Fingernail Onychomycosis: A Laboratory-Based Retrospective Study with Species Profiling and Antifungal Susceptibility of Yeasts
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population and Demographics
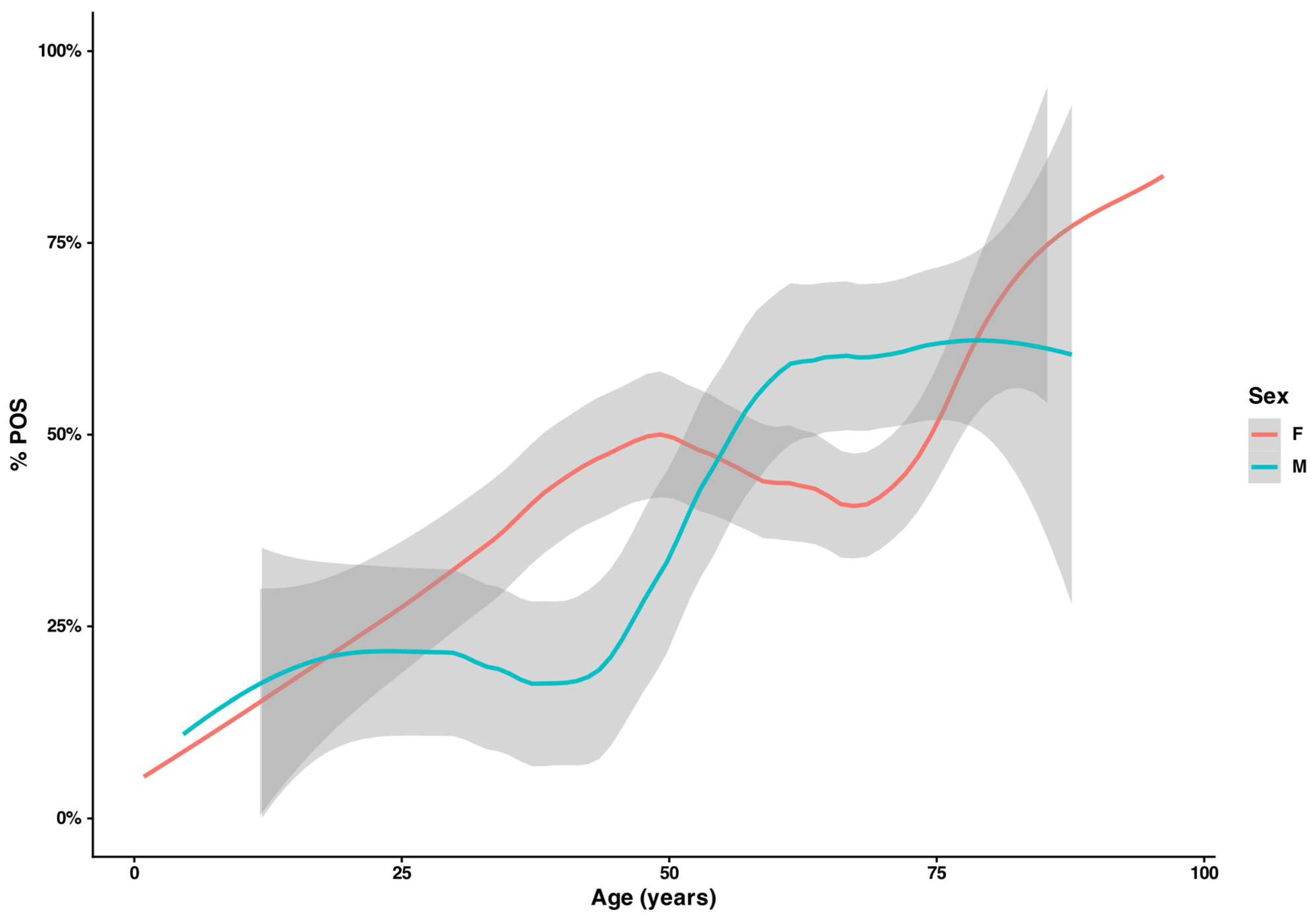
3.2. Demographic Differences Across Diagnostic Categories
3.3. Microscopy Findings
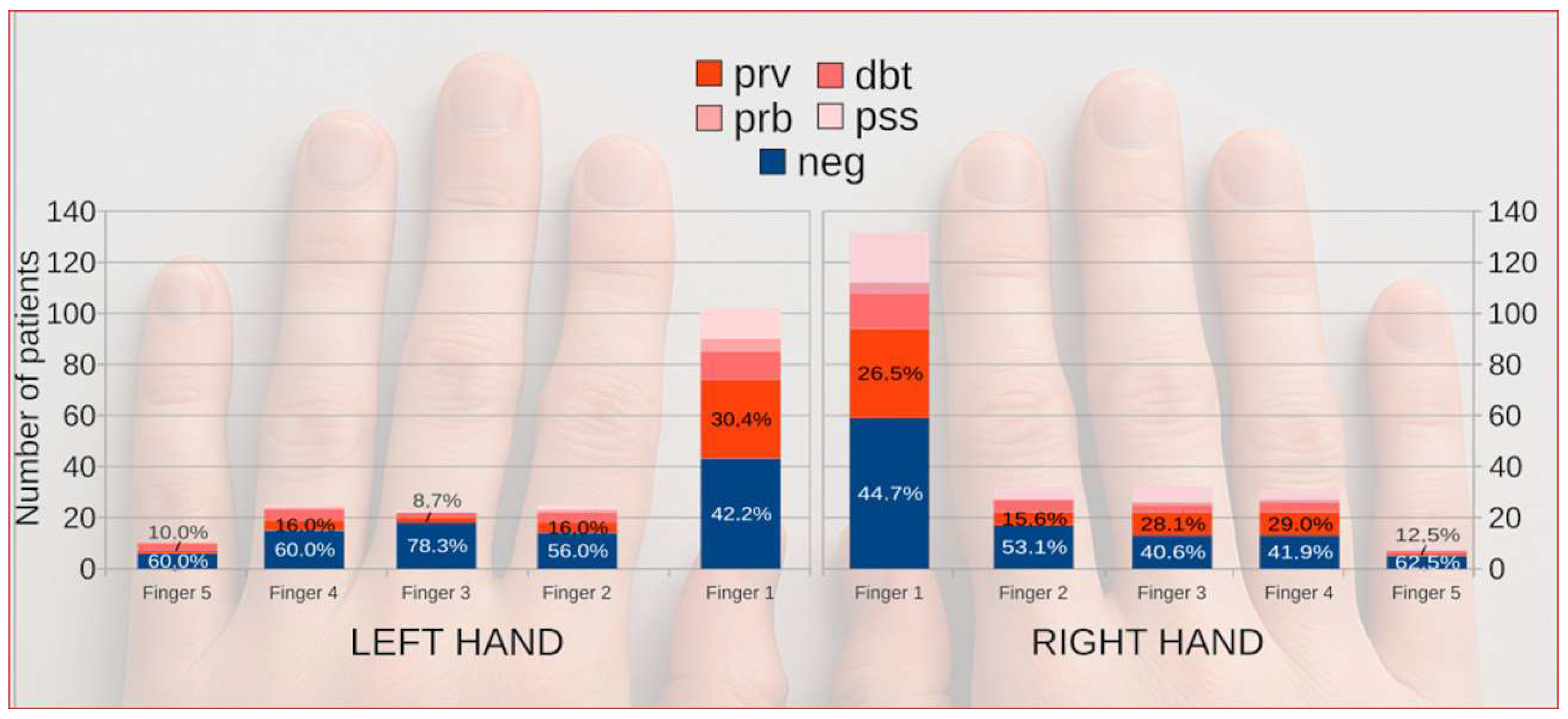
3.4. Lateralization and Digit-Specific Occurrence of Onychomycosis
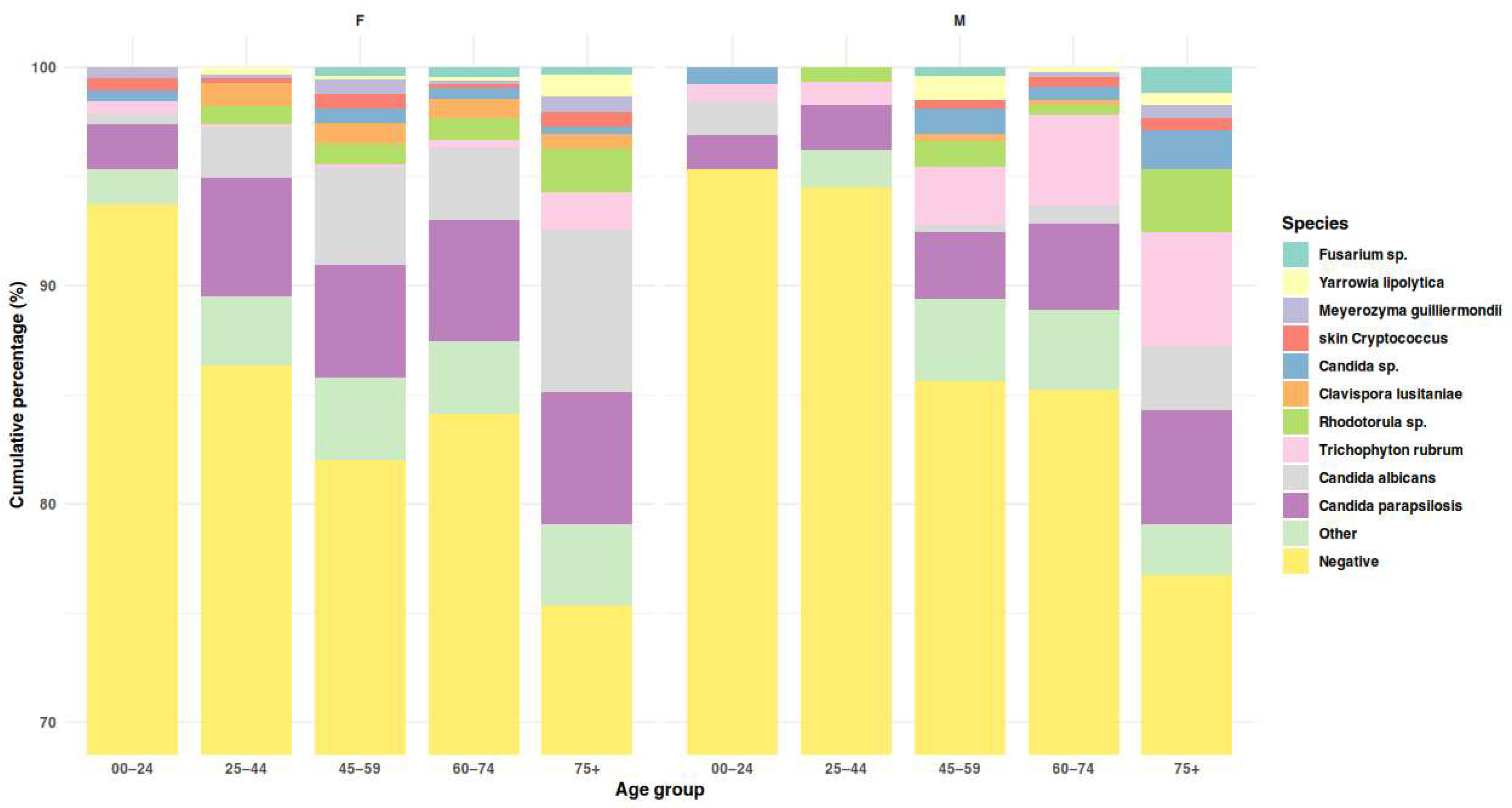
3.5. Etiological Agents
3.6. Analysis of Periungual Sampling Records
3.7. Yeasts Antifungal Susceptibility
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMB | Amphotericin B |
| AMO | Amorolfine |
| ATCC | American Type Culture Collection |
| BP | Break point |
| CFU | Colony-forming unit |
| CFW | Calcofluor white |
| CI | Confidence interval |
| CLSI | Clinical and Laboratory Standards Institute |
| CPX | Ciclopirox |
| DbtPOS | Doubtful positive |
| DSM | Deutsche Sammlung von Mikroorganismen |
| ECO | Econazole |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| FLU | Fluconazole |
| GM | Geometric mean |
| ID | Identification |
| ITR | Itraconazole |
| ITS | Internal transcribed spacer region |
| KOH | Potassium hydroxide |
| MALDI-TOF | Matrix-assisted laser desorption/ionization time-of-flight |
| MIC, MIC50, MIC90 | Minimum inhibitory concentration, MIC inhibiting 50%/90% of isolates |
| NDM | Non-dermatophyte mold(s) |
| NEG | Negative (no evidence of onychomycosis) |
| OR | Odds ratio |
| POS | Posaconazole |
| PrbPOS | Probable positive |
| PrvPOS | Proven positive |
| PssPOS | Possible positive |
| RPMI-1640/RPMI | Roswell Park Memorial Institute 1640 medium |
| TER | Terbinafine |
| VOR | Voriconazole |
References
- Bodman, M.A.; Syed, H.A.; Krishnamurthy, K. Onychomycosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Falotico, J.M.; Lipner, S.R. Updated Perspectives on the Diagnosis and Management of Onychomycosis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1933–1957. [Google Scholar] [CrossRef]
- Gallo, L.; Cinelli, E.; Fabbrocini, G.; Vastarella, M. A 15-year Retrospective Study on the Prevalence of Onychomycosis in Psoriatic vs Non-psoriatic Patients: A New European Shift from Dermatophytes towards Yeast. Mycoses 2019, 62, 659–664. [Google Scholar] [CrossRef]
- Barac, A.; Stjepanovic, M.; Krajisnik, S.; Stevanovic, G.; Paglietti, B.; Milosevic, B. Dermatophytes: Update on Clinical Epidemiology and Treatment. Mycopathologia 2024, 189, 101. [Google Scholar] [CrossRef]
- Shemer, A.; Daniel, R.; Lyakhovitsky, A.; Aghion-Svirsky, V.; Kassem, R.; Rigopoulos, D.; Farhi, R.; Galili, E. Clinical Significance of Candida Isolation from Dystrophic Fingernails. Mycoses 2020, 63, 964–969. [Google Scholar] [CrossRef]
