A Simple and Cost-Effective Retractor for Transorbital Neurosurgery: Technical Note and Application in Lacrimal Keyhole Approaches
Abstract
1. Introduction
2. Materials and Methods
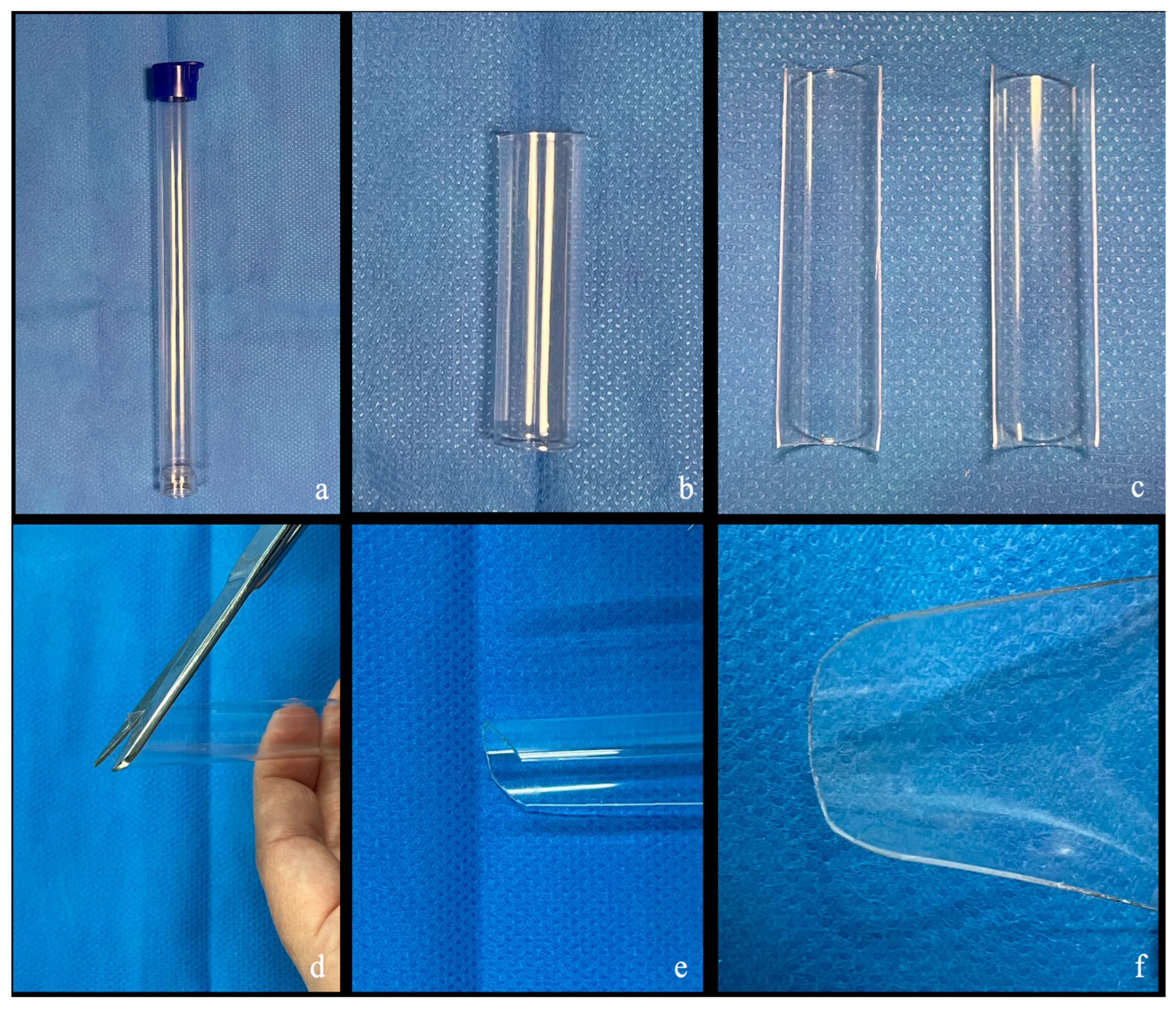
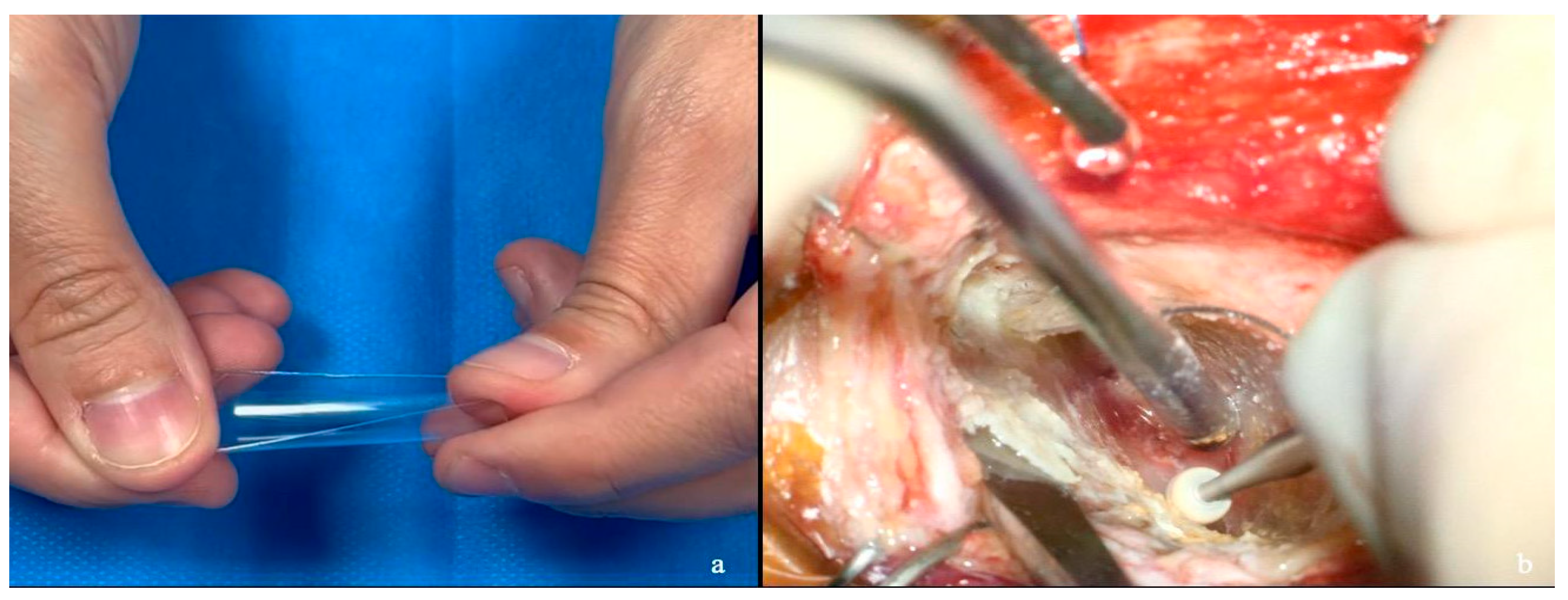
2.1. Semi-Rigid Handmade Retractor Customization
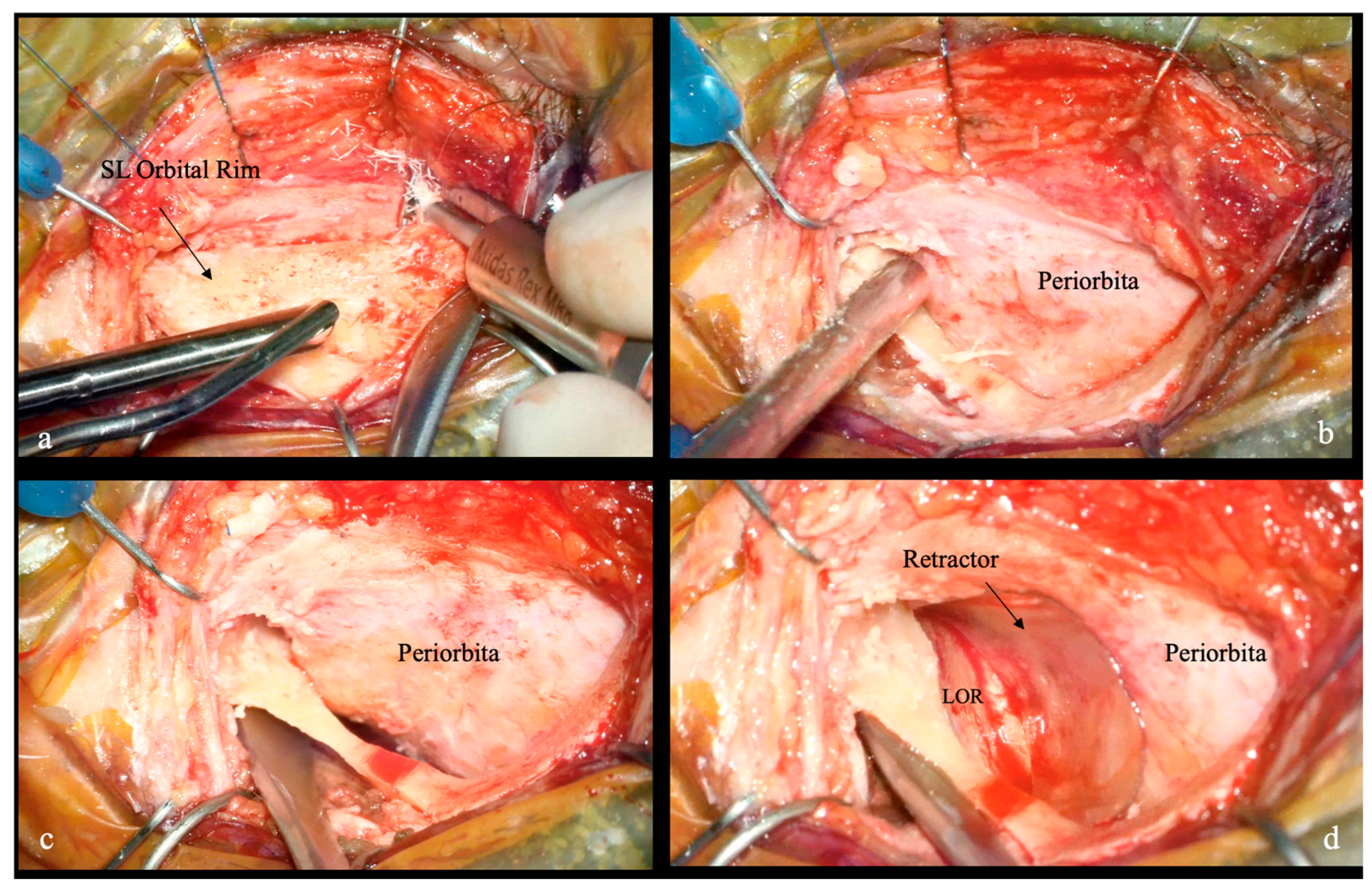
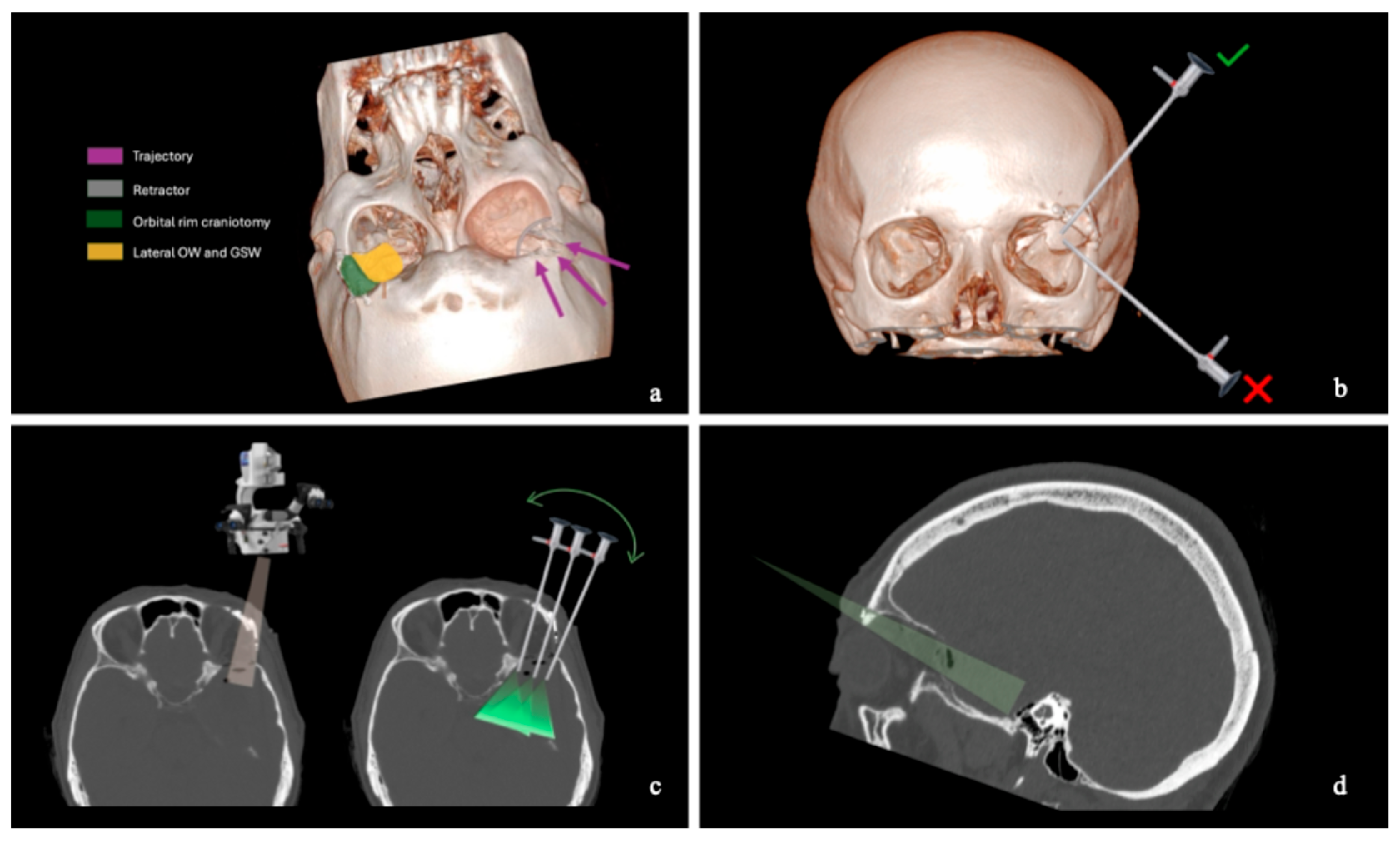
2.2. Skin and Craniotomy
2.3. Bony Phase
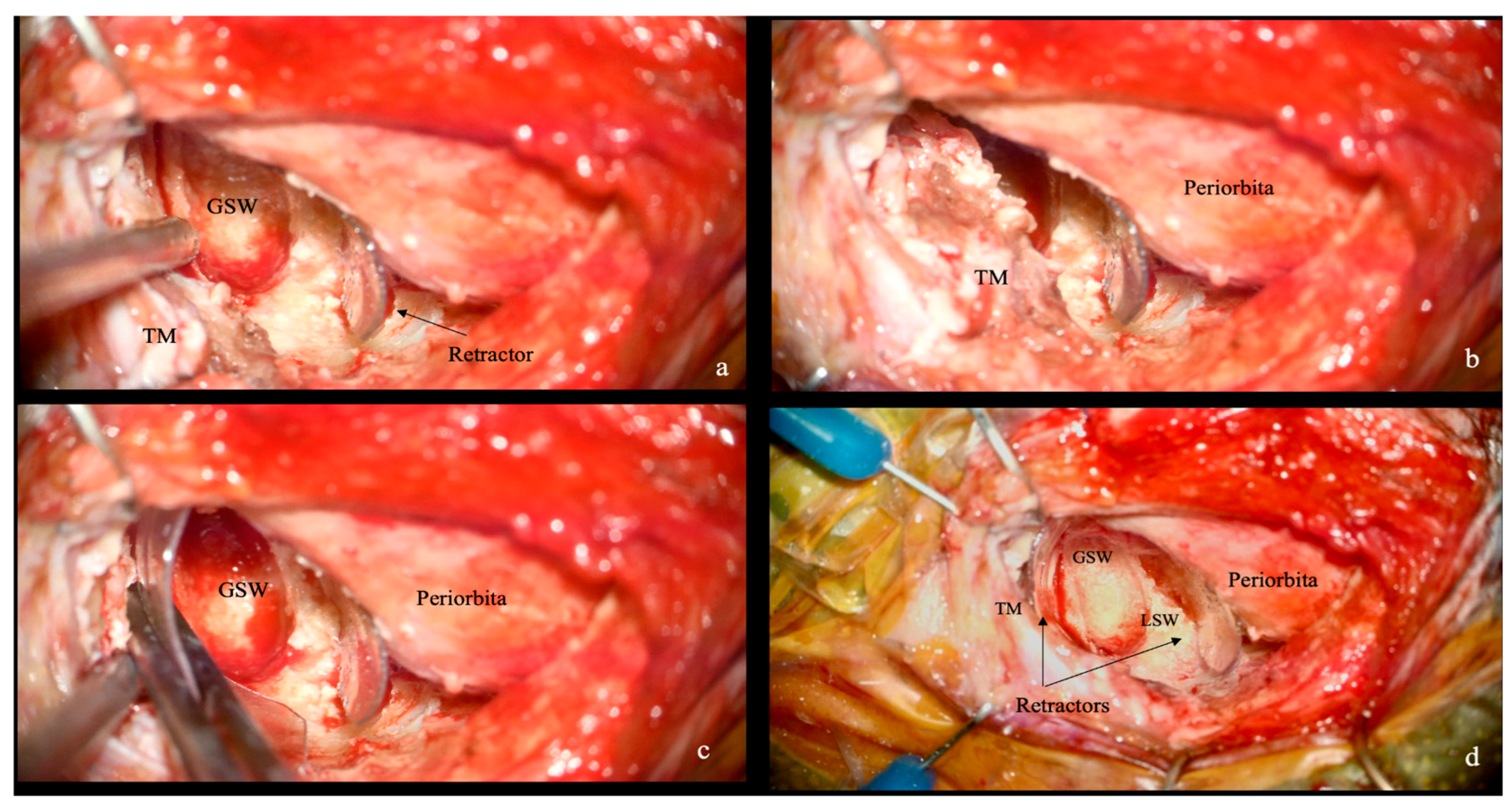
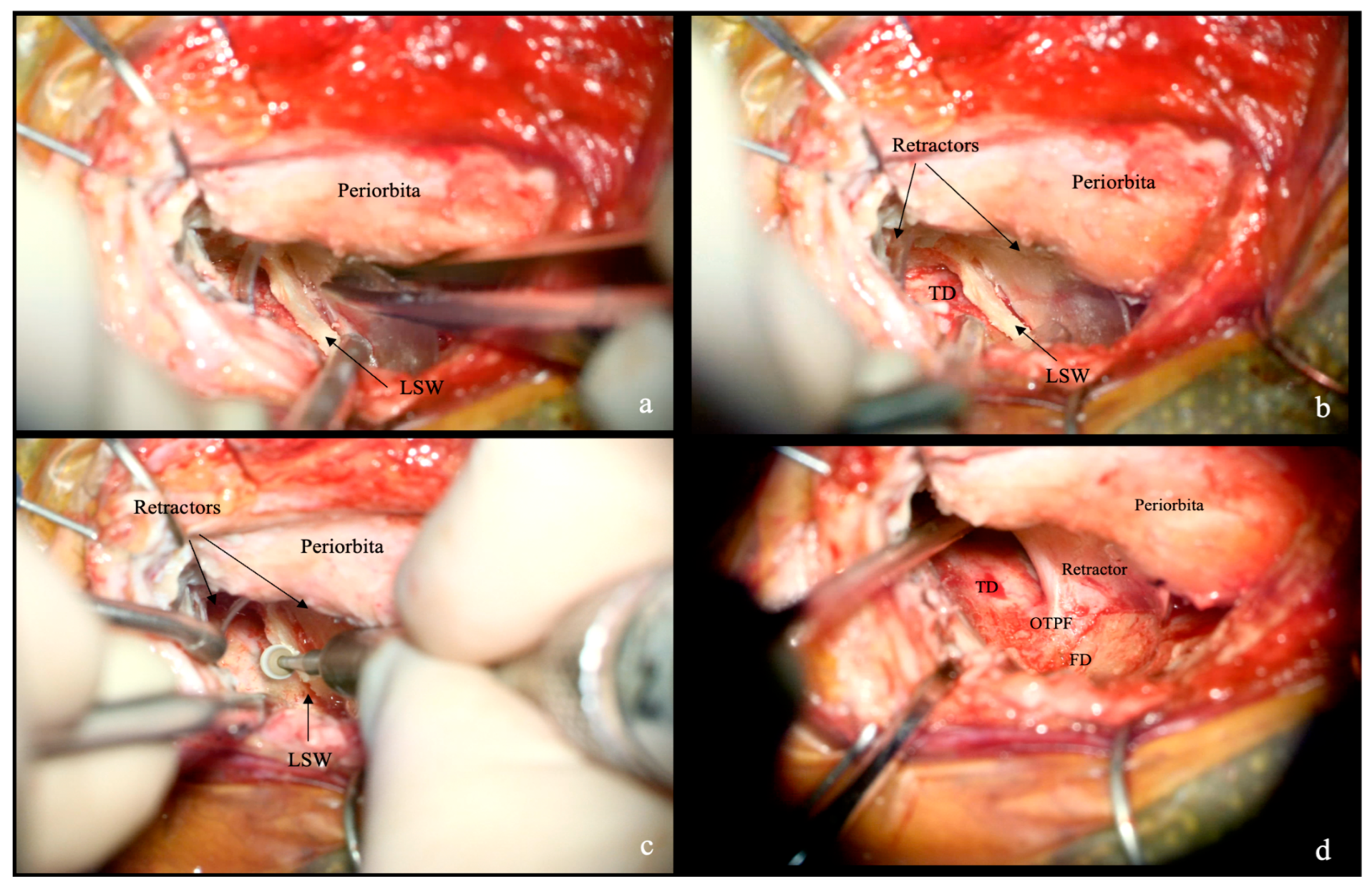
2.4. Extradural and Intradural Phases
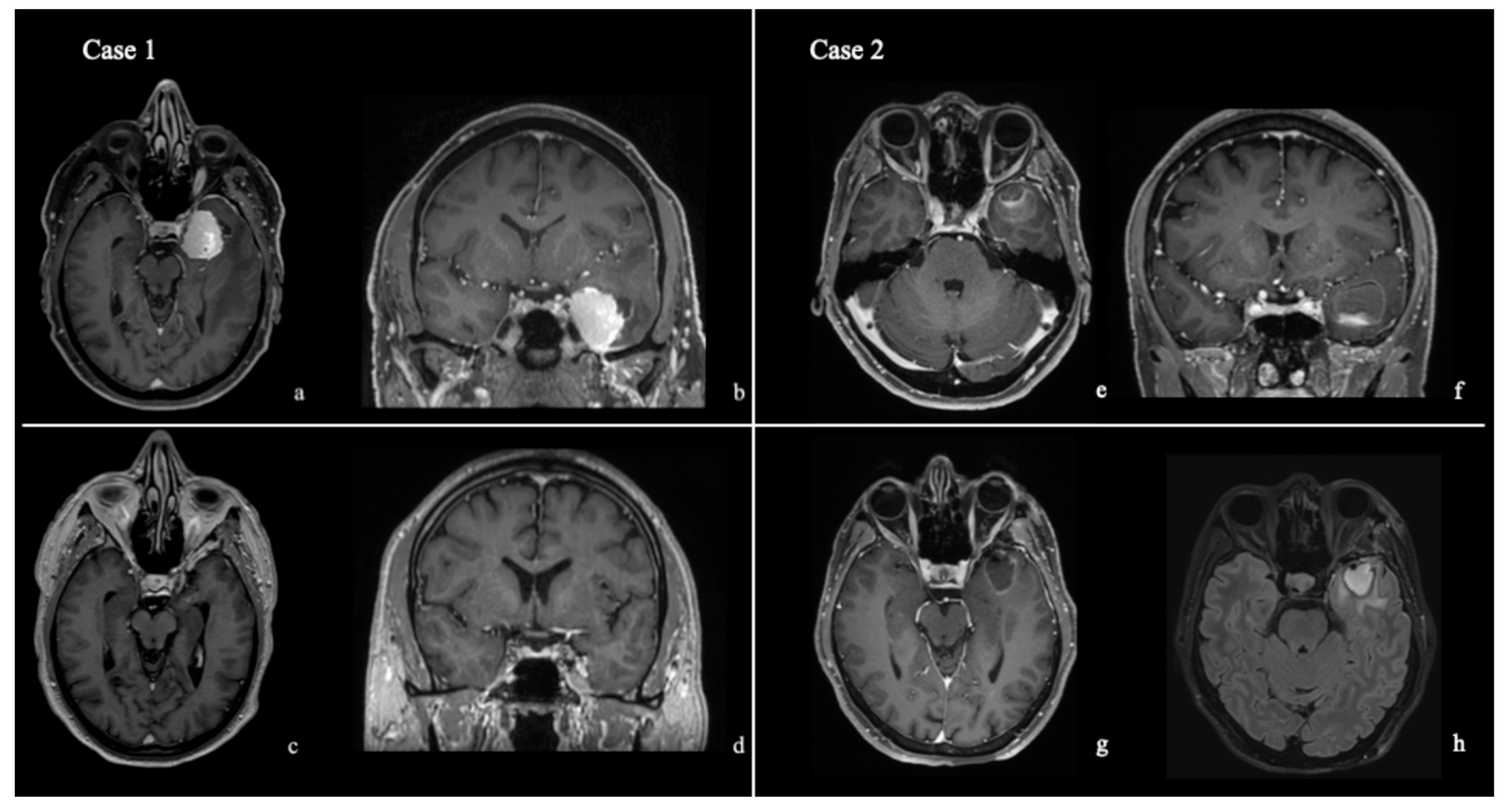
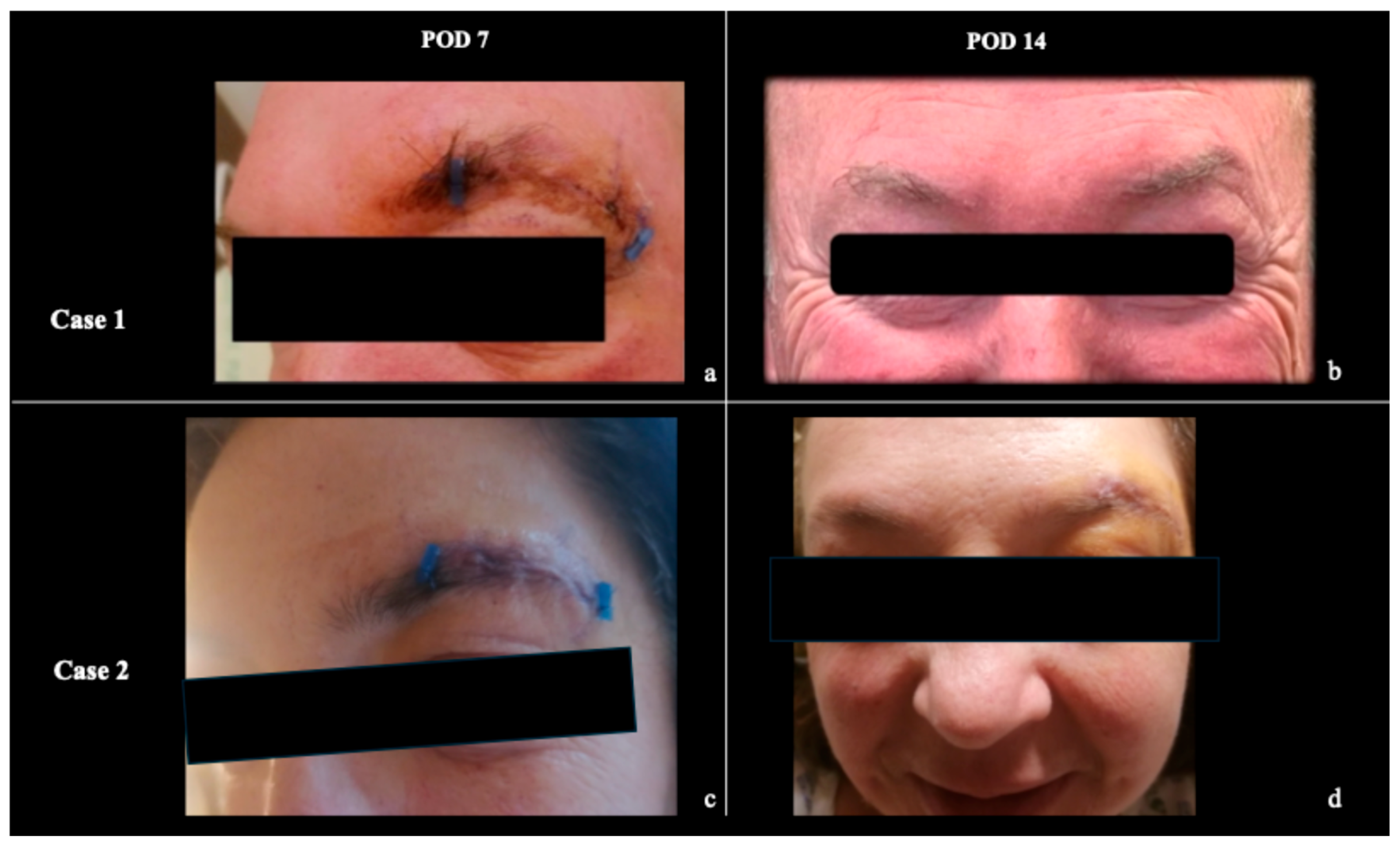
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TOAs | Transorbital approaches |
| TELKA | transorbital endoscopic lacrimal keyhole approach |
| TM | Temporalis muscle |
| GSW | Great sphenoidal wing |
| OTPF | Orbito-temporal periosteal fold |
| LSW | Lesser sphenoidal wing |
| MRI | Magnetic resonance imaging |
| SOF | Supraorbital fissure |
| LOR | Lateral orbital roof |
