Effects of Smoking on Neurocognitive Outcomes in Patients with Carbon Monoxide Poisoning
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Variables and Definitions
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Characteristics of Patients After Propensity Score Matching
3.3. Primary Outcomes in Mathced Cohorts
3.4. Additional Analysis for Neurocognitive Outcomes at 6 Months After Acute CO Poisoning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMD | Standardized Mean Difference |
| CO | Carbon Monoxide |
| GCS | Glasgow Coma Scale |
| ED | Emergency Department |
| HBO2 | Hyperbaric Oxygen |
| CO-Hb | Carboxyhemoglobin |
| PSM | Propensity Score Matching |
| CI | Confidence Interval |
References
- Hampson, N.B. Carbon monoxide poisoning mortality in the United States from 2015-2021. Clin. Toxicol. 2023, 61, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Bronstein, A.C.; Glidden, E.; Malone, M.; Chang, A.; Law, R.; Boehmer, T.K.; Strosnider, H.; Yip, F. Morbidity and mortality of unintentional carbon monoxide poisoning: United States 2005 to 2018. Ann. Emerg. Med. 2023, 81, 309–317. [Google Scholar] [CrossRef]
- Cho, D.H.; Ko, S.M.; Son, J.W.; Park, E.J.; Cha, Y.S. Myocardial injury and fibrosis from acute carbon monoxide poisoning: A prospective observational study. JACC Cardiovasc. Imaging 2021, 14, 1758–1770. [Google Scholar] [CrossRef]
- Huang, T.L.; Tung, M.C.; Lin, C.L.; Chang, K.H. Risk of acute kidney injury among patients with carbon monoxide poisoning. Medicine 2021, 100, e27239. [Google Scholar] [CrossRef] [PubMed]
- Hampson, N.B.; Piantadosi, C.A.; Thom, S.R.; Weaver, L.K. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am. J. Respir. Crit. Care Med. 2012, 186, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S. Delayed neurologic sequelae in carbon monoxide intoxication. Arch. Neurol. 1983, 40, 433–435. [Google Scholar] [CrossRef]
- Min, S.K. A brain syndrome associated with delayed neuropsychiatric sequelae following acute carbon monoxide intoxication. Acta Psychiatr. Scand. 1986, 73, 80–86. [Google Scholar] [CrossRef]
- Thom, S.R.; Keim, L.W. Carbon monoxide poisoning: A review epidemiology, pathophysiology, clinical findings, and treatment options including hyperbaric oxygen therapy. J. Toxicol. Clin. Toxicol. 1989, 27, 141–156. [Google Scholar] [CrossRef]
