Reduced Versus Full-Dose Direct Oral Anticoagulants for Long-Term Management of Venous Thromboembolism: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy, Study Selection, and Data Extraction
2.2. Quality Assessment and Risk of Bias
2.3. Data Synthesis and Analysis
3. Results
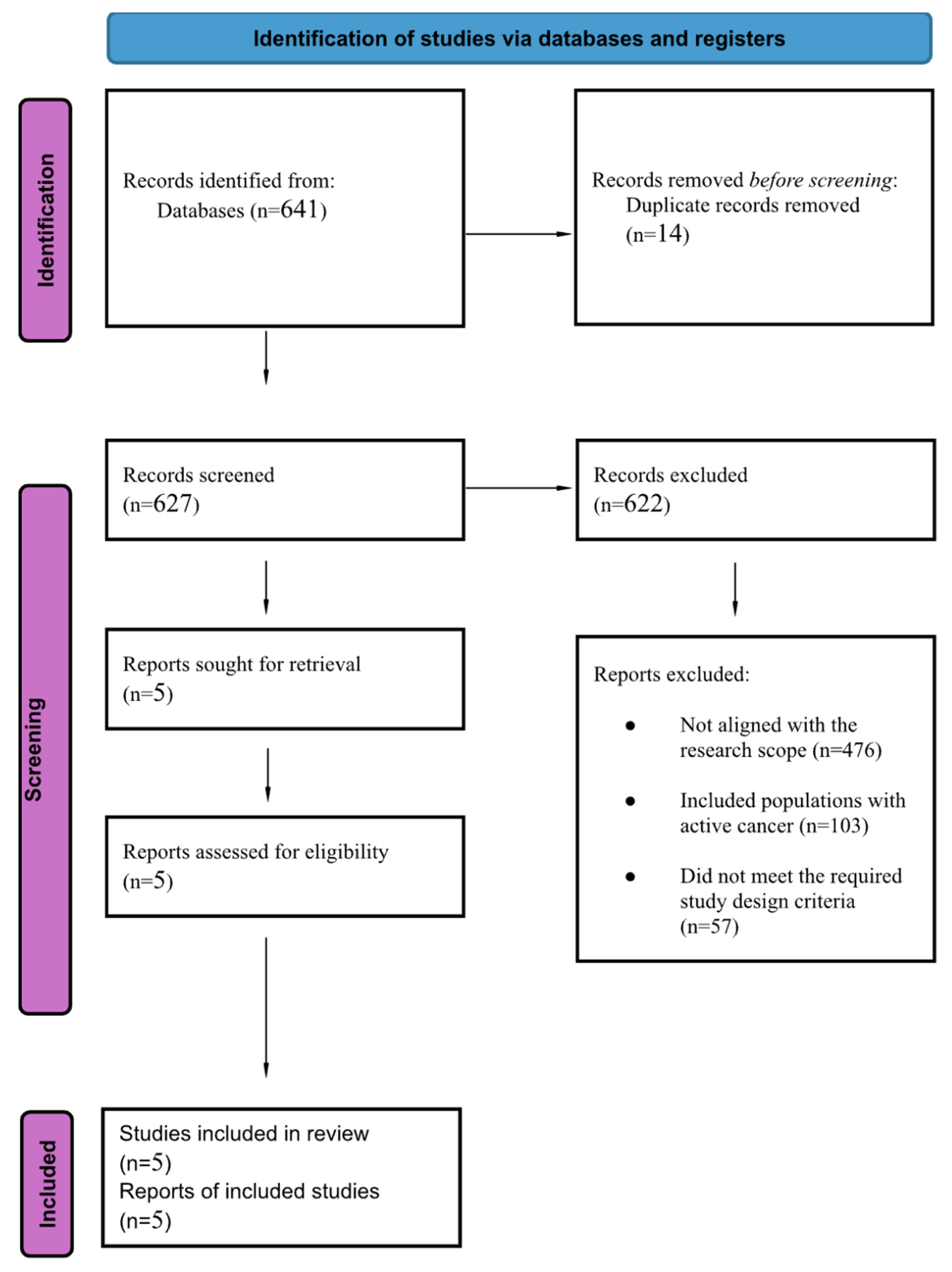
3.1. Search Results and Study Characteristics
3.2. Summary of the Included Studies
3.3. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VTE | Venous thromboembolism |
| DVT | Deep vein thrombosis |
| PE | Pulmonary embolism |
| DOAC(s) | Direct oral anticoagulant(s) |
| VKA(s) | Vitamin K antagonist(s) |
| MB | Major bleeding |
| CRNMB | Clinically relevant non-major bleeding |
| RCT | Randomized controlled trial |
| ESC | European Society of Cardiology |
| ISTH | International Society on Thrombosis and Haemostasis |
| RoB-2 | Revised Cochrane Risk-of-Bias tool for randomized trials |
| NOS | Newcastle–Ottawa Scale |
| HR | Hazard ratio |
| RR | Relative Risk |
| CI | Confidence interval |
| BID | Twice daily |
| OD | Once daily |
| PROSPERO | International Prospective Register of Systematic Reviews |
| EVE | Extending venous thromboembolism secondary prevention with apixaban in cancer patients trial |
| API-CAT | Extended Reduced-Dose Apixaban for Cancer-Associated Venous Thromboembolism trial |
| NR | Not Reported |
| NA | Not Applicable |
References
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous thromboembolism: A public health concern. Am. J. Prev. Med. 2010, 38, S495–S501. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Porcari, A.; Raskob, G.E.; Weitz, J.I. Apixaban for extended treatment of venous thromboembolism (AMPLIFY-EXT). N. Engl. J. Med. 2013, 368, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Lensing, A.W.; Prins, M.H.; Bauersachs, R.; Beyer-Westendorf, J.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism (EINSTEIN-CHOICE). N. Engl. J. Med. 2017, 376, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Couturaud, F.; Schmidt, J.; Sanchez, O.; Ballerie, A.; Sevestre, M.A.; Meneveau, N.; Bertoletti, L.; Connault, J.; Benhamou, Y.; Constans, J.; et al. Reduced-dose versus full-dose direct oral anticoagulants for extended treatment of venous thromboembolism (RENOVE Trial). Lancet 2025, 405, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.; Bikdeli, B.; Pandey, A.K.; Krishnathasan, D.; Khairani, C.D.; Bejjani, A.; Morrison, R.H.; Hogan, H.; Rashedi, S.; Pfeferman, M.; et al. Apixaban for Extended Treatment of Provoked Venous Thromboembolism (HI-PRO Trial). N. Engl. J. Med. 2025, 393, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, E.; Pannunzio, A.; Brogi, T.; Palumbo, I.M.; Menichelli, D.; Marucci, S.; Tretola, L.; Mastroianni, C.M.; Pastori, D.; Pignatelli, P. Three Year Follow-Up of Reduced Dose of Direct Oral Anticoagulants for Extended Treatment of Venous Thromboembolism: An Ambispective Cohort Study. Diagnostics 2025, 15, 2283. