From Liver to Kidney: The Overlooked Burden of Nonalcoholic Fatty Liver Disease in Chronic Kidney Disease
Abstract
1. Introduction
2. Methodology of Literature Search
3. Epidemiology
4. Mechanisms Linking NAFLD to CKD: Hormonal, Inflammatory, and Dietary Pathways
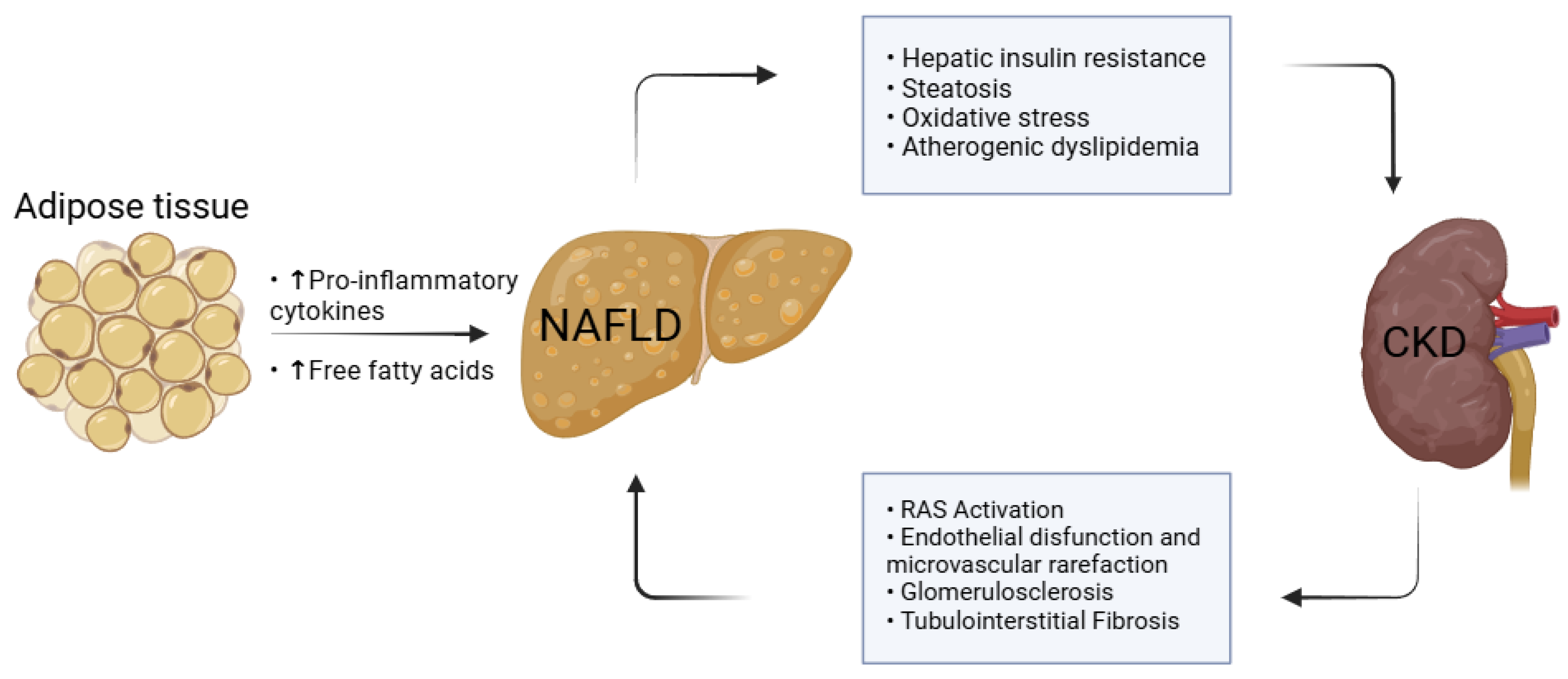
4.1. Hepatic–Renal Crosstalk and the Role of Adipose Tissue
4.2. Free Fatty Acids and Thyroid–Renal Axis
4.3. Dietary Pathways and Vitamin D Axis
5. Prevention and Management
6. Discussions
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morelli, M.C.; Rendina, M.; La Manna, G.; Alessandria, C.; Pasulo, L.; Lenci, I.; Bhoori, S.; Messa, P.; Biancone, L.; Gesualdo, L.; et al. Position paper on liver and kidney diseases from the Italian Association for the Study of Liver (AISF), in collaboration with the Italian Society of Nephrology (SIN). Dig. Liver Dis. 2021, 53, S49–S86. [Google Scholar] [CrossRef] [PubMed]
- Cornec-Le Gall, E.; Torres, V.E.; Harris, P.C. Genetic Complexity of Autosomal Dominant Polycystic Kidney and Liver Diseases. J. Am. Soc. Nephrol. 2018, 29, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Donato, F.M.; Messa, P. Association Between Hepatitis C Virus and Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2018, 17, 364–391. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Donato, F.M.; Messa, P. Association Between Hepatitis B Virus and Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann. Hepatol. 2017, 16, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Schattenberg, J.M.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: An updated meta-analysis. Gut 2022, 71, 156–162. [Google Scholar] [CrossRef]
- Umbro, I.; Baratta, F.; Angelico, F.; Del Ben, M. Nonalcoholic Fatty Liver Disease and the Kidney: A Review. Biomedicines 2021, 9, 1370. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37, 81–84. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar]
- Akbari, A.; Clase, C.M.; Acott, P.; Battistella, M.; Bello, A.; Feltmate, P.; Grill, A.; Karsanji, M.; Komenda, P.; Madore, F.; et al. Canadian Society of Nephrology Commentary on the KDIGO Clinical Practice Guideline for CKD Evaluation and Management. Am. J. Kidney Dis. 2015, 65, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Chonchol, M.; Zoppini, G.; Abaterusso, C.; Bonora, E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: Is there a link? J. Hepatol. 2011, 54, 1020–1029. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Chonchol, M.; Rodella, S.; Zoppini, G.; Lippi, G.; Zenari, L.; Bonora, E. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia 2010, 53, 1341–1348. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 297–310. [Google Scholar] [CrossRef]
- Sirota, J.C.; McFann, K.; Targher, G.; Chonchol, M.; Jalal, D.I. Association between Nonalcoholic Liver Disease and Chronic Kidney Disease: An Ultrasound Analysis from NHANES 1988–1994. Am. J. Nephrol. 2012, 36, 466–471. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, S.; Wang, M.; Huang, J.; Liu, S.; Wu, S.; Zhang, H.; Wu, Z.; Liu, W.-Y.; Zhang, D.-C.; et al. Association between NAFLD and risk of prevalent chronic kidney disease: Why there is a difference between east and west? BMC Gastroenterol. 2020, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lim, H.; Kim, Y.N.; Kim, J.Y.; Kim, H.W.; Chang, T.I.; Han, S.H. Non-Alcoholic Fatty Liver Disease and Its Association with Kidney and Cardiovascular Outcomes in Moderate to Advanced Chronic Kidney Disease. Am. J. Nephrol. 2024, 56, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hydes, T.J.; Kennedy, O.J.; Buchanan, R.; Cuthbertson, D.J.; Parkes, J.; Fraser, S.D.S.; Roderick, P. The impact of non-alcoholic fatty liver disease and liver fibrosis on adverse clinical outcomes and mortality in patients with chronic kidney disease: A prospective cohort study using the UK Biobank. BMC Med. 2023, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA 2014, 311, 806. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.B.; Byrne, C.D. CKD and Nonalcoholic Fatty Liver Disease. Am. J. Kidney Dis. 2014, 64, 638–652. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Han, E. Beyond Liver Disease: Non-Alcoholic Fatty Liver Disease and Advanced Liver Fibrosis in Kidney Disease. Diabetes Metab. J. 2022, 46, 564–566. [Google Scholar] [CrossRef]
