Endovascular Repair of Blunt Aortic Trauma: A Multidisciplinary Approach and a Retrospective Multicenter Study
Abstract
1. Introduction
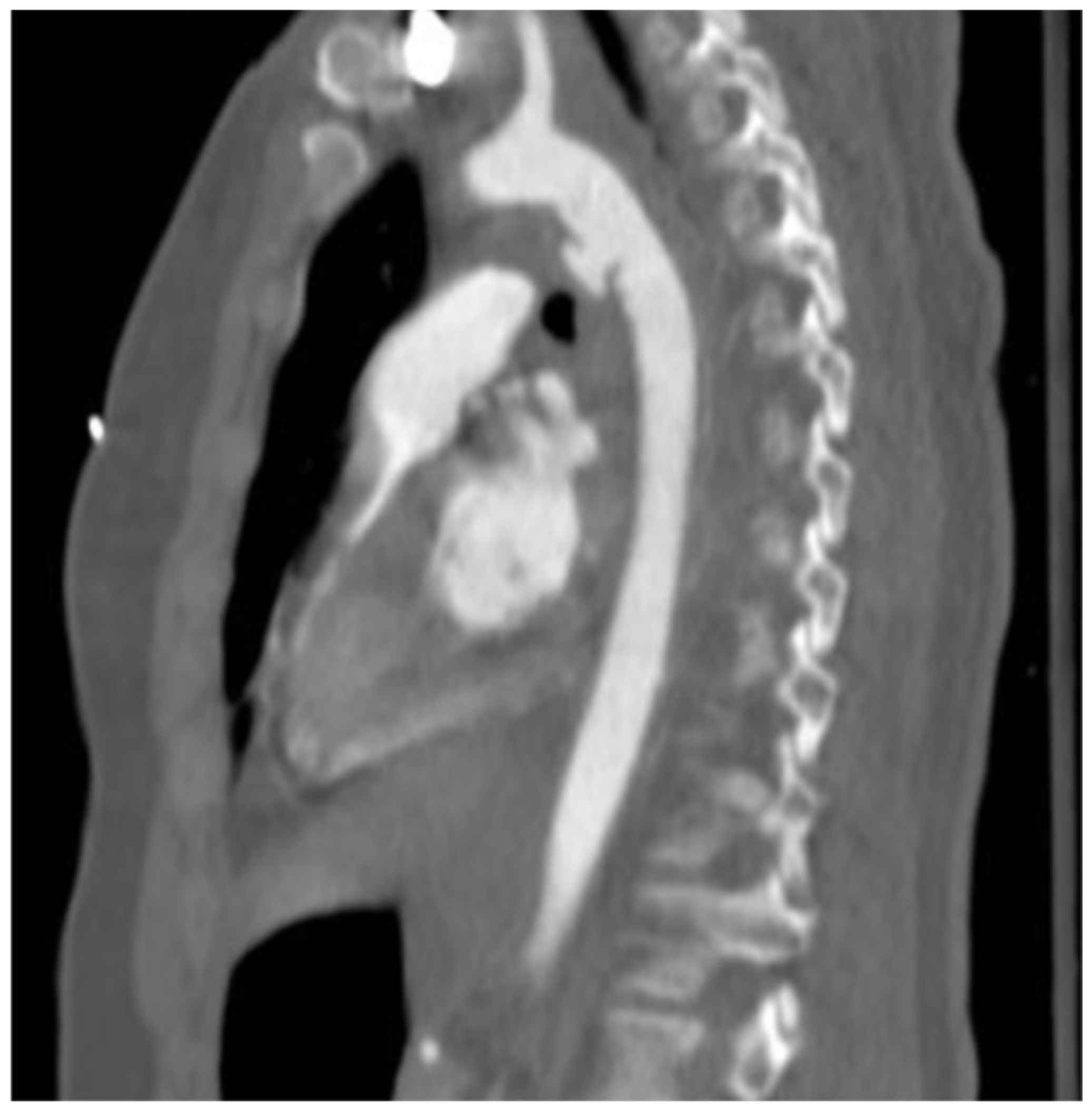
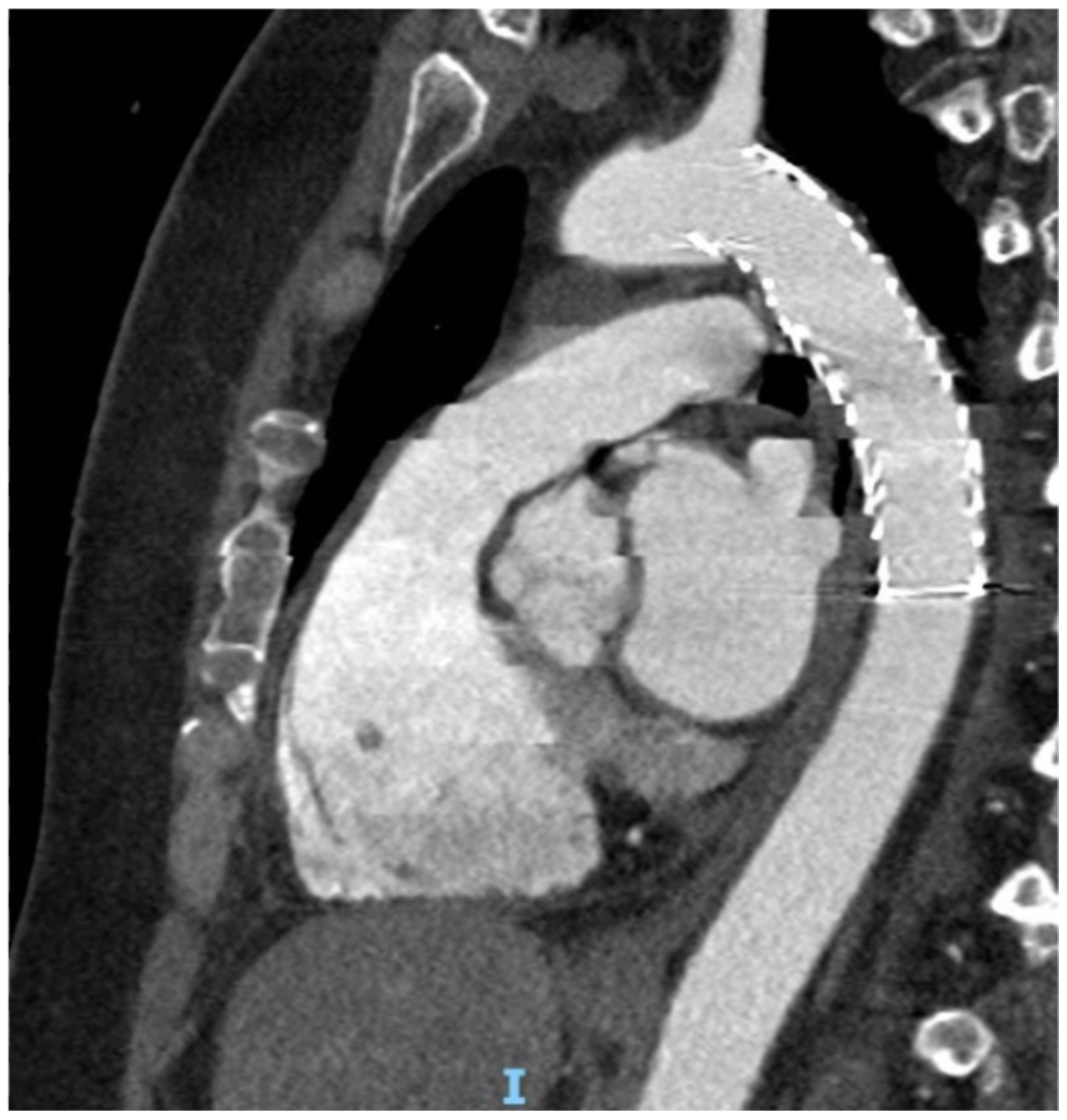
- Type I: intimal tear
- Type II: intramural hematoma
- Type III: pseudoaneurysm
- Type IV: rupture
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTAI | Blunt traumatic thoracic aortic injury |
| CTA | Computed Tomography angiography |
| NOM | Nonoperative management |
| AAST | American Association for the Surgery of Trauma |
| OR | open repair |
| TEVAR | endovascular repair |
| CSF | cerebrospinal fluid |
| SCI | spinal cord ischemia |
References
- Riambau, V.; Böcklera, D.; Brunkwall, J. Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- Long, D.; Hessel, M. A case of traumatic aortic transection presenting with hemorrhagic shock. J. Emerg. Med. 2020, 58, e201–e205. [Google Scholar] [CrossRef] [PubMed]
- Lotfollahzadeh, S.; Seligson, M. Aortic Rupture; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Harky, A.; Bleetman, D.; Chan, J.S.; Eriksen, P.; Chaplin, G.; MacCarthy-Ofosu, B.; Theologou, T.; Ambekar, S.; Roberts, N.; Oo, A. A systematic review and meta-analysis of endovascular versus open surgical repair for the traumatic ruptured thoracic aorta. J. Vasc. Surg. 2020, 71, 270–282. [Google Scholar] [CrossRef]
- Mouawad, N.J.; Paulisin, J. Blunt thoracic aortic injury-concepts and management. J. Cardiothorac. Surg. 2020, 15, 62. [Google Scholar] [CrossRef]
- Speziale, F. Chirurgia Vascolare, 3rd ed.; Medica, M., Ed.; SICVE: Bologna, Italy, 2022. [Google Scholar]
- DuBose, J.J.; Savage, S.A.; Fabian, T.C.; Menaker, J.; Scalea, T.; Holcomb, J.B.; Skarupa, D.; Poulin, N.; Chourliaras, K.; Inaba, K.; et al. The American Association for the Surgery of Trauma PROspective Observational Vascular Injury Treatment (PROOVIT) registry: Multicenter data on modern vascular injury diagnosis, management, and outcomes. J. Trauma Acute Care Surg. 2015, 78, 215–222. [Google Scholar] [CrossRef]