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 November 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- McBane, R.D., II; Loprinzi, C.L.; Zemla, T.; Tafur, A.; Sanfilippo, K.; Liu, J.J.; Garcia, D.A.; Heun, J.; Gundabolu, K.; Onitilo, A.A.; et al. Extending venous thromboembolism secondary prevention with apixaban in cancer patients: The EVE Trial. J. Thromb. Haemost. 2024, 22, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Mahé, I.; Carrier, M.; Mayeur, D.; Chidiac, J.; Vicaut, E.; Falvo, N.; Sanchez, O.; Grange, C.; Monreal, M.; López-Núñez, J.J.; et al. Extended Reduced-Dose Apixaban for Cancer-Associated Venous Thromboembolism: API-CAT Trial. N. Engl. J. Med. 2025, 392, 1363–1373. [Google Scholar] [CrossRef] [PubMed]

| Observational Studies Using the Newcastle–Ottawa Scale (NOS) | |||||
|---|---|---|---|---|---|
|
Study
(Author, Year) |
Selection
(0–4) |
Comparability
(0–2) |
Outcome
(0–3) |
Total
(0–9) | Quality |
| Valeriani et al., 2025 [7] | 3 | 0 | 2 | 5/9 | Good |
| Study | Study Design | Setting/Country Region | Age, Mean/Median (Years) | Sample Size (n) | Female % | Intervention Arm | Follow-Up Duration |
|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT (2013) [3] | Randomized, double-blind, placebo-controlled | Multicenter, 28 countries (Global) | ≈56.7 ± 15.4 years | 2486 | 42–44% | Apixaban 2.5 mg BID vs. Apixaban 5 mg BID vs. Placebo | 12 months |
| EINSTEIN CHOICE (2017) [4] | Randomized, double-blind, active-controlled | Multicenter, 31 countries (Global) | ≈57–59 ± 14.7 years | 3396 | 45% | Rivaroxaban 20 mg once daily vs. Rivaroxaban 10 mg once daily vs. Aspirin 100 once daily (control) mg | 12 months |
| RENOVE (2025) [5] | Randomized, open-label, non-inferiority | Multicenter, Europe, Australia, Canada | ≈62.7 ± 14.3 years | 2768 | 35% | Reduced-dose DOAC (apixaban 2.5 mg BID or rivaroxaban 10 mg OD) vs. Full-dose DOAC (apixaban 5 mg BID or rivaroxaban 20 mg OD) | Median 37 months (24.0–48.3) |
| HI-PRO (2025) [6] | Randomized, double-blind, placebo-controlled | Single center, United States (Boston) | 59.5 ± 15.2 years | 600 | 57% | Apixaban 2.5 mg BID vs. placebo | 12 months |
| Diagnostics Cohort (2025) [7] | Ambispective (prospective + retrospective) cohort | Single center, Italy | 72 ± 15 years | 140 | 52.1% | Reduced-dose DOACs: Apixaban 2.5 mg BID, Rivaroxaban 10 mg OD, Dabigatran 110 mg daily, Edoxaban 30 mg daily | 2.7 ± 2.1 years |
| AMPLIFY-EXT (2013) [3] | |||||||