- Cojocariu, C.; Singeap, A.-M.; Girleanu, I.; Chiriac, S.; Muzica, C.M.; Sfarti, C.V.; Cuciureanu, T.; Huiban, L.; Stanciu, C.; Trifan, A. Nonalcoholic Fatty Liver Disease-Related Chronic Kidney Disease. Can. J. Gastroenterol. Hepatol. 2020, 2020, 6630296. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Rinella, M.E. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Curr. Hepatol. Rep. 2016, 15, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Suh, Y.J.; Cho, Y.; Ahn, S.H.; Seo, S.; Hong, S.; Lee, Y.; Choi, Y.J.; Lee, E.; Kim, S.H. Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Diabetes Metab. J. 2022, 46, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Cohney, S.; De Michieli, F.; Pinach, S.; Saba, F.; Gambino, R. Fatty Liver and Chronic Kidney Disease: Novel Mechanistic Insights and Therapeutic Opportunities. Diabetes Care 2016, 39, 1830–1845. [Google Scholar] [CrossRef]
- Yi, M.; Peng, W.; Feng, X.; Teng, F.; Tang, Y.; Kong, Q.; Chen, Z. Extrahepatic morbidities and mortality of NAFLD: An umbrella review of meta-analyses. Aliment. Pharmacol. Ther. 2022, 56, 1119–1130. [Google Scholar] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2019, 73, A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Elendu, C.; Elendu, R.C.; Enyong, J.M.; Ibhiedu, J.O.; Ishola, I.V.; Egbunu, E.O.; Meribole, E.S.; Lawal, S.O.; Okenwa, C.J.; Okafor, G.C.; et al. Comprehensive review of current management guidelines of chronic kidney disease. Medicine 2023, 102, e33984. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Targher, G.; Baffy, G. Nonalcoholic Fatty Liver Disease and Chronic Kidney Disease: Epidemiology, Pathogenesis, and Clinical and Research Implications. Int. J. Mol. Sci. 2022, 23, 13320. [Google Scholar] [CrossRef]
- Cheung, A.; Ahmed, A. Nonalcoholic Fatty Liver Disease and Chronic Kidney Disease: A Review of Links and Risks. Clin. Exp. Gastroenterol. 2021, 14, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kiapidou, S.; Liava, C.; Kalogirou, M.; Akriviadis, E.; Sinakos, E. Chronic kidney disease in patients with non-alcoholic fatty liver disease: What the Hepatologist should know? Ann. Hepatol. 2020, 19, 134–144. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Hemmelgarn, B.R.; Tonelli, M. Early recognition and prevention of chronic kidney disease. Lancet 2010, 375, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Liver Fat in the Pathogenesis of Insulin Resistance and Type 2 Diabetes. Dig. Dis. 2010, 28, 203–209. [Google Scholar] [PubMed]
- Park, H.; Dawwas, G.K.; Liu, X.; Nguyen, M.H. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: A propensity-matched cohort study. J. Intern. Med. 2019, 286, 711–722. [Google Scholar] [PubMed]
- Mikolasevic, I.; Racki, S.; Bubic, I.; Jelic, I.; Stimac, D.; Orlic, L. Chronic Kidney Disease and Nonalcoholic Fatty Liver Disease Proven by Transient Elastography. Kidney Blood Press Res. 2013, 37, 305–310. [Google Scholar]
- Musso, G.; Cassader, M.; Cohney, S.; Pinach, S.; Saba, F.; Gambino, R. Emerging Liver–Kidney Interactions in Nonalcoholic Fatty Liver Disease. Trends Mol. Med. 2015, 21, 645–662. [Google Scholar]
- Marcuccilli, M.; Chonchol, M. NAFLD and Chronic Kidney Disease. Int. J. Mol. Sci. 2016, 17, 562. [Google Scholar] [CrossRef]
- De Vries, A.P.J.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; D’Agati, V.D.; Lamb, H.J.; Barlovic, D.P.; Hojs, R.; et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar]
- Ix, J.H.; Sharma, K. Mechanisms Linking Obesity, Chronic Kidney Disease, and Fatty Liver Disease: The Roles of Fetuin-A, Adiponectin, and AMPK. J. Am. Soc. Nephrol. 2010, 21, 406–412. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The Metabolic Syndrome as a Predictor of Nonalcoholic Fatty Liver Disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Deng, Y.; Wang, J.; Zhao, H.; Zhang, J.; Xie, W. The association between NAFLD and risk of chronic kidney disease: A cross-sectional study. Ther. Adv. Chronic Dis. 2021, 12, 20406223211048650. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Aronis, K.N.; Kountouras, J.; Raptis, D.D.; Vasiloglou, M.F.; Mantzoros, C.S. Circulating leptin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Diabetologia 2016, 59, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the art paper Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 2, 191–200. [Google Scholar] [CrossRef]