| CT | Computed tomography |
References
- Jeon, C.; Hong, C.-K.; Woo, K.I.; Hong, S.D.; Nam, D.-H.; Lee, J.-I.; Choi, J.W.; Seol, H.J.; Kong, D.-S. Endoscopic Transorbital Surgery for Meckel’s Cave and Middle Cranial Fossa Tumors: Surgical Technique and Early Results. J. Neurosurg. 2019, 131, 1126–1135. [Google Scholar] [CrossRef]
- Kong, D.-S.; Kim, Y.H.; Hong, C.-K. Optimal Indications and Limitations of Endoscopic Transorbital Superior Eyelid Surgery for Spheno-Orbital Meningiomas. J. Neurosurg. 2021, 134, 1472–1479. [Google Scholar] [CrossRef]
- Di Somma, A.; Andaluz, N.; Cavallo, L.M.; de Notaris, M.; Dallan, I.; Solari, D.; Zimmer, L.A.; Keller, J.T.; Zuccarello, M.; Prats-Galino, A.; et al. Endoscopic Transorbital Superior Eyelid Approach: Anatomical Study from a Neurosurgical Perspective. J. Neurosurg. 2018, 129, 1203–1216. [Google Scholar] [CrossRef]
- Dallan, I.; Castelnuovo, P.; Locatelli, D.; Turri-Zanoni, M.; AlQahtani, A.; Battaglia, P.; Hirt, B.; Sellari-Franceschini, S. Multiportal Combined Transorbital Transnasal Endoscopic Approach for the Management of Selected Skull Base Lesions: Preliminary Experience. World Neurosurg. 