- Weaver, L.K.; Hopkins, R.O.; Chan, K.J.; Churchill, S.; Elliott, C.G.; Clemmer, T.P.; Orme, J.F., Jr.; Thomas, F.O.; Morris, A.H. Hyperbaric oxygen for acute carbon monoxide poisoning. N. Engl. J. Med. 2002, 347, 1057–1067. [Google Scholar] [CrossRef]
- Weaver, L.K.; Valentine, K.J.; Hopkins, R.O. Carbon monoxide poisoning: Risk factors for cognitive sequelae and the role of hyperbaric oxygen. Am. J. Respir. Crit. Care Med. 2007, 176, 491–497. [Google Scholar] [CrossRef]
- Pan, K.T.; Shen, C.H.; Lin, F.G.; Chou, Y.C.; Croxford, B.; Leonardi, G.; Huang, K.L. Prognostic factors of carbon monoxide poisoning in Taiwan: A retrospective observational study. BMJ Open 2019, 9, e031135. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Castelli, M.; Nazerian, P.; Vanni, S.; Del Panta, M.; Gambassi, F.; Botti, P.; Missanelli, A.; Grifoni, S. Delayed neuropsychological sequelae after carbon monoxide poisoning: Predictive risk factors in the Emergency Department. A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.C.; Mao, Y.C.; Hung, Y.M.; Lee, C.H.; Yang, C.C. Predictive role of QTc prolongation in carbon monoxide poisoning-related delayed neuropsychiatric sequelae. Biomed. Res. Int. 2018, 2018, 2543018. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Q.; Jia, J.; Xiang, D.; Xi, Y. Multicenter retrospective analysis of the risk factors for delayed neurological sequelae after acute carbon monoxide poisoning. Am. J. Emerg. Med. 2021, 46, 165–169. [Google Scholar] [CrossRef]
- Sert, E.T.; Kokulu, K.; Mutlu, H. Clinical predictors of delayed neurological sequelae in charcoal-burning carbon monoxide poisoning. Am. J. Emerg. Med. 2021, 48, 12–17. [Google Scholar] [CrossRef]
- Han, S.; Choi, S.; Nah, S.; Lee, S.U.; Cho, Y.S.; Kim, G.W.; Lee, Y.H. Cox regression model of prognostic factors for delayed neuropsychiatric sequelae in patients with acute carbon monoxide poisoning: A prospective observational study. Neurotoxicology 2021, 82, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Lee, J.H. Serum lactate as a predictor of neurologic outcome in ED patients with acute carbon monoxide poisoning. Am. J. Emerg. Med. 2019, 37, 823–827. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.; Kang, S.; Paik, J.H.; Kim, H.; Cha, Y.S. Derivation and validation of a score for predicting poor neurocognitive outcomes in acute carbon monoxide poisoning. JAMA Netw. Open 2022, 5, e2210552. [Google Scholar] [CrossRef]