- Tesauro, M.; Mascali, A.; Franzese, O.; Cipriani, S.; Cardillo, C.; Di Daniele, N. Chronic Kidney Disease, Obesity, and Hypertension: The Role of Leptin and Adiponectin. Int. J. Hypertens. 2012, 2012, 943605. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Cheng, C.K.; Li, Q. Tubular injury in diabetic kidney disease: Molecular mechanisms and potential therapeutic perspectives. Front. Endocrinol. 2023, 14, 1238927. [Google Scholar] [CrossRef]
- Dogru, T.; Kirik, A.; Gurel, H.; Rizvi, A.A.; Rizzo, M.; Sonmez, A. The Evolving Role of Fetuin-A in Nonalcoholic Fatty Liver Disease: An Overview from Liver to the Heart. Int. J. Mol. Sci. 2021, 22, 6627. [Google Scholar] [CrossRef]
- Jung, T.W.; Yoo, H.J.; Choi, K.M. Implication of hepatokines in metabolic disorders and cardiovascular diseases. BBA Clin. 2016, 5, 108–113. [Google Scholar] [CrossRef]
- Lousa, I.; Reis, F.; Santos-Silva, A.; Belo, L. The Signaling Pathway of TNF Receptors: Linking Animal Models of Renal Disease to Human CKD. Int. J. Mol. Sci. 2022, 23, 3284. [Google Scholar] [CrossRef]
- Santos, J.P.M.D.; Maio, M.C.D.; Lemes, M.A.; Laurindo, L.F.; Haber, J.F.D.S.; Bechara, M.D.; Prado, P.S.D.; Rauen, E.C.; Costa, F.; Pereira, B.C.D.A.; et al. Non-Alcoholic Steatohepatitis (NASH) and Organokines: What Is Now and What Will Be in the Future. Int. J. Mol. Sci. 2022, 23, 498. [Google Scholar] [CrossRef]
- Guzzi, F.; Cirillo, L.; Roperto, R.M.; Romagnani, P.; Lazzeri, E. Molecular Mechanisms of the Acute Kidney Injury to Chronic Kidney Disease Transition: An Updated View. Int. J. Mol. Sci. 2019, 20, 4941. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Secor, J.D.; Fligor, S.C.; Tsikis, S.T.; Yu, L.J.; Puder, M. Free Fatty Acid Receptors as Mediators and Therapeutic Targets in Liver Disease. Front. Physiol. 2021, 12, 656441. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A. Association of NAFLD/NASH, and MAFLD/MASLD with chronic kidney disease: An updated narrative review. Metab. Target Organ. Damage 2024, 4, 16. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Iglesias, P.; Díez, J.J. Thyroid dysfunction and kidney disease. Eur. J. Endocrinol. 2009, 160, 503–515. [Google Scholar] [CrossRef]
- Van Tienhoven-Wind, L.J.N.; Dullaart, R.P.F. Low–normal thyroid function and the pathogenesis of common cardio-metabolic disorders. Eur. J. Clin. Investig. 2015, 45, 494–503. [Google Scholar] [CrossRef]
- Rhee, C.M.; Kalantar-Zadeh, K.; Streja, E.; Carrero, J.-J.; Ma, J.Z.; Lu, J.L.; Kovesdy, C.P. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 282–287. [Google Scholar] [CrossRef]
- Pagadala, M.R.; Zein, C.O.; Dasarathy, S.; Yerian, L.M.; Lopez, R.; McCullough, A.J. Prevalence of Hypothyroidism in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2012, 57, 528–534. [Google Scholar] [CrossRef]
- Chen, P.-C.; Kao, W.-Y.; Cheng, Y.-L.; Wang, Y.-J.; Hou, M.-C.; Wu, J.-C.; Su, C.-W. The correlation between fatty liver disease and chronic kidney disease. J. Formos. Med. Assoc. 2020, 119, 42–50. [Google Scholar] [PubMed]
- Nah, E.-H.; Shin, S.K.; Cho, S.; Park, H.; Kim, S.; Kwon, E.; Cho, H.-I. Chronic kidney disease in nonalcoholic fatty liver disease at primary healthcare centers in Korea. PLoS ONE 2022, 17, e0279367. [Google Scholar]
- Tanaka, M.; Mori, K.; Takahashi, S.; Higashiura, Y.; Ohnishi, H.; Hanawa, N.; Furuhashi, M. Metabolic dysfunction–associated fatty liver disease predicts new onset of chronic kidney disease better than fatty liver or nonalcoholic fatty liver disease. Nephrol. Dial. Transplant. 2023, 38, 700–711. [Google Scholar]
- Lau, K.; Lorbeer, R.; Haring, R.; Schmidt, C.O.; Wallaschofski, H.; Nauck, M.; John, U.; Baumeister, S.E.; Völzke, H. The association between fatty liver disease and blood pressure in a population-based prospective longitudinal study. J. Hypertens. 2010, 28, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Gonçalves, S.; Carepa, F.; Coutinho, J.; Costa, A.; Cortez-Pinto, H. Impaired renal function in morbid obese patients with nonalcoholic fatty liver disease. Liver Int. 2012, 32, 241–248. [Google Scholar] [PubMed]