2015, 84, 97–107. [Google Scholar] [CrossRef]
- De Rosa, A.; Pineda, J.; Cavallo, L.M.; Di Somma, A.; Romano, A.; Topczewski, T.E.; Somma, T.; Solari, D.; Enseñat, J.; Cappabianca, P.; et al. Endoscopic Endo- and Extra-Orbital Corridors for Spheno-Orbital Region: Anatomic Study with Illustrative Case. Acta Neurochir. 2019, 161, 1633–1646. [Google Scholar] [CrossRef]
- Vural, A.; Carobbio, A.L.C.; Ferrari, M.; Rampinelli, V.; Schreiber, A.; Mattavelli, D.; Doglietto, F.; Buffoli, B.; Rodella, L.F.; Taboni, S.; et al. Transorbital Endoscopic Approaches to the Skull Base: A Systematic Literature Review and Anatomical Description. Neurosurg. Rev. 2021, 44, 2857–2878. [Google Scholar] [CrossRef]
- Greenberg, I.M. Self-Retaining Retractor and Handrest System for Neurosurgery. Neurosurgery 1981, 8, 205–208. [Google Scholar] [CrossRef]
- Karımzada, G.; Evleksiz Karımzada, D.; Erol, G.; Gülsuna, B.; Kuzucu, P.; Güngör, A.; Kutlay, A.M.; Şahin, M.M.; Çeltikçi, E. Transorbital Neuroendoscopic Surgery for Treatment of Sphenoid Wing Meningiomas Extending to the Cavernous Sinus: Clinical Implications and a Technical Illustration. Neurosurg. Focus 2024, 56, E8. [Google Scholar] [CrossRef]
- Bander, E.D.; Jones, S.H.; Kovanlikaya, I.; Schwartz, T.H. Utility of Tubular Retractors to Minimize Surgical Brain Injury in the Removal of Deep Intraparenchymal Lesions: A Quantitative Analysis of FLAIR Hyperintensity and Apparent Diffusion Coefficient Maps. J. Neurosurg. 2016, 124, 1053–1060. [Google Scholar] [CrossRef]
- Zammar, S.G.; Cappelli, J.; Zacharia, B.E. Utility of Tubular Retractors Augmented with Intraoperative Ultrasound in the Resection of Deep-Seated Brain Lesions: Technical Note. Cureus 2019, 11, e4272. [Google Scholar] [CrossRef]
- Kashimura, H.; Ogasawara, K.; Kubo, Y.; Kakino, S.; Sasoh, M.; Takahashi, H.; Suzuki, K.; Ogawa, A. Brain Retraction Technique Using Gelatin Sponge in the Subtemporal Approach. Neurol. Med. Chir. 2008, 48, 143–146. [Google Scholar] [CrossRef]