- Gallucci, G.; Tartarone, A.; Lerose, R.; Lalinga, A.V.; Capobianco, A.M. Cardiovascular risk of smoking and benefits of smoking cessation. J. Thorac. Dis. 2020, 12, 3866–3876. [Google Scholar] [CrossRef]
- Shah, R.S.; Cole, J.W. Smoking and stroke: The more you smoke the more you stroke. Expert Rev. Cardiovasc. Ther. 2010, 8, 917–932. [Google Scholar] [CrossRef]
- Venkatason, P.; Salleh, N.M.; Zubairi, Y.; Hafidz, I.; Ahmad, W.A.; Han, S.K.; Zuhdi, A.S. The bizzare phenomenon of smokers’ paradox in the immediate outcome post acute myocardial infarction: An insight into the Malaysian National Cardiovascular Database-Acute Coronary Syndrome (NCVD-ACS) registry year 2006–2013. Springerplus 2016, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Weisz, G.; Cox, D.A.; Garcia, E.; Tcheng, J.E.; Griffin, J.J.; Guagliumi, G.; Stuckey, T.D.; Rutherford, B.D.; Mehran, R.; Aymong, E.; et al. Impact of smoking status on outcomes of primary coronary intervention for acute myocardial infarction--the smoker’s paradox revisited. Am. Heart J. 2005, 150, 358–364. [Google Scholar] [CrossRef]
- Weng, W.C.; Huang, W.Y.; Chien, Y.Y.; Wu, C.L.; Su, F.C.; Hsu, H.J.; Lee, T.H.; Peng, T.I. The impact of smoking on the severity of acute ischemic stroke. J. Neurol. Sci. 2011, 308, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.J. Hypoxia and aging. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Daijo, H.; Hoshino, Y.; Kai, S.; Suzuki, K.; Nishi, K.; Matsuo, Y.; Harada, H.; Hirota, K. Cigarette smoke reversibly activates hypoxia-inducible factor 1 in a reactive oxygen species-dependent manner. Sci. Rep. 2016, 6, 34424. [Google Scholar] [CrossRef]
- Weaver, L.K. Carbon monoxide poisoning. In Hyperbaric Oxygen Therapy Indications, 14th ed.; Moon, R.E., Ed.; Best Publishing Company: North Palm Beach, FL, USA, 2019; pp. 81–104. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef]
- Kim, S.J.; Thom, S.R.; Kim, H.; Hwang, S.O.; Lee, Y.; Park, E.J.; Lee, S.J.; Cha, Y.S. Effects of adjunctive therapeutic hypothermia combined with hyperbaric oxygen therapy in acute severe carbon monoxide poisoning. Crit. Care Med. 2020, 48, e706–e714. [Google Scholar] [CrossRef]
- Lee, Y.; Cha, Y.S.; Kim, S.H.; Kim, H. Effect of hyperbaric oxygen therapy initiation time in acute carbon monoxide poisoning. Crit. Care Med. 2021, 49, e910–e919. [Google Scholar] [CrossRef]
