; D’Achille, J. Salvage of a costochondral graft for microtia after postoperative infection. Ann. Plast. Surg. 2014, 72, 56–58. [Google Scholar] [CrossRef]
- Saadi, R.A.; Snyder, D.; Shokri, T.; Lighthall, J.G. Postoperative outcomes of autologous rib graft for microtia repair in children: A NSQIP study. Int. J. Pediatr. Otorhinolaryngol. 2021, 146, 110733. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, R.S.; Volpicelli, E.J.; Martins, D.B.; Park, S.H.; Dubina, E.; Ishiyama, A.; Bradley, J.P.; Lee, J.C. Evaluation of 4 Outcomes Measures in Microtia Treatment: Exposures, Infections, Aesthetics, and Psychosocial Ramifications. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1460. [Google Scholar] [CrossRef] [PubMed]
- Jovic, T.H.; Gibson, J.A.G.; Jovic, M.; Dobbs, T.D.; Griffiths, R.; Akbari, A.; Whitaker, I.S. The psychosocial impact of microtia and ear reconstruction: A national data-linkage study. Front. Pediatr. 2023, 11, 1148975. [Google Scholar] [CrossRef]
- Park, C.; Yoo, Y.S.; Park, H.J.; Park, Y.S. An analysis of the bacterial flora found in the external auditory canals of microtia patients: Results and clinical applications. Ann. Plast. Surg. 2010, 65, 197–200. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Q.; Jiang, F.; Wu, Y.; Su, X.; Zhuang, J.; Zhan, S. Distribution of Pathogenic Bacteria and Antimicrobial Resistance after Plastic Surgery for Microtia. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5442. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Schimizzi, A.M.; Del Prete, M.S.; Barchiesi, F.; D’Errico, M.M.; Petrelli, E.; Scalise, G. Epidemiology and microbiology of surgical wound infections. J. Clin. Microbiol. 2000, 38, 918–922. [Google Scholar] [CrossRef]
- Rezaei, A.R.; Zienkiewicz, D.; Rezaei, A.R. Surgical site infections: A comprehensive review. J. Trauma Inj. 2025, 38, 71–81. [Google Scholar] [CrossRef]
- Youbong, T.J.; De Pontfarcy, A.; Rouyer, M.; Strazzula, A.; Chakvetadze, C.; Flateau, C.; Sayegh, S.; Noel, C.; Pitsch, A.; Abbadi, A.; et al. Bacterial Epidemiology of Surgical Site Infections after Open Fractures of the Lower Limb: A Retrospective Cohort Study. Antibiotics 2021, 10, 1513. [Google Scholar] [CrossRef]
- Sasaki, K.; Sasaki, M.; Oshima, J.; Nishijima, A.; Aihara, Y.; Sekido, M. Salvaging exposed microtia cartilage framework with negative pressure wound therapy. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1355–1401. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Yun, I.S.; Chung, S. Salvage of Ear Framework Exposure in Total Auricular Reconstruction. Ann. Plast. Surg. 2017, 78, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, J.F.; van Hövell Tot Westerflier, C.V.A.; Gould, D.J.; Tahiri, Y.T. Secondary Salvage of the Unsatisfactory Microtia Reconstruction. Plast. Reconstr. Surg. 2020, 145, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Fukui, K.; Yoshino, K.; Noguchi, M.; Murakami, R. Salvage of Ear Framework Exposure Following Autologous Microtia Reconstruction: Repair Strategy for Each Location of Exposure. Cleft Palate Craniofac. J. 2023, 60, 1172–1175. [Google Scholar] [CrossRef]
- Nagata, S. Modification of the stages in total reconstruction of the auricle. Plast. Reconstr. Surg. 1994, 93, 221–266. [Google Scholar] [CrossRef]
- Firmin, F.; Marchac, A. A novel algorithm for autologous ear reconstruction. Semin. Plast. Surg. 2011, 25, 257–264. [Google Scholar] [CrossRef]
- Argenta, L.C.; Morykwas, M.J. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann. Plast. Surg. 1997, 38, 563–576. [Google Scholar] [CrossRef]
- Eto, A.; Sasaki, K.; Togashi, S.; Aizawa, T.; Sekido, M. Usefulness of vacuum-assisted closure therapy for the treatment of exposed cartilage after repair of microtia with costal cartilage. J. Jpn. Plast. Reconstr. Aesthet. Surg. 2012, 32, 686–689. [Google Scholar]
- Concepcion, L.G.; Beatriz, B.; Elena, T. Use of innovative negative pressure therapy for cartilage exposure in microtia reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, e3–e4. [Google Scholar]
- Borchardt, R.A.; Tzizik, D. Update on surgical site infections: The new CDC guidelines. JAAPA 2018, 31, 52–54. [Google Scholar] [CrossRef]