- Bly, R.A.; Ramakrishna, R.; Ferreira, M.; Moe, K.S. Lateral Transorbital Neuroendoscopic Approach to the Lateral Cavernous Sinus. J. Neurol. Surg. B Skull Base 2014, 75, 11–17. [Google Scholar] [CrossRef]
- Piper, K.; Saez-Alegre, M.; George, Z.; Srivastava, A.; Felbaum, D.R.; Jean, W.C. Transorbital Surgical Corridor: An Anatomic Analysis of Ocular Globe Retraction and the Associated Exposure for the Transpalpebral Orbital Rim Preserving Endoscopic Orbitotomy (TORPEDO) Approach. Oper. Neurosurg. 2024, 26, 196–202. [Google Scholar] [CrossRef]
- Kim, W.; Moon, J.H.; Kim, E.H.; Hong, C.-K.; Han, J.; Hong, J.B. Optimization of Orbital Retraction during Endoscopic Transorbital Approach via Quantitative Measurement of the Intraocular Pressure-[SevEN 006]. BMC Ophthalmol. 2021, 21, 76. [Google Scholar] [CrossRef]
- Fava, A.; Jiang, T.; Vu, T.H.; Abbritti, R.; Froelich, S. Transorbital Eyebrow Lacrimal Keyhole Approach for Resection of a Meningioma of the Lateral Wall of the Cavernous Sinus. Neurosurg. Focus Video 2025, 12, V7. [Google Scholar] [CrossRef]