- Huijun, H.; Qiang, S.; Dazhi, G.; Yu, Z.; Yan, L.; Shuyi, P.; Xuejun, S. Sex differences may affect the severity of poisoning and prognosis after carbon monoxide poisoning: A retrospective study. Undersea Hyperb. Med. 2016, 43, 207–215. [Google Scholar]
- Hampson, N.B.; Rudd, R.A.; Hauff, N.M. Increased long-term mortality among survivors of acute carbon monoxide poisoning. Crit. Care Med. 2009, 37, 1941–1947. [Google Scholar] [CrossRef]
- Liao, W.C.; Cheng, W.C.; Wu, B.R.; Chen, W.C.; Chen, C.Y.; Chen, C.H.; Tu, C.Y.; Hsia, T.C. Outcome and prognostic factors of patients treated in the intensive care unit for carbon monoxide poisoning. J. Formos. Med. Assoc. 2019, 118, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Lee, J.C.; Lin, K.C.; Lin, H.J.; Su, S.B.; Hsu, C.C.; Guo, H.R. Exposure duration and history of hypertension predicted neurological sequelae in patients with carbon monoxide poisoning. Epidemiology 2019, 30 (Suppl. S1), S76–S81. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.H.; Hung, H.M.; Leong, W.C.; Chen, H.H.; Lin, J.L.; Huang, W.H.; Yang, H.Y.; Weng, C.H.; Lin, C.M.; Lee, S.H.; et al. Outcome of patients with carbon monoxide poisoning at a far-east poison center. PLoS ONE 2015, 10, e0118995. [Google Scholar] [CrossRef]
- Patel, B.; Omeh, J.; Tackling, G.; Gupta, R.; Sahadeo, T.; Villcant, V.; Dussie, T.; Atnas, M.; Hai, O.; Zeltser, R.; et al. The clinical association between carbon monoxide poisoning and myocardial injury as measured by elevated troponin I levels. J. Clin. Med. 2023, 12, 5529. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am. Stat. 1985, 39, 33–38. [Google Scholar] [CrossRef]
- Nah, S.; Choi, S.; Lee, S.U.; Kim, G.W.; Lee, Y.H.; Han, S. Effects of smoking on delayed neuropsychiatric sequelae in acute carbon monoxide poisoning: A prospective observational study. Medicine 2021, 100, e26032. [Google Scholar] [CrossRef] [PubMed]
- Barbash, G.I.; Reiner, J.; White, H.D.; Wilcox, R.G.; Armstrong, P.W.; Sadowski, Z.; Morris, D.; Aylward, P.; Woodlief, L.H.; Topol, E.J. Evaluation of paradoxic beneficial effects of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: Mechanism of the “smoker’s paradox” from the GUSTO-I trial, with angiographic insights. J. Am. Coll. Cardiol. 1995, 26, 1222–1229. [Google Scholar] [CrossRef]