- Azizzadeh, A.; Keyhani, K.; Miller, C.C., 3rd; Coogan, S.M.; Safi, H.J.; Estrera, A.L. Blunt traumatic aortic injury: Initial experience with endovascular repair. J. Vasc. Surg. 2009, 49, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Fabian, T.C.; Richardson, J.D.; Croce, M.A.; Smith, J.S., Jr.; Rodman, G., Jr.; Kearney, P.A.; Flynn, W.; Ney, A.L.; Cone, J.B.; Luchette, F.A.; et al. Prospective study of blunt aortic injury: Multicenter trial of the American Association for the Surgery of Trauma. J. Trauma 1997, 42, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Estrera, A.L.; Gochnour, D.C.; Azizzadeh, A.; Miller, C.C.; Coogan, S.; Charlton-Ouw, K.; Holcomb, J.B.; Safi, H.J. Progress in the treatment of blunt thoracic aortic injury: 12-Year single-institution experience. Ann. Thorac. Surg. 2010, 90, 64–71. [Google Scholar] [CrossRef]
- Hemmila, M.R.; Arbabi, S.; Rowe, S.A.; Brandt, M.-M.; Wang, S.C.; Taheri, P.A.; Wahl, W.L. Delayed repair for blunt thoracic aortic injury: Is it really equivalent to early repair? J. Trauma 2004, 56, 13–23. [Google Scholar] [CrossRef]
- Demetriades, D.; Velmahos, G.C.; Scalea, T.M.; Jurkovich, G.J.; Karmy-Jones, R.; Teixeira, P.G.; Hemmila, M.R.; O’Connor, J.V.; McKenney, M.O.; Moore, F.O.; et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: Results of an American Association for the Surgery of Trauma Multicenter Study. J. Trauma 2008, 64, 561–570. [Google Scholar] [CrossRef]
- Murad, M.H.; Rizvi, A.Z.; Malgor, R.; Carey, J.; Alkatib, A.A.; Erwin, P.J.; Lee, W.A.; Fairman, R.M. Comparative effectiveness of the treatments for aortic transection. J. Vasc. Surg. 2011, 53, 193–199.e21. [Google Scholar]
- Neschis, D.G.; Scalea, T.M.; Flinn, W.R.; Griffith, B.P. Blunt aortic injury. N. Engl. J. Med. 2008, 359, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Von Oppel, O.U.; Dunne, T.T.; De Groot, M.K.; Zilla, P. Traumatic aortic rupture: Twenty-year metaanalysis of mortality and risk of paraplegia. Ann. Thorac. Surg. 1994, 58, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, E.K.; Forauer, A.R.; Silas, A.M.; Gemery, J.M. Endovascular stentgraft or open surgical repair for blunt thoracic aortic trauma: Systematic review. J. Vasc. Interv. Radiol. 2008, 19, 1153–1164. [Google Scholar] [CrossRef]
- Cardarelli, M.G.; McLaughlin, J.S.; Downing, S.W.; Brown, J.M.; Attar, S.; Griffith, B.P. Management of traumatic aortic rupture: A 30-year experience. Ann. Surg. 2002, 236, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.B.; Thompson, J.K.; Crafton, C.J.; Delvecchio, C.; Giglia, J.S. Timing of endovascular repair of blunt traumatic thoracic aortic transections. J. Vasc. Surg. 2006, 43, 684–688. [Google Scholar] [CrossRef]