- Nenoff, P.; Reinel, D.; Mayser, P.; Abeck, D.; Bezold, G.; Bosshard, P.P.; Brasch, J.; Daeschlein, G.; Effendy, I.; Ginter-Hanselmayer, G.; et al. S1 Guideline Onychomycosis. JDDG J. Dtsch. Dermatol. Ges. 2023, 21, 678–692. [Google Scholar] [CrossRef]
- Figueiredo, V.T.; de Assis Santos, D.; Resende, M.A.; Hamdan, J.S. Identification and in Vitro Antifungal Susceptibility Testing of 200 Clinical Isolates of Candida Spp. Responsible for Fingernail Infections. Mycopathologia 2007, 164, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.; Kimchi, A.; Kritzman, A.; Inbar, R.; Segal, Z. The Frequency of Candida parapsilosis in Onychomycosis. An Epidemiological Survey in Israel. Mycoses 2000, 43, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Piraccini, B.M.; Ghetti, E.; Colombo, M.D. Topical Steroids versus Systemic Antifungals in the Treatment of Chronic Paronychia: An Open, Randomized Double-Blind and Double Dummy Study. J. Am. Acad. Dermatol. 2002, 47, 73–76. [Google Scholar] [CrossRef]
- Negi, M.; Tsuboi, R.; Matsui, T.; Ogawa, H. Isolation and Characterization of Proteinase from Candida albicans: Substrate Specificity. J. Investig. Dermatol. 1984, 83, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Yoshiura, K.; Negi, M.; Ogawa, H. Keratinolytic Proteinase Produced by Candida albicans. Sabouraudia 1984, 22, 175–183. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Mazotto, A.M.; de Melo, A.C.N.; Vieira, F.H.C.; Duarte, T.R.; Macrae, A.; Nishikawa, M.M.; da Silva Bon, E.P. Identification of a Candida parapsilosis Strain Producing Extracellular Serine Peptidase with Keratinolytic Activity. Mycopathologia 2010, 169, 57–65. [Google Scholar] [CrossRef]
- Duarte, T.R.; Oliveira, S.S.; Macrae, A.; Cedrola, S.M.L.; Mazotto, A.M.; Souza, E.P.; Melo, A.C.N.; Vermelho, A.B. Increased Expression of Keratinase and Other Peptidases by Candida parapsilosis Mutants. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas Biol. 2011, 44, 212–216. [Google Scholar] [CrossRef]
- Gupta, A.K.; Daigle, D.; Carviel, J.L. The Role of Biofilms in Onychomycosis. J. Am. Acad. Dermatol. 2016, 74, 1241–1246. [Google Scholar] [CrossRef]
- Methods Document on MIC Testing of Yeasts Updated by AFST. Available online: https://www.eucast.org/news-detail/methods-document-on-mic-testing-of-yeasts-updated-by-afst/ (accessed on 9 November 2025).
- The Breakpoint Table for Antifungal Agents and the Presentation of ECOFFs Updated. Available online: https://www.eucast.org/news-detail/the-breakpoint-table-for-antifungal-agents-and-the-presentation-of-ecoffs-updated/ (accessed on 9 November 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Jaworek, A.K.; Hałubiec, P.; Sroka, D.; Grabarczyk, I.; Kachnic, N.; Wojas-Pelc, A.; Szepietowski, J.C. Demographic and Pathogen Profiles of Superficial Fungal Infections—A Single-Centre Observational Study in Poland. Mycoses 2024, 67, e70009. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.K.; Hałubiec, P.; Wojas-Pelc, A.; Szepietowski, J.C. Analysis of Causative Factors and Potential Predictors of Onychomycosis: A Retrospective Single-Center Study in Poland. J. Fungi 2025, 11, 131. [Google Scholar] [CrossRef]