- Targher, G.; Chonchol, M.; Bertolini, L.; Rodella, S.; Zenari, L.; Lippi, G.; Franchini, M.; Zoppini, G.; Muggeo, M. Increased Risk of CKD among Type 2 Diabetics with Nonalcoholic Fatty Liver Disease. J. Am. Soc. Nephrol. 2008, 19, 1564–1570. [Google Scholar]
- Yilmaz, Y.; Alahdab, Y.O.; Yonal, O.; Kurt, R.; Kedrah, A.E.; Celikel, C.A.; Ozdogan, O.; Duman, D.; Imeryuz, N.; Avsar, E.; et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: Association with liver fibrosis. Metabolism 2010, 59, 1327–1330. [Google Scholar] [PubMed]
- Söderberg, C.; Stål, P.; Askling, J.; Glaumann, H.; Lindberg, G.; Marmur, J.; Hultcrantz, R. Decreased Survival of Subjects With Elevated Liver Function Tests During A 28-Year Follow-Up. Hepatology 2010, 51, 595–602. [Google Scholar]
- Campos, G.M.; Bambha, K.; Vittinghoff, E.; Rabl, C.; Posselt, A.M.; Ciovica, R.; Tiwari, U.; Ferrel, L.; Pabst, M.; Bass, N.M.; et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology 2008, 47, 1916–1923. [Google Scholar]
- Triozzi, J.L.; Richardson, P.A.; Gregg, L.P.; Navaneethan, S.D. Incidence and predictors of non-alcoholic fatty liver disease among patients with chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 1546–1548. [Google Scholar]
- Adrian, T.; Sørensen, I.M.H.; Knop, F.K.; Bro, S.; Ballegaard, E.L.F.; Nordestgaard, B.G.; Fuchs, A.; Kofoed, K.F.; Kühl, J.T.; Sigvardsen, P.E.; et al. Prevalence of non-alcoholic fatty liver disease in patients with chronic kidney disease: A cross-sectional study. Nephrol. Dial. Transplant. 2022, 37, 1927–1934. [Google Scholar] [PubMed]
- Choe, A.R.; Ryu, D.-R.; Kim, H.Y.; Lee, H.A.; Lim, J.; Kim, J.S.; Lee, J.K.; Kim, T.H.; Yoo, K. Noninvasive indices for predicting nonalcoholic fatty liver disease in patients with chronic kidney disease. BMC Nephrol. 2020, 21, 50. [Google Scholar]
- Roderburg, C.; Krieg, S.; Krieg, A.; Demir, M.; Luedde, T.; Kostev, K.; Loosen, S.H. Non-alcoholic fatty liver disease (NAFLD) is associated with an increased incidence of chronic kidney disease (CKD). Eur. J. Med. Res. 2023, 28, 153. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Kang, D.; Sinn, D.H.; Gu, S.; Cho, S.J.; Lee, J.E.; Huh, W.; Paik, S.W.; Ryu, S.; Chang, Y.; et al. Nonalcoholic fatty liver disease accelerates kidney function decline in patients with chronic kidney disease: A cohort study. Sci. Rep. 2018, 8, 4718. [Google Scholar]
- Takahashi, S.; Tanaka, M.; Furuhashi, M.; Moniwa, N.; Koyama, M.; Higashiura, Y.; Osanami, A.; Gocho, Y.; Ohnishi, H.; Numata, K.; et al. Fatty liver index is independently associated with deterioration of renal function during a 10-year period in healthy subjects. Sci. Rep. 2021, 11, 8606. [Google Scholar]
- Kasem, H.E.S.; Abdelatty, E.A.; Yahia, A.M.M.; Abdalla, E.M. The association between non-alcoholic fatty liver disease and chronic kidney disease in Egyptian patients. Egypt Liver J. 2023, 13, 63. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Perez-Pozo, S.E.; Sautin, Y.Y.; Manitius, J.; Sanchez-Lozada, L.G.; Feig, D.I.; Shafiu, M.; Segal, M.; Glassock, R.J.; Shimada, M.; et al. Hypothesis: Could Excessive Fructose Intake and Uric Acid Cause Type 2 Diabetes? Endocr. Rev. 2009, 30, 96–116. [Google Scholar] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, Uric Acid, and the Etiology of Diabetes and Obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar]
- Mosca, A.; Nobili, V.; De Vito, R.; Crudele, A.; Scorletti, E.; Villani, A.; Alisi, A.; Byrne, C.D. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J. Hepatol. 2017, 66, 1031–1036. [Google Scholar]
- Lanaspa, M.A.; Cicerchi, C.; Garcia, G.; Li, N.; Roncal-Jimenez, C.A.; Rivard, C.J.; Hunter, B.; Andrés-Hernando, A.; Ishimoto, T.; Sánchez-Lozada, L.G.; et al. Counteracting Roles of AMP Deaminase and AMP Kinase in the Development of Fatty Liver. PLoS ONE 2012, 7, e48801. [Google Scholar]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.-J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [PubMed]
- Lanaspa, M.A.; Ishimoto, T.; Li, N.; Cicerchi, C.; Orlicky, D.J.; Ruzycki, P.; Rivard, C.; Inaba, S.; Roncal-Jimenez, C.A.; Bales, E.S.; et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 2013, 4, 2434. [Google Scholar] [PubMed]
