Overcoming Barriers in Neurosurgical Education: Introducing a Simulator for Insular Glioma Resection with Fluorescence Imaging (SIGMA)
Abstract
1. Introduction
2. Materials and Methods
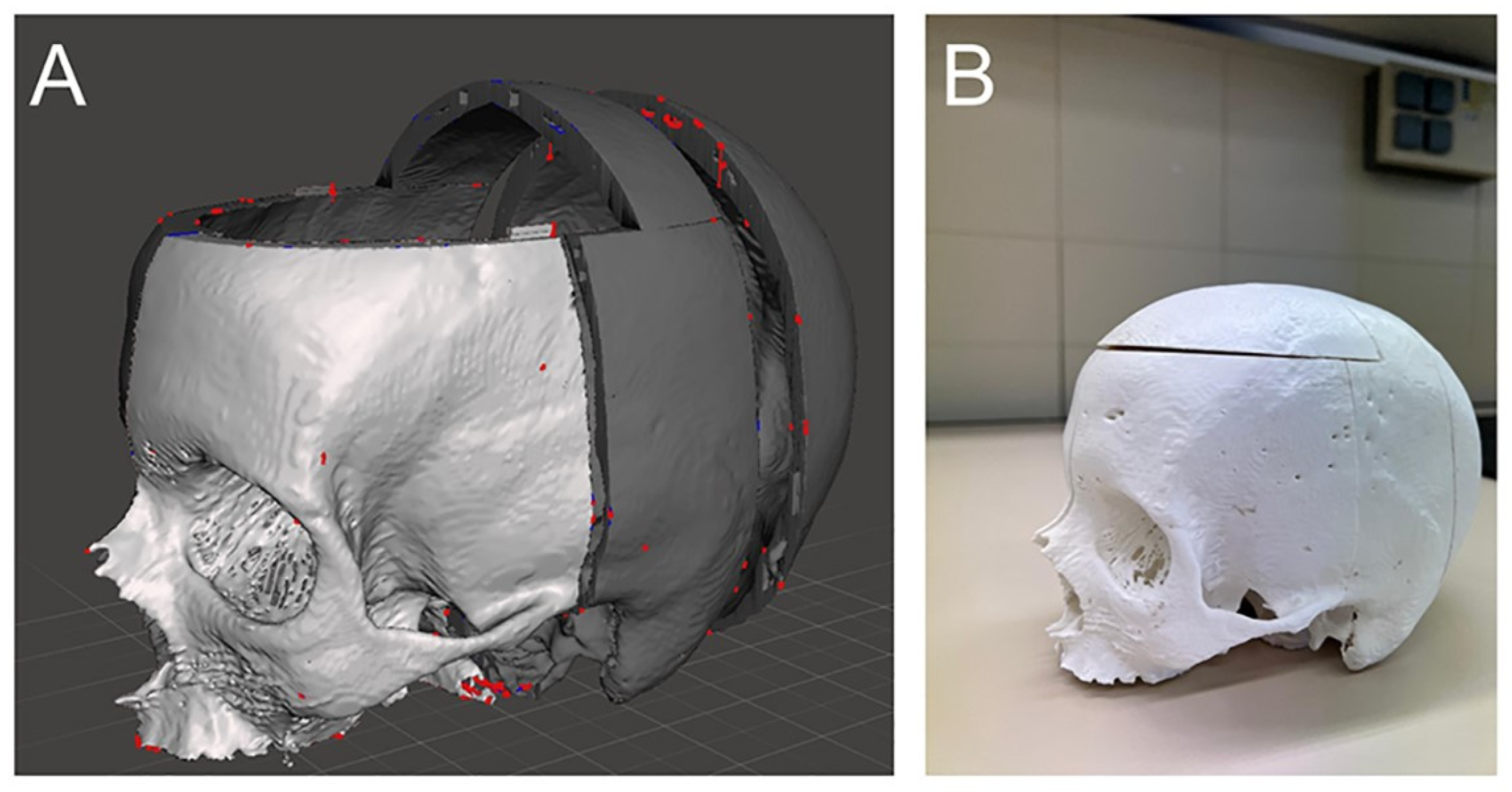
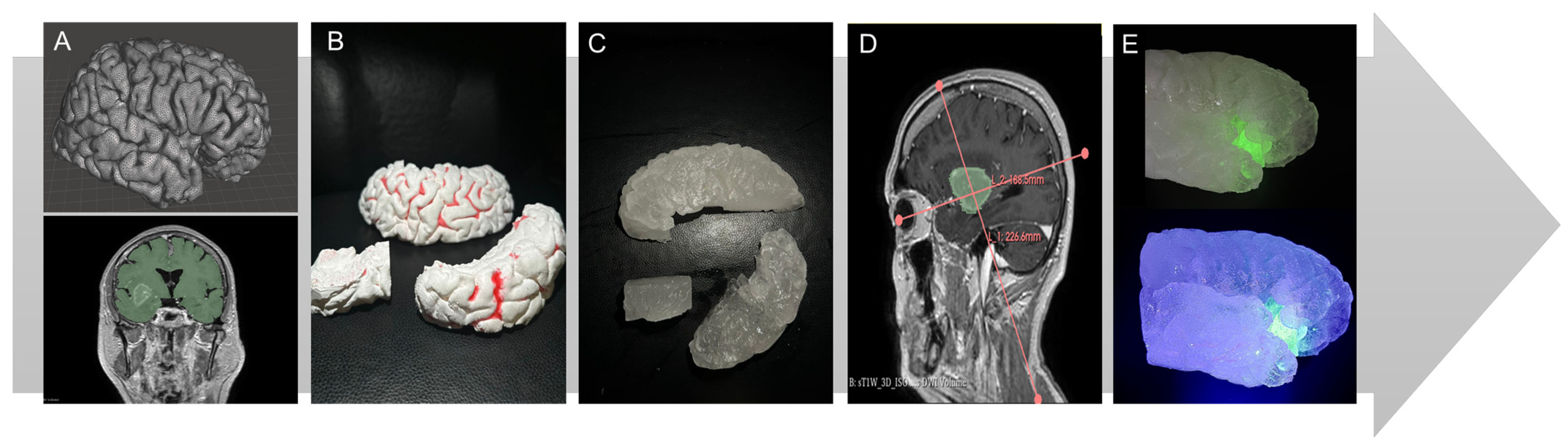