- Matano, F.; Passeri, T.; Abbritti, R.; Camara, B.; Mastantuoni, C.; Noya, C.; Giammattei, L.; Devaux, B.; Mandonnet, E.; Froelich, S. Eyebrow Incision with a Crescent-Shaped Orbital Rim Craniotomy for Microscopic and Endoscopic Transorbital Approach to the Anterior and Middle Cranial Fossa: A Cadaveric Study and Case Presentation. Brain Spine 2022, 2, 100891. [Google Scholar] [CrossRef]
- Ferlendis, L.; Watanabe, N.; Fava, A.; Jiang, T.; Passeri, T.; Froelich, S. Mononostril Endoscopic Endonasal Chopstick Technique for Low Petroclival Meningioma with Sphenoidal Sinus Cranialization and Rostral Mucosal Closure. Oper. Neurosurg. 2025. [Google Scholar] [CrossRef]
- Noiphithak, R.; Yanez-Siller, J.C.; Revuelta Barbero, J.M.; Otto, B.A.; Carrau, R.L.; Prevedello, D.M. Comparative Analysis Between Lateral Orbital Rim Preservation and Osteotomy for Transorbital Endoscopic Approaches to the Cavernous Sinus: An Anatomic Study. Oper. Neurosurg. 2019, 16, 86–93. [Google Scholar] [CrossRef]