- Bortone, A.S.; Schena, S.; D’agostino, D.; Dialetto, G.; Paradiso, V.; Mannatrizio, G.; Fiore, T.; Cotrufo, M.; Schinosa, L.d.L.T. Immediate versus delayed endovascular treatment of post-traumatic aortic pseudoaneurysms and type B dissections: Retrospective analysis and premises to the upcoming European trial. Circulation 2002, 106, I234–I240. [Google Scholar] [CrossRef]
- Jang, M.O.; Kim, J.H.; Oh, S.K.; Lee, M.G.; Park, K.H.; Sim, D.S.; Hong, Y.J.; Ahn, Y.; Jeong, M.H. Endovascular stent in traumatic thoracic aortic dissection. Korean Circ. J. 2012, 42, 341–344. [Google Scholar] [CrossRef]
- Volodos, N.L. Historical perspective: The first steps in endovascular aortic repair: How it all began. J. Endovasc. Ther. 2013, 20, 13–23. [Google Scholar] [CrossRef]
- Arbabi, C.N.; DuBose, J.; Charlton-Ouw, K.; Starnes, B.W.; Saqib, N.; Quiroga, E.; Miller, C.; Azizzadeh, A. Outcomes and practice pattern of medical management of blunt thoracic aortic injury from the Aortic Trauma Foundation global registry. J. Vasc. Surg. 2022, 75, 625–631. [Google Scholar] [CrossRef]
- Aucoin, V.J.; Motyl, C.M.; Novak, Z.; Eagleton, M.J.; Farber, M.A.; Gasper, W.; Oderich, G.S.; Mendes, B.; Schanzer, A.; Tenorio, E.; et al. Predictors and outcomes of spinal cord injury following complex branched/fenestrated endovascular aortic repair in the US Aortic Research Consortium. J. Vasc. Surg. 2023, 77, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Aucoin, V.J.; Bolaji, B.; Novak, Z.; Spangler, E.L.; Sutzko, D.C.; McFarland, G.E.; Pearce, B.J.; Passman, M.A.; Scali, S.T.; Beck, A.W. Trends in the use of cerebrospinal drains and outcomes related to spinal cord ischemia after thoracic endovascular aortic repair and complex endovascular aortic repair in the Vascular Quality Initiative database. J. Vasc. Surg. 2021, 74, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Aucoin, V.J.; Eagleton, M.J.; Farber, M.A.; Oderich, G.S.; Schanzer, A.; Timaran, C.H.; Schneider, D.B.; Sweet, M.P.; Beck, A.W. Spinal cord protection practices used during endovascular repair of complex aortic aneurysms by the U.S. Aortic Research Consortium. J. Vasc. Surg. 2021, 73, 323–330. [Google Scholar] [CrossRef]