- Yetley, E.A. Assessing the vitamin D status of the US population. Am. J. Clin. Nutr. 2008, 88, 558S–564S. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Carotti, S.; Labbadia, G.; Gentilucci, U.V.; Muda, A.O.; Angelico, F.; Silecchia, G.; Leonetti, F.; Fraioli, A.; Picardi, A.; et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: Relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology 2012, 56, 2180–2187. [Google Scholar] [CrossRef]
- Beilfuss, A.; Sowa, J.-P.; Sydor, S.; Beste, M.; Bechmann, L.P.; Schlattjan, M.; Syn, W.-K.; Wedemeyer, I.; Mathé, Z.; Jochum, C.; et al. Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptor-dependently and independently. Gut 2015, 64, 791–799. [Google Scholar]
- Wang, X.X.; Jiang, T.; Shen, Y.; Santamaria, H.; Solis, N.; Arbeeny, C.; Levi, M. Vitamin D receptor agonist doxercalciferol modulates dietary fat-induced renal disease and renal lipid metabolism. Am. J. Physiol. -Ren. Physiol. 2011, 300, F801–F810. [Google Scholar] [CrossRef]
- Xu, L.; Wan, X.; Huang, Z.; Zeng, F.; Wei, G.; Fang, D.; Deng, W.; Li, Y. Impact of Vitamin D on Chronic Kidney Diseases in Non-Dialysis Patients: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e61387. [Google Scholar]
- Parikh, A.; Chase, H.S.; Vernocchi, L.; Stern, L. Vitamin D resistance in chronic kidney disease (CKD). BMC Nephrol. 2014, 15, 47. [Google Scholar]
- Sanchez-Lozada, L.G.; Andres-Hernando, A.; Garcia-Arroyo, F.E.; Cicerchi, C.; Li, N.; Kuwabara, M.; Roncal-Jimenez, C.A.; Johnson, R.J.; Lanaspa, M.A. Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J. Biol. Chem. 2019, 294, 4272–4281. [Google Scholar]
- Brown, K.; Broadhurst, K.; Kladney, R. Immunodetection of aldose reductase in normal and diseased human liver. Histol. Histopathol. 2005, 20, 429–436. [Google Scholar] [PubMed]
- Hara, M.; Tanaka, S.; Torisu, K.; Matsukuma, Y.; Tsuchimoto, A.; Tokumoto, M.; Ooboshi, H.; Nakano, T.; Tsuruya, K.; Kitazono, T. Non-invasive fibrosis assessments of non-alcoholic fatty liver disease associated with low estimated glomerular filtration rate among CKD patients: The Fukuoka Kidney disease Registry Study. Clin. Exp. Nephrol. 2021, 25, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.W.; Kim, S.G.; Jang, J.Y.; Yoo, J.-J.; Jeong, S.W.; Kim, Y.S.; Kim, B.S. Two-dimensional shear wave elastography for assessing liver fibrosis in patients with chronic liver disease: A prospective cohort study. Korean J. Intern. Med. 2022, 37, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Lazarus, J.V.; Younossi, Z.M. Healthcare and socioeconomic costs of NAFLD: A global framework to navigate the uncertainties. J. Hepatol. 2023, 79, 209–217. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Friedman, S.L.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Villa-Jimenez, O.; Lazo-del Vallin, S.; Diago, M.; Adams, L.A.; Romero-Gomez, M.; et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2017, 45, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Liu, Y.; Bai, S.; Jiang, J.; Zhou, H.; Luan, J.; Cao, L.; Lv, Y.; Zhang, Q.; et al. Adherence to healthy lifestyle was associated with an attenuation of the risk of chronic kidney disease from metabolic dysfunction–associated fatty liver disease: Results from two prospective cohorts. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102873. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Lakshmanan, M.C.; Rayner, B.; Busch, R.S.; Zimmermann, A.G.; Woodward, D.B.; Botros, F.T. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M.; Toyoda, H.; Yasuda, S.; Tada, T.; Hayashi, H.; Nishigaki, Y.; Suzuki, Y.; Naiki, T.; Morishita, A.; et al. Common Drug Pipelines for the Treatment of Diabetic Nephropathy and Hepatopathy: Can We Kill Two Birds with One Stone? Int. J. Mol. Sci. 2020, 21, 4939. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Allen, A.M.; Jarvis, H.; Zelber-Sagi, S.; Cusi, K.; Dillon, J.F.; Caussy, C.; Francque, S.M.; Younossi, Z.; Alkhouri, N.; et al. A multistakeholder approach to innovations in NAFLD care. Commun. Med. 2023, 3, 1. [Google Scholar] [CrossRef]
- Bosch, C.; Carriazo, S.; Soler, M.J.; Ortiz, A.; Fernandez-Fernandez, B. Tirzepatide and prevention of chronic kidney disease. Clin. Kidney J. 2023, 16, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Heda, R.; Yazawa, M.; Shi, M.; Bhaskaran, M.; Aloor, F.Z.; Thuluvath, P.J.; Satapathy, S.K. Non-alcoholic fatty liver and chronic kidney disease: Retrospect, introspect, and prospect. World J. Gastroenterol. 2021, 27, 1864–1882. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Leiter, L.A.; Forst, T.; Polidori, D.; Balis, D.A.; Xie, J.; Sha, S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016, 42, 25–32. [Google Scholar] [CrossRef]