- Itani, K.M.F.; Dellinger, E.P.; Mazuski, J.; Solomkin, J.; Allen, G.; Blanchard, J.C.; Kelz, R.; Berríos-Torres, S.I. Surgical Site Infection Research Opportunities. Surg. Infect. 2017, 18, 401–408. [Google Scholar] [CrossRef]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Orgill, D.P.; Bayer, L.R. Negative pressure wound therapy: Past, present and future. Int. Wound J. 2013, 10, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Giacobbe, D.R.; Castaldo, N. Management of Infections Caused by Multidrug-resistant Gram-negative Pathogens: Recent Advances and Future Directions. Arch. Med. Res. 2021, 52, 817–827. [Google Scholar] [CrossRef] [PubMed]

| Case | Age/Sex | Time of Wound Problem Occurrence | Wound Status at Presentation | Pathogen of Wound Culture | Gram Stain | MDR Pathogen | Treatment Modalities | Treatment Duration | Final Result | Follow-Up Periods |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10/M | Postoperative 10 days after RCG | Skin flap erythema, purulent discharge | Coagulase-negative Staphylococcus | G(+) cocci | No | IV antibiotics, NPWT, staged debridement | 18 days | Healed with loss of less than 10% cartilage framework | 6 months |
| 2 | 51/F | Postoperative 7 days after RCG | Skin flap erythema, swelling, purulent discharge | Serratia marcescens | G(−) rod | No | IV antibiotics, NPWT | 7 days | Healed with no loss of cartilage framework | 12 months |
| 3 | 64/M | Postoperative 20 years after AE | Minimal cartilage exposure, purulent discharge, erythema | Pseudomonas aeruginosa | G(−) rod | Yes | IV antibiotics, NPWT, staged debridement | 14 days | Healed with loss of less than 10% cartilage framework | 6 months |
| 4 | 50/F | Postoperative 2 months after AE | Skin graft partial necrosis, cartilage exposure | Enterobacter hormaechei, Enterobacter cloacae | G(−) rod | Yes | IV antibiotics, NPWT, staged debridement, STFF + FTSG | 24 days | Lower two-thirds of framework preserved with upper helix loss | 20 months |
| 5 | 58/M | Postoperative 8 days after RCG | Skin flap erythema with purulent discharge | Staphylococcus aureus | G(+) cocci | No | IV antibiotics, NPWT | 7 days | Healed without significant cartilage loss | 9 months |
| 6 | 52/F | Postoperative 14 days after RCG | Localized wound dehiscence with purulent discharge | Coagulase-negative Staphylococcus | G(+) cocci | No | IV antibiotics + NPWT | 10 days | Healed without significant cartilage loss | 10 months |
| 7 | 48/F | Postoperative 11 days after RCG | Partial wound breakdown with minimal cartilage exposure | Staphylococcus aureus | G(+) cocci | No | IV antibiotics, NPWT | 8 days | Healed without significant cartilage loss | 15 months |
| 8 | 58/M | Postoperative 12 days after RCG | Skin flap erythema with purulent discharge | Enterobacter cloacae complex | G(−) rod | No | IV antibiotics, NPWT | 17 days | Healed without significant cartilage loss | 8 months |
| 9 | 54/F | Postoperative 2 months after RCG | Delayed wound breakdown with purulent discharge | Pseudomonas aeruginosa | G(−) rod | No | IV antibiotics, NPWT | 21 days | Healed without significant cartilage loss | 12 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Oh, K.S.; Cho, W.; Kim, J.; Kim, K.N. Clinical Salvage Approaches for Surgical Site Infection After Autologous Microtia Reconstruction. J. Clin. Med. 2026, 15, 1064. https://doi.org/10.3390/jcm15031064
Oh KS, Cho W, Kim J, Kim KN. Clinical Salvage Approaches for Surgical Site Infection After Autologous Microtia Reconstruction. Journal of Clinical Medicine. 2026; 15(3):1064. https://doi.org/10.3390/jcm15031064
Chicago/Turabian StyleOh, Kap Sung, Wonseok Cho, Junekyu Kim, and Kyu Nam Kim. 2026. "Clinical Salvage Approaches for Surgical Site Infection After Autologous Microtia Reconstruction" Journal of Clinical Medicine 15, no. 3: 1064. https://doi.org/10.3390/jcm15031064
APA StyleOh, K. S., Cho, W., Kim, J., & Kim, K. N. (2026). Clinical Salvage Approaches for Surgical Site Infection After Autologous Microtia Reconstruction. Journal of Clinical Medicine, 15(3), 1064. https://doi.org/10.3390/jcm15031064