- Patel, H.J.; Williams, D.M.; Upchurch, G.R.; Dasika, N.L.; Deeb, G.M. A 15 year comparative analysis of open and endovascular repair for the ruptured descending thoracic aorta. J. Vasc. Surg. 2009, 50, 1265–1270. [Google Scholar] [PubMed]
| BTAI (n. 45) | |
|---|---|
| Age | 50 ± 3 |
| Male | 29 (64.4%) |
| Type I lesion | 5 (11.1%) |
| Type II lesion | 12 (26.7%) |
| Type III lesion | 25 (55.5%) |
| Type IV lesion | 3 (6.7%) |
| Hemorrhagic shock | 11 (24.4%) |
| Isolated lesion | 12 (26%) |
| Orthopedics injuries | 30 (66.7%) |
| Abdominal injuries | 17 (37.8%) |
| Thoracic injuries | 3 (6,7%) |
| Neurosurgical injuries | 8 (17.8%) |
| Endovascular treatment | 40 (88.8%) |
| BTAI (n. 45) | |
|---|---|
| General Anesthesia | 45 (100%) |
| Medtronic Captivia | 28 (62.2%) |
| Gore TAG | 17 (37%) |
| LSA Coverage | 13 (28.9%) |
| Procedural success rate | 45 (100%) |
| Type I Endoleak | 0 |
| Type II Endoleak | 1 (2.2%) |
| SCI | 0 |
| STROKE | 1 (2.2%) |
| In Hospital Mortality (not aortic related) | 6 (13.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Di Sario, I.; Franceschini, E.; Gatta, E.; Pagliariccio, G. Endovascular Repair of Blunt Aortic Trauma: A Multidisciplinary Approach and a Retrospective Multicenter Study. J. Clin. Med. 2026, 15, 68. https://doi.org/10.3390/jcm15010068
Di Sario I, Franceschini E, Gatta E, Pagliariccio G. Endovascular Repair of Blunt Aortic Trauma: A Multidisciplinary Approach and a Retrospective Multicenter Study. Journal of Clinical Medicine. 2026; 15(1):68. https://doi.org/10.3390/jcm15010068
Chicago/Turabian StyleDi Sario, Ilenia, Enrico Franceschini, Emanuele Gatta, and Gabriele Pagliariccio. 2026. "Endovascular Repair of Blunt Aortic Trauma: A Multidisciplinary Approach and a Retrospective Multicenter Study" Journal of Clinical Medicine 15, no. 1: 68. https://doi.org/10.3390/jcm15010068
APA StyleDi Sario, I., Franceschini, E., Gatta, E., & Pagliariccio, G. (2026). Endovascular Repair of Blunt Aortic Trauma: A Multidisciplinary Approach and a Retrospective Multicenter Study. Journal of Clinical Medicine, 15(1), 68. https://doi.org/10.3390/jcm15010068