- Gregoriou, S.; Mpali, N.; Vrioni, G.; Hatzidimitriou, E.; Chryssou, S.-E.; Rigopoulos, D. Epidemiology of Onychomycosis in an Academic Nail Unit in South Greece during a Three-Year Period. Ski. Appendage Disord. 2020, 6, 102–107. [Google Scholar] [CrossRef]
- Haghani, I.; Shokohi, T.; Hajheidari, Z.; Khalilian, A.; Aghili, S.R. Comparison of Diagnostic Methods in the Evaluation of Onychomycosis. Mycopathologia 2013, 175, 315–321. [Google Scholar] [CrossRef]
- Dass, S.; Vinayaraj, E.V.; Pavavni, K.; Pallam, A.; Rao, M. Comparison of KOH, Calcofluor White and Fungal Culture for Diagnosing Fungal Onychomycosis in an Urban Teaching Hospital, Hyderabad. Indian J. Microbiol. Res. 2015, 2, 148. [Google Scholar] [CrossRef]
- Makled, A.F.; Ghonaim, M.M.; Ali, S.A.M.; ElHefnawy, S.M.; Sabal, M.S.; Elbrolosy, A.M. Comparison of Fungal Fluorescent Staining and ITS rDNA PCR-Based Sequencing with Conventional Methods for Diagnosis of Onychomycosis. J. Pure Appl. Microbiol. 2022, 16, 1337–1349. [Google Scholar] [CrossRef]
- Brzózka, P.; Kolodziejski, W. Sex-Related Chemical Differences in Keratin from Fingernail Plates: A Solid-State Carbon-13 NMR Study. RSC Adv. 2017, 7, 28213–28223. [Google Scholar] [CrossRef]
- Brasch, J.; Flader, S. Human Androgenic Steroids Affect Growth of Dermatophytes in Vitro. Mycoses 1996, 39, 387–392. [Google Scholar] [CrossRef]
- Iorizzo, M.; Piraccini, B.M.; Alessandrini, A.; Bruni, F.; Vollono, L.; Pampaloni, F.; Di Chiacchio, N.G.; Di Chiacchio, N.; Jimenez-Cauhe, J.; Grover, C.; et al. Clinical and Onychoscopy Patterns in Fingernail Onychomycosis—A Study By The International Dermoscopy Society “Trichoscopy and Onychoscopy” Task Force. Dermatol. Pract. Concept. 2025, 15, 4887. [Google Scholar] [CrossRef]
- Papini, M.; Piraccini, B.M.; Difonzo, E.; Brunoro, A. Epidemiology of Onychomycosis in Italy: Prevalence Data and Risk Factor Identification. Mycoses 2015, 58, 659–664. [Google Scholar] [CrossRef]
- Dulski, A.; Edwards, C.W. Paronychia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rather, S.; Keen, A.; Shah, F.Y.; Yaseen, A.; Farooq, S.; Bakhshi, A. Candidal Onychomycosis: Clinicoepidemiological Profile, Prevailing Strains, and Antifungal Susceptibility Pattern–A Study from a Tertiary Care Hospital. Indian J. Dermatol. 2021, 66, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Bahunuthula, R.K.; Thappa, D.M.; Kumari, R.; Singh, R.; Munisamy, M.; Parija, S.C. Evaluation of Role of Candida in Patients with Chronic Paronychia. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 485. [Google Scholar] [CrossRef]