- Barbash, G.I.; White, H.D.; Modan, M.; Diaz, R.; Hampton, J.R.; Heikkila, J.; Kristinsson, A.; Moulopoulos, S.; Paolasso, E.A.; Van der Werf, T.; et al. Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction. Experience gleaned from the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Circulation 1993, 87, 53–58. [Google Scholar] [CrossRef]
- Ali, S.F.; Smith, E.E.; Bhatt, D.L.; Fonarow, G.C.; Schwamm, L.H. Paradoxical association of smoking with in-hospital mortality among patients admitted with acute ischemic stroke. J. Am. Heart Assoc. 2013, 2, e000171. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bailen, M.; de Hoyos, E.A.; Reina-Toral, A.; Torres-Ruiz, J.M.; Alvarez-Bueno, M.; Gomez Jimenez, F.J.; Group, A. Paradoxical effect of smoking in the Spanish population with acute myocardial infarction or unstable angina: Results of the ARIAM Register. Chest 2004, 125, 831–840. [Google Scholar] [CrossRef][Green Version]
- Elosua, R.; Vega, G.; Rohlfs, I.; Aldasoro, E.; Navarro, C.; Cabades, A.; Demissie, S.; Segura, A.; Fiol, M.; Moreno-Iribas, C.; et al. Smoking and myocardial infarction case-fatality: Hospital and population approach. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Smoking and risk of stroke. J. Cardiovasc. Risk 1999, 6, 207–211. [Google Scholar] [CrossRef]
- Wolf, P.A.; D’Agostino, R.B.; Kannel, W.B.; Bonita, R.; Belanger, A.J. Cigarette smoking as a risk factor for stroke: The Framingham Study. JAMA 1988, 259, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, C.; Vedin, J.A.; Elmfeldt, D.; Tibblin, G.; Wilhelmsen, L. Smoking and myocardial infarction. Lancet 1975, 1, 415–420. [Google Scholar] [CrossRef]
- Prescott, E.; Hippe, M.; Schnohr, P.; Hein, H.O.; Vestbo, J. Smoking and risk of myocardial infarction in women and men: Longitudinal population study. BMJ 1998, 316, 1043–1047. [Google Scholar] [CrossRef]
- Redfors, B.; Furer, A.; Selker, H.P.; Thiele, H.; Patel, M.R.; Chen, S.; Udelson, J.E.; Ohman, E.M.; Eitel, I.; Granger, C.B.; et al. Effect of smoking on outcomes of primary PCI in patients with STEMI. J. Am. Coll. Cardiol. 2020, 75, 1743–1754. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Liu, J.F.; Wang, L.; Li, B.Z.; Yan, X.J.; Liu, W.; Wu, K.; Xiang, R.L. “Smoking paradox” is not true in patients with ischemic stroke: A systematic review and meta-analysis. J. Neurol. 2021, 268, 2042–2054. [Google Scholar] [CrossRef]