| Drug | Recurrent VTE (%) | Unprovoked (%) | Arterial events (%) | Major bleeding (%) | CRNMB (%) | Minor bleeding (%) | p-Value |
| Apixaban 2.5 mg BID | 1.7% | 93.2% | NR | 0.2% | 3.0% | NR | <0.001 |
| Apixaban 5 mg BID | 1.7% | 90.7% | NR | 0.1% | 4.2% | NR | <0.001 |
| Placebo | 8.8% | 91.1% | NR | 0.5% | 2.3% | NR | <0.001 |
| EINSTEIN CHOICE (2017) [4] | |||||||
| Drug | Recurrent VTE (%) | Unprovoked (%) | Arterial events (%) | Major bleeding (%) | CRNMB (%) | Minor bleeding (%) | p-Value |
| Rivaroxaban 10 mg OD | 1.2% | 39.8% | NR | 0.4% | 2.0% | 14.5% | <0.001 |
| Rivaroxaban 20 mg OD | 1.5% | 42.6% | NR | 0.5% | 2.7% | 11.8% | <0.001 |
| Aspirin 100 mg | 4.4% | 41.4% | NR | 0.3% | 1.8% | 0.8% | <0.001 |
| RENOVE (2025) [5] | |||||||
| Drug | Recurrent VTE (%) | Unprovoked (%) | Arterial events (%) | Major bleeding (%) | CRNMB (%) | Minor bleeding (%) | p-Value |
| Reduced-dose DOAC | 2.2% | 60.5% | 0.5% | 2.1% | 8.6% | NR | 0.23 |
| Full-dose DOAC | 1.8% | 61.1% | 0.4% | 4% | 11.5% | NR | 0.23 |
| HI-PRO (2025) * [6] | |||||||
| Drug | Recurrent VTE (%) | Unprovoked (%) | Arterial events (%) | Major bleeding (%) | CRNMB (%) | Minor bleeding (%) | p-Value |
| Apixaban 2.5 mg BID | 1.3% | 0% | 0.4% | 0.3% | 4.8% | NA | <0.001 |
| Placebo | 10.0% | 0% | 0.4% | 0% | 1.7% | NA | <0.001 |
| Valeriani et al. (2025) [7] | |||||||
| Drug | Recurrent VTE (%) | Unprovoked (%) | Arterial events (%) | Major bleeding (%) | CRNMB (%) | Minor bleeding (%) | p-Value |
| DOAC (single-arm) | 0.7% | 37.1% | 2.9% | 2.9% | 1.4% | 2.1% | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Al Arifi, M.; Alshahrani, W.A.; Alshehri, A.M.; Al Yami, M.S. Reduced Versus Full-Dose Direct Oral Anticoagulants for Long-Term Management of Venous Thromboembolism: A Systematic Review. J. Clin. Med. 2026, 15, 770. https://doi.org/10.3390/jcm15020770
Al Arifi M, Alshahrani WA, Alshehri AM, Al Yami MS. Reduced Versus Full-Dose Direct Oral Anticoagulants for Long-Term Management of Venous Thromboembolism: A Systematic Review. Journal of Clinical Medicine. 2026; 15(2):770. https://doi.org/10.3390/jcm15020770
Chicago/Turabian StyleAl Arifi, Manar, Walaa A. Alshahrani, Abdulmajeed M. Alshehri, and Majed S. Al Yami. 2026. "Reduced Versus Full-Dose Direct Oral Anticoagulants for Long-Term Management of Venous Thromboembolism: A Systematic Review" Journal of Clinical Medicine 15, no. 2: 770. https://doi.org/10.3390/jcm15020770
APA StyleAl Arifi, M., Alshahrani, W. A., Alshehri, A. M., & Al Yami, M. S. (2026). Reduced Versus Full-Dose Direct Oral Anticoagulants for Long-Term Management of Venous Thromboembolism: A Systematic Review. Journal of Clinical Medicine, 15(2), 770. https://doi.org/10.3390/jcm15020770