- Al-Dwibe, H.; Ghazil, M.B.; Kalifa, Z. Candida and Other Yeasts as Nail Pathogens in Chronic Paronychia and Onycholysis of Fingernails. J. Adv. Med. Med. Res. 2018, 27, 1–5. [Google Scholar] [CrossRef]
- Morovati, H.; Kord, M.; Ahmadikia, K.; Eslami, S.; Hemmatzadeh, M.; Kurdestani, K.M.; Khademi, M.; Darabian, S. A Comprehensive Review of Identification Methods for Pathogenic Yeasts: Challenges and Approaches. Adv. Biomed. Res. 2023, 12, 187. [Google Scholar] [CrossRef]
- Pakshir, K.; Kamali, M.; Nouraei, H.; Zomorodian, K.; Motamedi, M.; Mahmoodi, M. Molecular Characterization and Antifungal Activity against Non-Dermatophyte Molds Causing Onychomycosis. Sci. Rep. 2021, 11, 20736. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, S.; Jabrodini, A.; Motamedi, M.; Zomorodian, K.; Kharazi, M.; Shabanzadeh, S.; Ghasemi, F.; Shariat, S.; Rezaei Arab, M. Species Distribution and Antifungal Susceptibility Profiles of Yeasts Isolated from Onychomycosis: A Cross-Sectional Study with Insights into Emerging Species. Antonie Van Leeuwenhoek 2023, 117, 6. [Google Scholar] [CrossRef]
- Afsarian, M.H.; Sharafi, Z. Molecular Identification of Candida Species Isolated from Onychomycosis with In Vitro Antifungal Susceptibility Profiles. Jundishapur J. Microbiol. 2023, 16, e139906. [Google Scholar] [CrossRef]
- Otašević, S.; Barac, A.; Pekmezovic, M.; Tasic, S.; Ignjatović, A.; Momčilović, S.; Stojanović, P.; Arsic Arsenijevic, V.; Hay, R. The Prevalence of Candida Onychomycosis in Southeastern Serbia from 2011 to 2015. Mycoses 2016, 59, 167–172. [Google Scholar] [CrossRef]
- Andres, M.; Jaworek, A.; Stec-Polak, M.; Radzimowska, J.; Wojas-Pelc, A. Infekcje grzybicze skóry i jej przydatków-analiza wyników badań mykologicznych Pracowni Mykologicznej w latach 2010–2014. Przegla̧d Lek. 2015, 72, 253–256. [Google Scholar]
- Munprom, K.; Bunyaratavej, S.; Pattanaprichakul, P.; Jirawattanadon, P.; Matthapan, L.; Prasong, W.; Panyawong, C.; Plengpanich, A.; Leeyaphan, C. Ex Vivo Fungal Nail Penetration Study: Effects of Causative Organisms, Nail Polish and Age. Mycoses 2025, 68, e70019. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Jeong, E.; Park, H.J.; Oh, S.T.; Lee, J.Y.; Cho, B. A Case of Total Dystrophic Onychomycosis Caused by Candida albicans in Diabetes Mellitus. Korean J. Med. Mycol. 2004, 9, 112–116. [Google Scholar]
- Domański, D.; Sikora, M.; Kuthan, R.; Augustynowicz-Kopeć, E.; Swoboda-Kopeć, E. New Species within Candida parapsilosis and Candida Glabrata. Med. Dośw. Mikrobiol. 2019, 71, 51–57. [Google Scholar] [CrossRef]
- Benedict, K.; Lipner, S.R.; Lockhart, S.R.; Gold, J.A.W. Low Positivity Rate and High Percentage of Nondermatophyte Molds in an Analysis of 35,257 Fungal Nail Culture Results from a United States National Commercial Laboratory, 2019–2022. JAAD Int. 2023, 12, 43–45. [Google Scholar] [CrossRef]