- Aune, E.; Roislien, J.; Mathisen, M.; Thelle, D.S.; Otterstad, J.E. The “smoker’s paradox” in patients with acute coronary syndrome: A systematic review. BMC Med. 2011, 9, 97. [Google Scholar] [CrossRef]
- Bonow, R.O.; Mann, D.L.; Zipes, D.P.; Libby, P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Elsevier Health Sciences: Philadelphia, PA, USA, 2011. [Google Scholar]
- Gourlay, S.G.; Rundle, A.C.; Barron, H.V. Smoking and mortality following acute myocardial infarction: Results from the National Registry of Myocardial Infarction 2 (NRMI 2). Nicotine Tob. Res. 2002, 4, 101–107. [Google Scholar] [CrossRef] [PubMed]
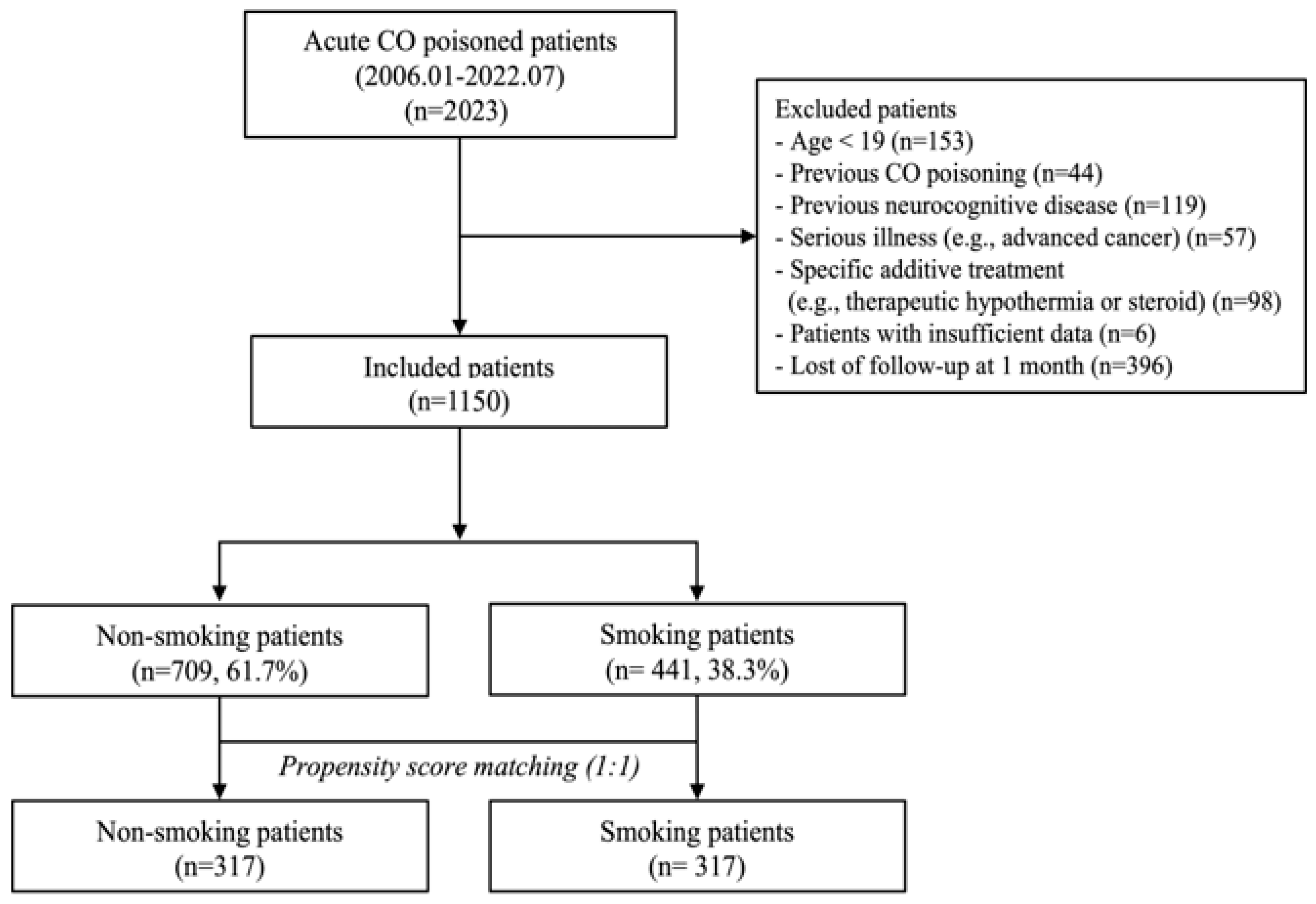
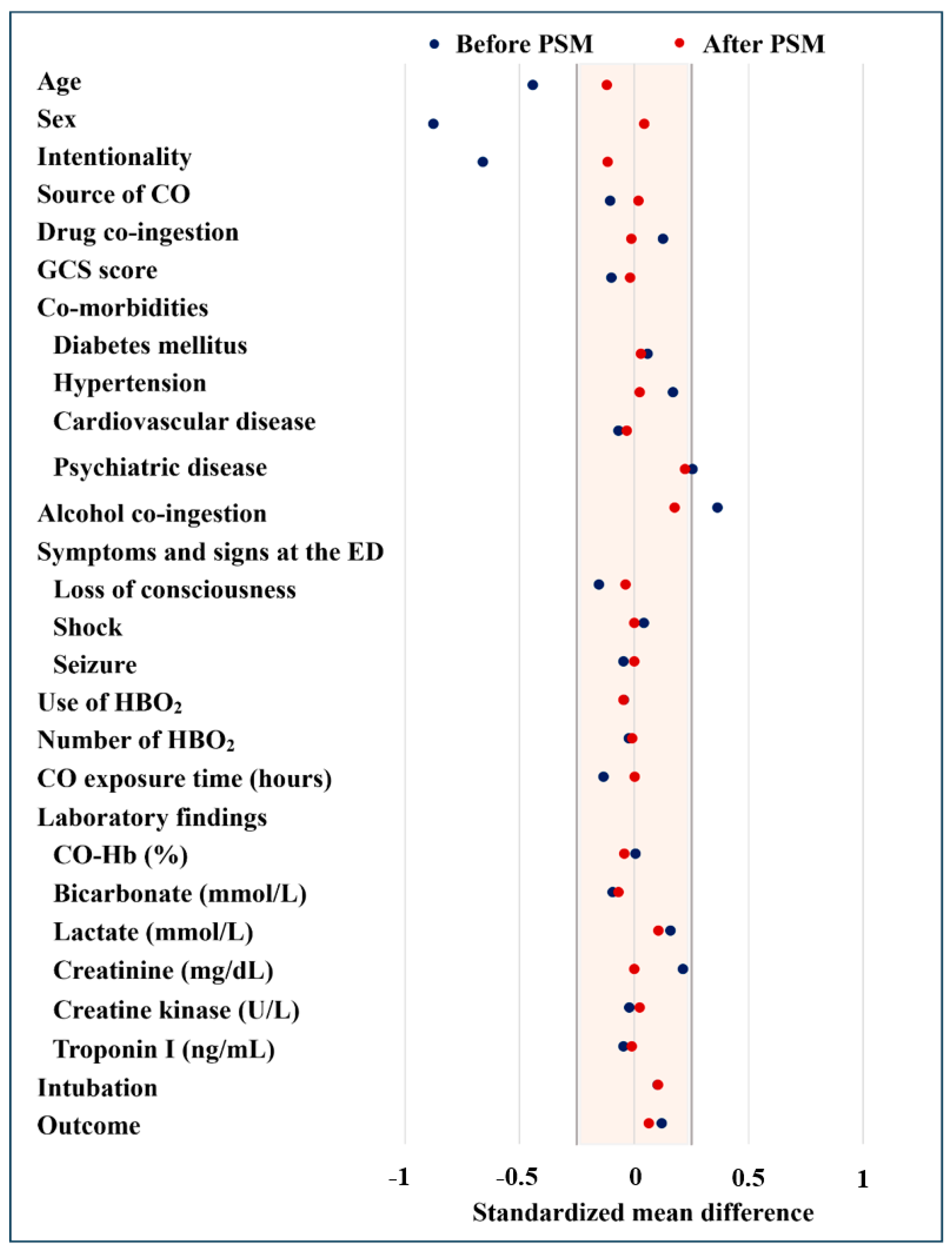

| Unmatched | Matched (1:1) | ||||||
|---|---|---|---|---|---|---|---|
| Non-Smoking (n = 709; 61.7%) | Smoking (n = 441; 38.3%) | p-Value | Non-Smoking (n = 317) | Smoking (n = 317) | SMD | p-Value | |
| Age | 50 (38–64) | 41 (32–54) | <0.001 | 46 (34–58) | 43 (33–56) | −0.120 | 0.076 |
| Sex | <0.001 | 0.044 | 0.504 | ||||
| Woman | 383 (54.0) | 60 (13.6) | 49 (15.5) | 55 (17.4) | |||
| Man | 326 (46.0) | 381 (86.4) | 268 (84.5) | 262 (82.6) | |||
| Intentionality | 166 (23.4) | 240 (54.4) | <0.001 | 123 (38.8) | 140 (44.2) | −0.115 | 0.086 |
| CO source | 0.538 | 0.019 | 0.142 | ||||
| Charcoal | 510 (71.9) | 330 (74.8) | 234 (73.8) | 229 (72.2) | |||
| Oil and gas | 97 (13.7) | 56 (12.7) | 43 (13.6) | 49 (15.5) | |||
| Fire | 102 (14.4) | 55 (12.5) | 40 (12.6) | 39 (12.3) | |||
| Drug co-ingestion | 44 (6.2) | 45 (10.2) | 0.014 | 30 (9.5) | 29 (9.1) | −0.011 | 1.000 |
| GCS score | 15 (12–15) | 15 (12–15) | 0.073 | 15 (12–15) | 15 (12–15) | −0.017 | 0.817 |
| Co-morbidities | |||||||
| Diabetes mellitus | 82 (11.6) | 40 (9.1) | 0.182 | 33 (10.4) | 30 (9.5) | 0.031 | 0.788 |
| Hypertension | 156 (22.0) | 59 (13.4) | <0.001 | 53 (16.7) | 50 (15.8) | 0.025 | 0.826 |
| Cardiovascular disease | 31 (4.4) | 13 (2.9) | 0.221 | 13 (4.1) | 11 (3.5) | −0.032 | 0.839 |
| Psychiatric disease | 61 (8.6) | 76 (17.2) | <0.001 | 35 (11.0) | 59 (18.6) | 0.222 | 0.011 |
| Alcohol co-ingestion | 25 (3.5) | 60 (13.6) | <0.001 | 20 (6.3) | 36 (11.4) | 0.177 | 0.029 |
| Symptoms and signs at the ED | |||||||
| Loss of consciousness | 349 (49.2) | 247 (56.0) | 0.025 | 167 (52.7) | 173 (54.6) | −0.038 | 0.673 |