- Scheen, A.J. Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: A common comorbidity associated with severe complications. Diabetes Metab. 2019, 45, 213–223. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple Hits, Including Oxidative Stress, as Pathogenesis and Treatment Target in Non-Alcoholic Steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol.-Ren. Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef]
- Targher, G.; Zoppini, G.; Moghetti, P.; Day, C. Disorders of Coagulation and Hemostasis in Abdominal Obesity: Emerging Role of Fatty Liver. Semin. Thromb. Hemost. 2010, 36, 041–048. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Miele, L.; Zoppini, G.; Pichiri, I.; Muggeo, M. Nonalcoholic Fatty Liver Disease as a Contributor to Hypercoagulation and Thrombophilia in the Metabolic Syndrome. Semin. Thromb. Hemost. 2009, 35, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased Hepatic and Circulating Interleukin-6 Levels in Human Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Emerging risk factors and markers of chronic kidney disease progression. Nat. Rev. Nephrol. 2009, 5, 677–689. [Google Scholar] [CrossRef]
- Massy, Z.A.; Stenvinkel, P.; Drueke, T.B. PROGRESS IN UREMIC TOXIN RESEARCH: The Role of Oxidative Stress in Chronic Kidney Disease. Semin. Dial. 2009, 22, 405–408. [Google Scholar] [PubMed]
- Vlassara, H.; Torreggiani, M.; Post, J.B.; Zheng, F.; Uribarri, J.; Striker, G.E. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int. 2009, 76, S3–S11. [Google Scholar]
- Carrero, J.J.; Park, S.; Axelsson, J.; Lindholm, B.; Stenvinkel, P. PROGRESS IN UREMIC TOXIN RESEARCH: Cytokines, Atherogenesis, and Hypercatabolism in Chronic Kidney Disease: A Dreadful Triad. Semin. Dial. 2009, 22, 381–386. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of Non-alcoholic Fatty Liver Disease with Chronic Kidney Disease: A Systematic Review and Meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar]
- Kaps, L.; Labenz, C.; Galle, P.R.; Weinmann-Menke, J.; Kostev, K.; Schattenberg, J.M. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. UEG J. 2020, 8, 942–948. [Google Scholar] [CrossRef]
- Parvanova, A.; Abbate, M.; Yañez, A.M.; Bennasar-Veny, M.; López-González, Á.A.; Ramírez-Manent, J.I.; Iliev, I.P.; Fresneda, S.; Arias-Fernandez, M.; Remuzzi, G.; et al. MAFLD and glomerular hyperfiltration in subjects with prediabetes, visceral obesity and “preserved” kidney function: A cross-sectional study. Diabetes Res. Clin. Pract. 2023, 201, 110729. [Google Scholar] [PubMed]
- Nardolillo, M.; Rescigno, F.; Bartiromo, M.; Piatto, D.; Guarino, S.; Marzuillo, P.; Miraglia Del Giudice, E.; Di Sessa, A. Interplay between metabolic dysfunction-associated fatty liver disease and renal function: An intriguing pediatric perspective. World J. Gastroenterol. 2024, 30, 2081–2086. [Google Scholar] [PubMed]
- Cai, X.; Sun, L.; Liu, X.; Zhu, H.; Zhang, Y.; Zheng, S.; Huang, Y. Non-alcoholic fatty liver disease is associated with increased risk of chronic kidney disease. Ther. Adv. Chronic Dis. 2021, 12, 20406223211024360. [Google Scholar]
| Study | Study Type and Population | Findings | Comments |
|---|---|---|---|
| Park et al., 2019 [45] | Propensity-matched cohort study using a large U.S. insurance database (262,619 NAFLD vs. 769,878 non-NAFLD patients). | NAFLD was associated with a 41% increased risk of advanced CKD, independent of diabetes, hypertension, obesity, and cirrhosis. | This study provides strong epidemiological evidence that NAFLD independently increases CKD risk, suggesting the need for routine renal function screening. |
| Nah et al., 2022 [72] | Cross-sectional study using data from 13 health-promotion centers in Korea (8909 participants). | NAFLD prevalence was 47.6%, with 12.4% diagnosed with CKD. NAFLD was linked to early CKD but not advanced CKD after adjusting for metabolic factors. | Highlights the impact of NAFLD on early CKD but suggests that metabolic factors play a greater role in advanced CKD. |