- Petranyuk, A.; Bykowska, B.; Wilkowska, A.; Nowicki, R. Onychomycosis in the Gdansk Area in Poland. Dermatol. Rev. 2021, 108, 258–268. [Google Scholar] [CrossRef]
- Gawdzik, A.; Nowogrodzka, K.; Hryncewicz-Gwóźdź, A.; Maj, J.; Szepietowski, J.; Jankowska-Konsur, A. Epidemiology of Dermatomycoses in Southwest Poland, Years 2011–2016. Adv. Dermatol. Allergol. Postep. Dermatol. Alergol. 2018, 36, 604–608. [Google Scholar] [CrossRef]
- Benvenuti, M.; Burlando, M.; Cozzani, E.C. Rhodotorula Mucilaginosa (A. Jörg.) F.C. Harrison 1928 and Onychomycosis: Three Case Reports for an Unusual and Underestimated Combo. FEMS Microbiol. Lett. 2025, 372, fnaf089. [Google Scholar] [CrossRef]
- Zhang, M.-J.; Liang, G.-Z.; Mei, H.; Song, G.; Liu, W. Onychomycosis Caused by Pichia guilliermondii: A Case Report and Mini-Review. Med. Mycol. Case Rep. 2020, 27, 72–76. [Google Scholar] [CrossRef]
- Major, J.R.; Warren, C.A.; Rowley, P.A. The Association of Yarrowia lipolytica with Onychomycosis. Med. Mycol. Case Rep. 2025, 49, 100715. [Google Scholar] [CrossRef]
- Jabrodini, A.; Eghtedarnejad, E.; Ghanbarzadeh, A.; Motamedi, M.; Jafari, M.; Kharazi, M.; Yazdanpanah, S.; Khodadadi, H. Molecular Identification and Antifungal Susceptibility Profile of Rare and Emerging Yeast Species Causing Onychomycosis. BMC Res. Notes 2025, 18, 167. [Google Scholar] [CrossRef]
- Ohara, S.; Noguchi, H.; Matsumoto, T.; Kubo, M.; Hayashi, D.; Kashiwada-Nakamura, K.; Yaguchi, T.; Kano, R. Emerging Antifungal-Resistant Onychomycosis in a Dermatology Clinic in Kumamoto, Japan. Med. Mycol. J. 2025, 66, 61–67. [Google Scholar] [CrossRef]
- Hedderwick, S.A.; McNeil, S.A.; Lyons, M.J.; Kauffman, C.A. Pathogenic Organisms Associated with Artificial Fingernails Worn by Healthcare Workers. Infect. Control Hosp. Epidemiol. 2000, 21, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Maleszka, R.; Adamski, Z.; Szepietowski, J.; Baran, E. Treatment of Superficial Fungal Infections–Recommendations of Experts of Mycological Section of Polish Dermatological Society. Dermatol. Rev. Prz. Dermatol. 2015, 102, 305–315. [Google Scholar] [CrossRef]
- da Cunha, M.M.L.; dos Santos, L.P.B.; Dornelas-Ribeiro, M.; Vermelho, A.B.; Rozental, S. Identification, Antifungal Susceptibility and Scanning Electron Microscopy of a Keratinolytic Strain of Rhodotorula Mucilaginosa: A Primary Causative Agent of Onychomycosis. FEMS Immunol. Med. Microbiol. 2009, 55, 396–403. [Google Scholar] [CrossRef] [PubMed]
| Category | Microscopy Criterion | Culture Criterion | Rationale (Clinical Mycology) |
|---|---|---|---|
| NEG (Negative) | No fungal elements | <5 CFU on media | Both primary tests are negative, so there is no laboratory evidence of onychomycosis. |