| Shock | 24 (3.4) | 10 (2.3) | 0.277 | 9 (2.8) | 9 (2.8) | 0 | 1.000 |
| Seizure | 11 (1.6) | 4 (0.9) | 0.349 | 3 (0.9) | 3 (0.9) | 0 | 1.000 |
| Use of HBO2 therapy | 586 (82.7) | 364 (82.5) | 0.961 | 267 (84.2) | 262 (82.6) | 0.046 | 0.635 |
| Number of HBO2 therapy sessions | 1 (1–2) | 1 (1–2) | 0.524 | 1 (1–2) | 1 (1–2) | −0.009 | 0.911 |
| CO exposure time (hours) | 4 (1–8) | 3 (1–8) | 0.007 | 3 (1–8) | 3 (1–8) | 0.002 | 0.974 |
| Laboratory findings | |||||||
| CO-Hb (%) | 16.1 (5.7–28.7) | 16.1 (6.6–30) | 0.324 | 19.4 (6.5–29.6) | 16.3(6.7–30) | −0.043 | 0.605 |
| Bicarbonate (mmol/L) | 21.8 (19.4–23.8) | 21.6 (19.4–23.3) | 0.099 | 21.5 (18.7–23.6) | 21.6 (18.8–23.4) | −0.069 | 0.388 |
| Lactate (mmol/L) | 2.0 (1.3–3.1) | 2.1 (1.4–3.5) | 0.014 | 2.2 (1.38–3.42) | 2.1 (1.37–3.69) | 0.106 | 0.207 |
| Creatinine (mg/dL) | 0.78 (0.625–0.96) | 0.87 (0.71–1.03) | <0.001 | 0.90 (0.74–1.06) | 0.89 (0.72–1.1) | 0 | 0.996 |
| Creatine kinase (U/L) | 130 (84–280) | 143 (96.5–256) | 0.027 | 147 (103–293) | 151 (99–311) | 0.024 | 0.745 |
| Troponin I (ng/mL) | 15 (12.67–146) | 15 (9–128) | 0.051 | 15 (11–115) | 15 (8–187) | −0.009 | 0.852 |
| Intubation | <0.001 | ||||||
| Yes | 51 (7.2) | 29 (6.6) | 21 (6.62) | 23 (7.26) | 0.104 | 0.600 | |
| Outcome | 86 (12.1) | 30 (6.8) | 0.004 | 31 (9.8) | 25 (7.9) | −0.064 | 0.461 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, E.; Park, Y.; Cha, Y.S.; Lee, J.S.; Lee, S.C.; Ahn, G.J. Effects of Smoking on Neurocognitive Outcomes in Patients with Carbon Monoxide Poisoning. J. Clin. Med. 2025, 14, 2497. https://doi.org/10.3390/jcm14072497
Ko E, Park Y, Cha YS, Lee JS, Lee SC, Ahn GJ. Effects of Smoking on Neurocognitive Outcomes in Patients with Carbon Monoxide Poisoning. Journal of Clinical Medicine. 2025; 14(7):2497. https://doi.org/10.3390/jcm14072497
Chicago/Turabian StyleKo, Eunsan, Yeonjae Park, Yong Sung Cha, Je Seop Lee, Sun Chul Lee, and Gyo Jin Ahn. 2025. "Effects of Smoking on Neurocognitive Outcomes in Patients with Carbon Monoxide Poisoning" Journal of Clinical Medicine 14, no. 7: 2497. https://doi.org/10.3390/jcm14072497
APA StyleKo, E., Park, Y., Cha, Y. S., Lee, J. S., Lee, S. C., & Ahn, G. J. (2025). Effects of Smoking on Neurocognitive Outcomes in Patients with Carbon Monoxide Poisoning. Journal of Clinical Medicine, 14(7), 2497. https://doi.org/10.3390/jcm14072497