| Mikolasevic et al., 2013 [46] | Fibroscan-based study on 62 CKD patients in Croatia assessing NAFLD prevalence and severity. | 85.5% of CKD patients had NAFLD. Liver steatosis severity correlated with serum creatinine and inversely with eGFR. | Demonstrates a strong NAFLD–CKD link using Fibroscan, supporting noninvasive liver assessment in CKD patients. |
| Tanaka et al., 2023 [73] | Prospective study on 13,159 Japanese adults followed for 10 years. | MAFLD was found to predict new-onset CKD better than NAFLD or simple fatty liver (FL). | This study highlights MAFLD as a stronger predictor of CKD than traditional NAFLD definitions, suggesting that metabolic dysfunction plays a crucial role. |
| Lau et al., 2010 [74] | Longitudinal study on 3191 individuals from Germany. | FL disease was significantly associated with hypertension and increased blood pressure over time. | Suggests a close link between liver fat accumulation and hypertension, supporting early intervention strategies. |
| Machado et al., 2011 [75] | Cross-sectional study on 148 morbidly obese patients from Lisbon, Portugal. | NASH was associated with lower eGFR, indicating renal impairment. Advanced fibrosis correlated with greater CKD risk. | Provides strong evidence for a direct liver–kidney connection, particularly in severe obesity and advanced liver disease. |
| Targher et al., 2008 [76] | Prospective study on 1760 type 2 diabetes patients for over 6.5 years. | NAFLD increased the risk of CKD by 49% even after adjusting for metabolic risk factors. | Reinforces the independent role of NAFLD in CKD development among diabetics. |
| Yilmaz et al., 2010 [77] | Study on 87 biopsy-confirmed NAFLD patients from Turkey. | Microalbuminuria was more prevalent in patients with higher liver fibrosis scores, linking NAFLD severity to kidney dysfunction. | Indicates that early renal damage in NAFLD may be identified through microalbuminuria, suggesting potential screening markers. |
| Söderberg et al., 2010 [78] | 28-year cohort study on 256 Swedish patients with elevated liver enzymes, focusing on NAFLD-related mortality. | NAFLD increased overall mortality by 69%, with NASH patients facing the highest risk. Liver disease was the third most common cause of death. | Highlights the long-term mortality impact of NAFLD, particularly NASH, reinforcing the need for monitoring. |
| Campos et al., 2008 [79] | Study on metabolic and inflammatory markers in patients with NAFLD and their potential link to CKD. | Metabolic dysfunction and inflammation in NAFLD patients were associated with kidney function decline. | Supports the role of metabolic and inflammatory pathways in linking NAFLD to CKD progression. |
| Triozzi et al., 2021 [80] | Retrospective cohort study on 1,155,901 CKD patients without NAFLD at baseline, followed from 2005 to 2016. | NAFLD incidence was higher in earlier CKD stages, associated with metabolic factors like BMI and diabetes. The use of ACE inhibitors was linked to a reduced likelihood of NAFLD, whereas statin therapy appeared to increase the risk. | Emphasizes the metabolic drivers of NAFLD in CKD and highlights ACE inhibitors’ potential protective role. |
| Adrian et al., 2022 [81] | Cross-sectional study evaluating hepatic liver fat content via CT in 291 CKD patients vs. 866 controls. | No significant association between CKD stage and moderate–severe hepatic steatosis. Diabetes and obesity were major risk factors. | Suggests CKD does not inherently increase NAFLD risk but shares common metabolic pathways. |
| Choe et al., 2020 [82] | Retrospective cross-sectional study on 819 CKD patients assessing NAFLD risk using noninvasive serum markers. | NAFLD was observed in 15.7% of CKD patients in the derivation group and 20.2% in the validation group. A predictive model using BMI, renal function, triglyceride-glucose index, serum ALT, and hemoglobin achieved an AUROC of 0.850. | This study provides a validated model for predicting NAFLD in CKD patients, emphasizing its comparable prevalence to the general population. |
| Hydes et al., 2023 [24] | Prospective cohort study using the UK Biobank on 18,073 CKD patients, evaluating NAFLD impact on adverse outcomes. | NAFLD was associated with increased risks of cardiovascular events (HR 1.20) and all-cause mortality (HR 1.31). Advanced liver fibrosis further elevated risks. | This large-scale study highlights NAFLD’s role in worsening CKD outcomes, reinforcing the need for early screening and intervention. |