| PssPOS (Possible +) | No fungal elements | ≥5 CFU of a clinically relevant yeast (species formerly classified in the Candida genus, e.g., C. parapsilosis, C. albicans) | A sizeable yeast load without microscopic confirmation may represent an early or focal infection; dermatological correlation and/or repeat culture are recommended. |
| DbtPOS (Doubtful +) | Fungal elements present (yeast cells or hyphae) | (i) No growth of clinically relevant fungi and only scant growth (<5 CFU) of common contaminants; or (ii) non-correspondence between microscopy and culture (e.g., yeast cells in microscopy with dermatophyte growth, or abundant hyphae in microscopy while a yeast species that does not produce hyphae/pseudohyphae is isolated) | Fungal structures are seen, yet culture is absent or discordant—may reflect non-viable organisms (prior therapy, desiccation) or suboptimal culture; the result is inconclusive. |
| PrbPOS (Probable +) | Fungal elements morphologically consistent with the culture isolate | <5 CFU of a clinically relevant yeast (i), or ≥5 CFU of a colonizing yeast (e.g., Rhodotorula, Naganishia) (ii) | (i) Microscopy shows fungal elements but only scant growth occurs—typical of treated or small samples. (ii) Alternatively, abundant growth of yeasts usually regarded as colonizers may indicate an emerging infection. Both scenarios suggest infection but do not prove it. |
| PrvPOS (Proven +) | Yeast cells, hyphal elements, or atypical fungal structures consistent with the cultured organism; may be negative in dermatophyte infections | (i) ≥5 CFU of a yeast species; or (ii) any growth of Trichophyton spp.; or (iii) growth of a non-dermatophytic mold matching the atypical microscopy | Full concordance between tests (i), isolation of a recognized primary pathogen (ii), or consistent mold morphology when no previous treatment (iii) establishes etiological certainty. |
| Case Category | 0–24 | 25–44 | 45–59 | 60–74 | 75+ | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | |
| Total POS | 9 (18.8%) | 6 (18.8%) | 53 (37.1%) | 16 (21.9%) | 86 (46.5%) | 27 (40.9%) | 125 (42.7%) | 69 (60.0%) | 47 (63.5%) | 26 (60.5%) | 326 (43.8%) | 145 (43.8%) |
| PrvPOS 1 | 4 (8.3%) | 1 (3.1%) | 22 (15.4%) | 6 (8.2%) | 38 (20.5%) | 12 (18.2%) | 66 (22.5%) | 33 (28.7%) | 22 (29.7%) | 17 (39.5%) | 152 (20.4%) | 69 (20.8%) |
| PrbPOS 2 | 0 (0.0%) | 1 (3.1%) | 3 (2.1%) | 2 (2.7%) | 7 (3.8%) | 1 (1.5%) | 7 (2.4%) | 3 (2.6%) | 2 (2.7%) | 0 (0.0%) | 19 (2.5%) | 7 (2.1%) |
| DbtPOS 3 | 3 (6.2%) | 3 (9.4%) | 12 (8.4%) | 7 (9.6%) | 10 (5.4%) | 7 (10.6%) | 16 (5.5%) | 25 (21.7%) | 5 (6.8%) | 7 (16.3%) | 46 (6.2%) | 50 (15.1%) |
| PssPOS 4 | 2 (4.2%) | 1 (3.1%) | 16 (11.2%) | 1 (1.4%) | 31 (16.8%) | 7 (10.6%) | 36 (12.3%) | 8 (7.0%) | 18 (24.3%) | 2 (4.7%) | 103 (13.8%) | 19 (5.7%) |