- Kelly, P.J. Future Perspectives in Stereotactic Neurosurgery: Stereotactic Microsurgical Removal of Deep Brain Tumors. J. Neurosurg. Sci. 1989, 33, 149–154. [Google Scholar]
- Castelnuovo, P.; Turri-Zanoni, M.; Battaglia, P.; Locatelli, D.; Dallan, I. Endoscopic Endonasal Management of Orbital Pathologies. Neurosurg. Clin. N. Am. 2015, 26, 463–472. [Google Scholar] [CrossRef]
- Locatelli, D.; Restelli, F.; Alfiero, T.; Campione, A.; Pozzi, F.; Balbi, S.; Arosio, A.; Castelnuovo, P. The Role of the Transorbital Superior Eyelid Approach in the Management of Selected Spheno-Orbital Meningiomas: In-Depth Analysis of Indications, Technique, and Outcomes from the Study of a Cohort of 35 Patients. J. Neurol. Surg. B Skull Base 2022, 83, 145–158. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ferlendis, L.; Fava, A.; Passeri, T.; Abbritti, R.; Froelich, S. A Simple and Cost-Effective Retractor for Transorbital Neurosurgery: Technical Note and Application in Lacrimal Keyhole Approaches. J. Clin. Med. 2026, 15, 482. https://doi.org/10.3390/jcm15020482
Ferlendis L, Fava A, Passeri T, Abbritti R, Froelich S. A Simple and Cost-Effective Retractor for Transorbital Neurosurgery: Technical Note and Application in Lacrimal Keyhole Approaches. Journal of Clinical Medicine. 2026; 15(2):482. https://doi.org/10.3390/jcm15020482
Chicago/Turabian StyleFerlendis, Luca, Arianna Fava, Thibault Passeri, Rosaria Abbritti, and Sebastien Froelich. 2026. "A Simple and Cost-Effective Retractor for Transorbital Neurosurgery: Technical Note and Application in Lacrimal Keyhole Approaches" Journal of Clinical Medicine 15, no. 2: 482. https://doi.org/10.3390/jcm15020482
APA StyleFerlendis, L., Fava, A., Passeri, T., Abbritti, R., & Froelich, S. (2026). A Simple and Cost-Effective Retractor for Transorbital Neurosurgery: Technical Note and Application in Lacrimal Keyhole Approaches. Journal of Clinical Medicine, 15(2), 482. https://doi.org/10.3390/jcm15020482