| Roderburg et al., 2023 [83] | Retrospective study using the IQVIA database, analyzing 92,225 NAFLD and matched non-NAFLD patients over 10 years. | CKD incidence was significantly higher in NAFLD patients (19.1% vs. 11.1%). The risk was more pronounced in younger (18–50 years) and female patients. | This large real-world cohort confirms NAFLD as a strong independent risk factor for CKD, advocating for interdisciplinary care. |
| Jang et al., 2018 [84] | Cohort study of 1525 CKD patients investigating NAFLD’s impact on renal function decline. | NAFLD was linked to a faster decline in eGFR, with severity correlating with greater deterioration. Smoking and hypertension exacerbated this progression. | Provides strong evidence for NAFLD’s role in CKD progression, advocating for targeted interventions in high-risk groups. |
| Takahashi et al., 2021 [85] | 10-year longitudinal study on 14,163 healthy individuals assessing FL index as a predictor of CKD. | Higher FLI levels independently predicted CKD development, with the risk increasing across FLI tertiles. | Demonstrates the utility of FLI as a noninvasive predictor for CKD, emphasizing its clinical relevance in early risk assessment. |
| Kasem et al., 2023 [86] | Cross-sectional study on 430 Egyptian patients evaluating NAFLD and CKD associations. | CKD prevalence was significantly higher in NAFLD patients (38.1% vs. 7.4%), with hypertension and diabetes being major risk factors. | Confirms the bidirectional risk between NAFLD and CKD, underlining the need for integrated disease management strategies. |
| Category | Recommended Measures | Description |
|---|---|---|
| Screening and Monitoring | Serum Creatinine and Cystatin C | Assess kidney function and detect early renal impairment |
| eGFR | Evaluate kidney filtration capacity | |
| Urine Albumin-to-Creatinine Ratio and Protein Excretion | Detect early kidney damage and albuminuria | |
| Imaging: Renal Ultrasound and/or Computed Tomography | Assess structural kidney changes | |
| Blood Pressure Monitoring | Identify and manage hypertension, a key CKD risk factor | |
| Therapeutic Strategies | Lifestyle Modifications | Mediterranean diet, weight management, and regular physical activity |
| Optimizing Glycemic Control | Maintain blood glucose levels within target ranges to reduce renal stress | |
| Blood Pressure and Lipid Management | Control hypertension and dyslipidemia to slow CKD progression | |
| Pharmacologic Interventions | Use of evidence-based agents such as SGLT2 inhibitors, RAAS blockers, and GLP-1 receptor agonists | |
| Reducing Uric Acid Levels | Consider xanthine oxidase inhibitors in hyperuricemic patients | |
| Managing Gut Dysbiosis | Probiotics and dietary fiber to improve gut–liver–kidney axis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, R.G.; Boicean, A.; Anderco, P.; Ichim, C.; Iliescu-Glaja, M.; Todor, S.B.; Leonte, E.; Bloanca, V.A.; Crainiceanu, Z.P.; Popa, M.L. From Liver to Kidney: The Overlooked Burden of Nonalcoholic Fatty Liver Disease in Chronic Kidney Disease. J. Clin. Med. 2025, 14, 2486. https://doi.org/10.3390/jcm14072486
Bogdan RG, Boicean A, Anderco P, Ichim C, Iliescu-Glaja M, Todor SB, Leonte E, Bloanca VA, Crainiceanu ZP, Popa ML. From Liver to Kidney: The Overlooked Burden of Nonalcoholic Fatty Liver Disease in Chronic Kidney Disease. Journal of Clinical Medicine. 2025; 14(7):2486. https://doi.org/10.3390/jcm14072486
Chicago/Turabian StyleBogdan, Razvan George, Adrian Boicean, Paula Anderco, Cristian Ichim, Mihai Iliescu-Glaja, Samuel Bogdan Todor, Elisa Leonte, Vlad Adam Bloanca, Zorin Petrisor Crainiceanu, and Mirela Livia Popa. 2025. "From Liver to Kidney: The Overlooked Burden of Nonalcoholic Fatty Liver Disease in Chronic Kidney Disease" Journal of Clinical Medicine 14, no. 7: 2486. https://doi.org/10.3390/jcm14072486
APA StyleBogdan, R. G., Boicean, A., Anderco, P., Ichim, C., Iliescu-Glaja, M., Todor, S. B., Leonte, E., Bloanca, V. A., Crainiceanu, Z. P., & Popa, M. L. (2025). From Liver to Kidney: The Overlooked Burden of Nonalcoholic Fatty Liver Disease in Chronic Kidney Disease. Journal of Clinical Medicine, 14(7), 2486. https://doi.org/10.3390/jcm14072486