| NEG | 38 (79.2%) | 26 (81.2%) | 89 (62.2%) | 57 (78.1%) | 98 (53.0%) | 39 (59.1%) | 165 (56.3%) | 46 (40.0%) | 27 (36.5%) | 17 (39.5%) | 418 (56.2%) | 186 (56.2%) |
| POS vs. NEG statistics | χ2 = 0.002 p =0.965 | χ2 = 5.251 p = 0.022 | χ2 = 0.667 p = 0.414 | χ2 = 9.420 p =0.002 | χ2 = 0.108 p = 0.743 | χ2 = 0.000 p = 0.997 | ||||||
| Group of Fungi | Genus | Species | Number | Percentage | |
|---|---|---|---|---|---|
| YEAST | ASCOMYCETOUS | CANDIDA | Candida albicans complex | 122 | 18.83 |
| Candida parapsilosis complex | 208 | 32.10 | |||
| Candida tropicalis | 11 | 1.70 | |||
| subtotal CANDIDA | 341 | 52.62 | |||
| OTHER SPECIES | Clavispora lusitaniae | 27 | 4.17 | ||
| Debaryomyces hansenii | 7 | 1.08 | |||
| Geotrichum candidum | 8 | 1.23 | |||
| Hanseniospora spp. | 11 | 1.70 | |||
| Meyerozyma guilliermondii | 13 | 2.00 | |||
| Sungouiella intermedia | 8 | 1.23 | |||
| Wickerhamiella pararugosa | 10 | 1.54 | |||
| Yarrowia lipolytica | 13 | 2.00 | |||
| other | 18 | 3.68 | |||
| subtotal other yeast | 117 | 18.06 | |||
| Candida sp. (not species ID) | 23 | 3.55 | |||
| subtotal ascomycetous yeasts | 485 | 74.85 | |||
| BASIDIOMYCETOUS | RHODOTORULA | Rhodotorula spp. | 43 | 6.64 | |
| SKIN CRYTPOCOCCI | Filobasidium, Naganishia, Papillotrema | 24 | 3.7 | ||
| OTHER SPECIES | Trichosporon spp., Aureobasidium spp. | 2 | 0.92 | ||
| subtotal basidiomycetous yeasts | 73 | 11.27 | |||
| subtotal YEASTS | 558 | 86.12 | |||
| DERMATOPHYTES | other Trichophyton | 5 | 0.77 | ||
| TRICHOPHYTON | Trichophyton rubrum | 51 | 7.87 | ||
| subtotal DERMATOPHYTES | 56 | 8.64 | |||
| MOLDS | ASPERGILLUS | Aspergillus sp. | 10 | 1.54 | |
| FUSARIUM | Fusarium spp. | 13 | 2.01 | ||
| OTHER GENERA | 11 | 1.70 | |||
| subtotal MOLDS | 34 | 5.25 | |||
| TOTAL | 648 | 100 | |||
| Antifungal Agent (µg/mL) | C. parapsilosis (n = 48) | C. albicans (n = 25) | C. lusitaniae (n = 9) | M. guilliermondii (n = 5) | W. pararugosa (n = 5) | |
|---|---|---|---|---|---|---|
| Econazole | GM (range) | 0.250 (0.016–2.000) | 0.051 (0.016–2.000) | 0.068 (0.016–1.000) | 0.379 (0.125–1.000) | 0.758 (0.500–2.000) |
| MIC50/MIC90 | 0.250/1.000 | 0.016/1.400 | 0.031/1.000 | 0.250/1.000 | 0.500/2.000 | |
| Fluconazole | GM (range) | 0.459 (0.031–1.000) | 0.244 (0.016–16.000) | 0.429 (0.125–16.000) | 0.500 (0.125–8.000) | 5.278 (1.000–8.000) |
| MIC50/MIC90 | 0.500/1.000 | 0.125/3.800 | 0.250/16.000 | 0.250/8.000 | 8.000/8.000 | |
| CBP/ECOFF | 4/2 | 4/0.5 | nd/nd | nd/(16) | nd/nd | |
| Itraconazole | GM (range) | 0.043 (0.008–0.500) | 0.018 (0.008–1.000) | 0.047 (0.008–0.250) | 0.125 (0.031–1.000) | 0.250 (0.125–0.500) |
| MIC50/MIC90 | 0.031/0.313 | 0.016/0.119 | 0.031/0.250 | 0.063/1.000 | 0.250/0.500 | |
| CBP | 0.125 | 0.06/0.03 | nd/0.125 | nd/(1) | nd/nd | |
| Posaconazole | GM (range) | 0.009 (0.008–0.063) | 0.011 (0.008–1.000) | 0.008 (0.008–0.008) | 0.024 (0.008–0.063) | 0.072 (0.031–0.125) |
| MIC50/MIC90 | 0.008/0.016 | 0.008/0.044 | 0.008/0.008 | 0.031/0.063 | 0.063/0.125 | |
| CBP | 0.06/0.06 | 0.06/0.06 | nd/nd | nd/0.25 | nd/nd | |
| Voriconazole | GM (range) | 0.016 (0.008–0.125) | 0.017 (0.008–0.250) | 0.014 (0.008–0.125) | 0.041 (0.008–0.125) | 0.189 (0.125–0.500) |
| MIC50/MIC90 | 0.016/0.031 | 0.008/0.088 | 0.008/0.125 | 0.031/0.125 | 0.125/0.500 | |
| BP | 0.25/0.06 | 0.25/0.03 | nd/nd | nd/nd | nd/nd | |
| Terbinafine | GM (range) | 0.028 (0.008–8.000) | 0.608 (0.016–8.000) | 0.184 (0.016–1.000) | 0.110 (0.016–2.000) | 0.250 (0.125–1.000) |
| MIC50/MIC90 | 0.016/0.125 | 1.000/5.600 | 0.250/1.000 | 0.063/2.000 | 0.250/1.000 | |
| Amorolfine | GM (range) | 0.698 (0.016–8.000) | 0.109 (0.008–2.000) | 0.250 (0.125–1.000) | 0.574 (0.250–1.000) | 0.501 (0.063–8.000) |
| MIC50/MIC90 | 1.000/4.000 | 0.125/1.400 | 0.125/1.000 | 0.500/1.000 | 0.500/8.000 | |
| Ciclopirox | GM (range) | 0.515 (0.250–1.000) | 0.473 (0.250–0.500) | 0.500 (0.500–0.500) | 0.500 (0.500–0.500) | 0.574 (0.500–1.000) |
| MIC50/MIC90 | 0.500/0.500 | 0.500/0.500 | 0.500/0.500 | 0.500/0.500 | 0.500/1.000 | |
| Amphotericin B | GM (range) | 0.199 (0.063–1.000) | 0.179 (0.031–1.000) | 0.107 (0.063–0.250) | 0.190 (0.063–1.000) | 0.250 (0.250–0.250) |
| MIC50/MIC90 | 0.250/0.275 | 0.125/0.700 | 0.125/0.250 | 0.125/1.000 | 0.250/0.250 | |
| CBP | 1.0/1.0 | 1.0/1.0 | nd/(0.5) | nd/(0.5) | nd/nd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Krzyściak, P.; Tokarz, Z.; Pomorska-Wesołowska, M.; Skóra, M.; Jaworek, A.K.; Wójkowska-Mach, J. Fingernail Onychomycosis: A Laboratory-Based Retrospective Study with Species Profiling and Antifungal Susceptibility of Yeasts. J. Clin. Med. 2026, 15, 325. https://doi.org/10.3390/jcm15010325
Krzyściak P, Tokarz Z, Pomorska-Wesołowska M, Skóra M, Jaworek AK, Wójkowska-Mach J. Fingernail Onychomycosis: A Laboratory-Based Retrospective Study with Species Profiling and Antifungal Susceptibility of Yeasts. Journal of Clinical Medicine. 2026; 15(1):325. https://doi.org/10.3390/jcm15010325
Chicago/Turabian StyleKrzyściak, Paweł, Zuzanna Tokarz, Monika Pomorska-Wesołowska, Magdalena Skóra, Andrzej Kazimierz Jaworek, and Jadwiga Wójkowska-Mach. 2026. "Fingernail Onychomycosis: A Laboratory-Based Retrospective Study with Species Profiling and Antifungal Susceptibility of Yeasts" Journal of Clinical Medicine 15, no. 1: 325. https://doi.org/10.3390/jcm15010325
APA StyleKrzyściak, P., Tokarz, Z., Pomorska-Wesołowska, M., Skóra, M., Jaworek, A. K., & Wójkowska-Mach, J. (2026). Fingernail Onychomycosis: A Laboratory-Based Retrospective Study with Species Profiling and Antifungal Susceptibility of Yeasts. Journal of Clinical Medicine, 15(1), 325. https://doi.org/10.3390/jcm15010325