2.1. Sample Preparation and Evaluation
2.1.1. Hydrogel Preparation
PVA
Candle Gel
Gelatin
Agar
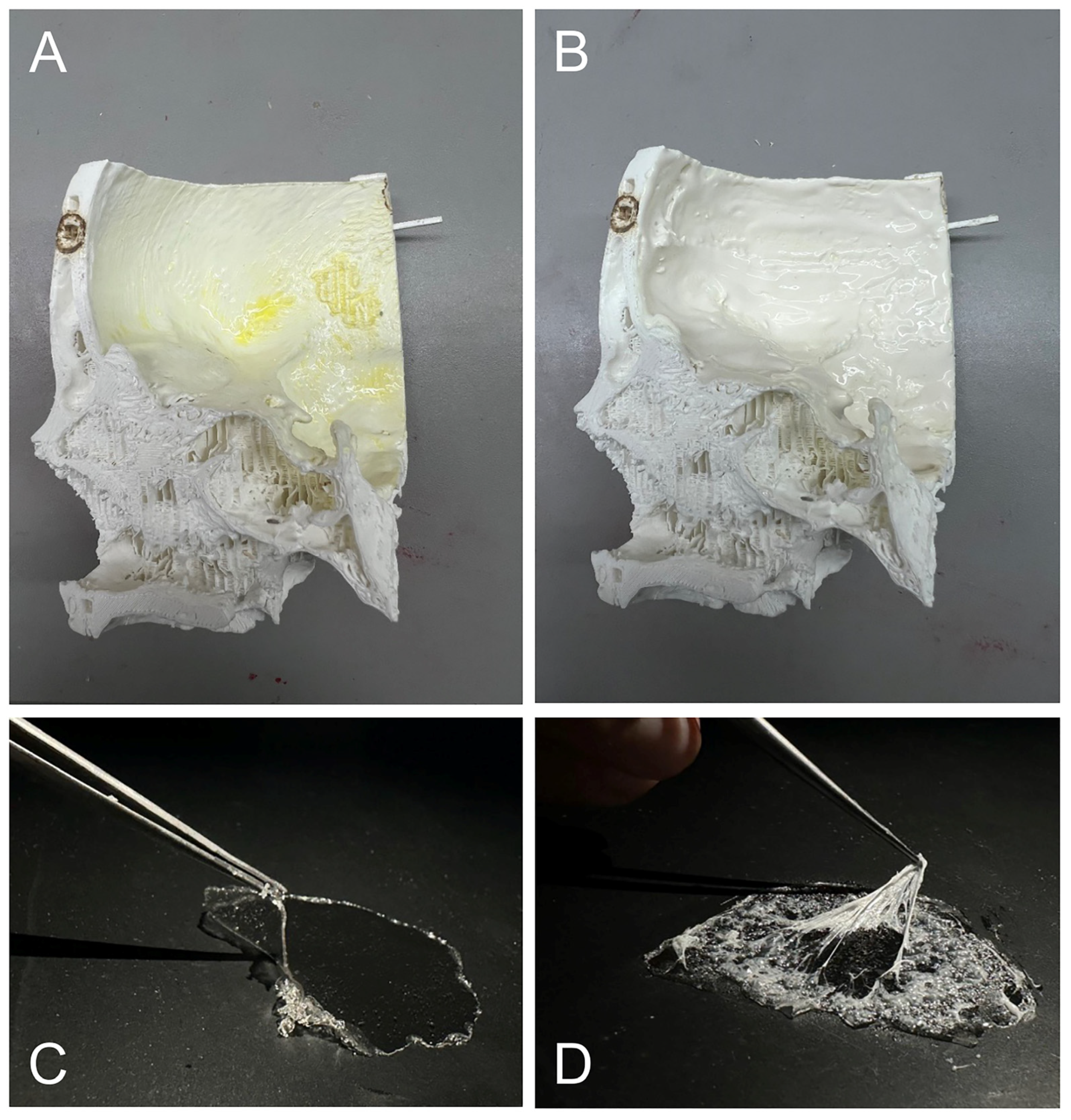
2.1.2. Subjective Evaluation of the Material Samples
2.1.3. Objective Evaluation of the Materials
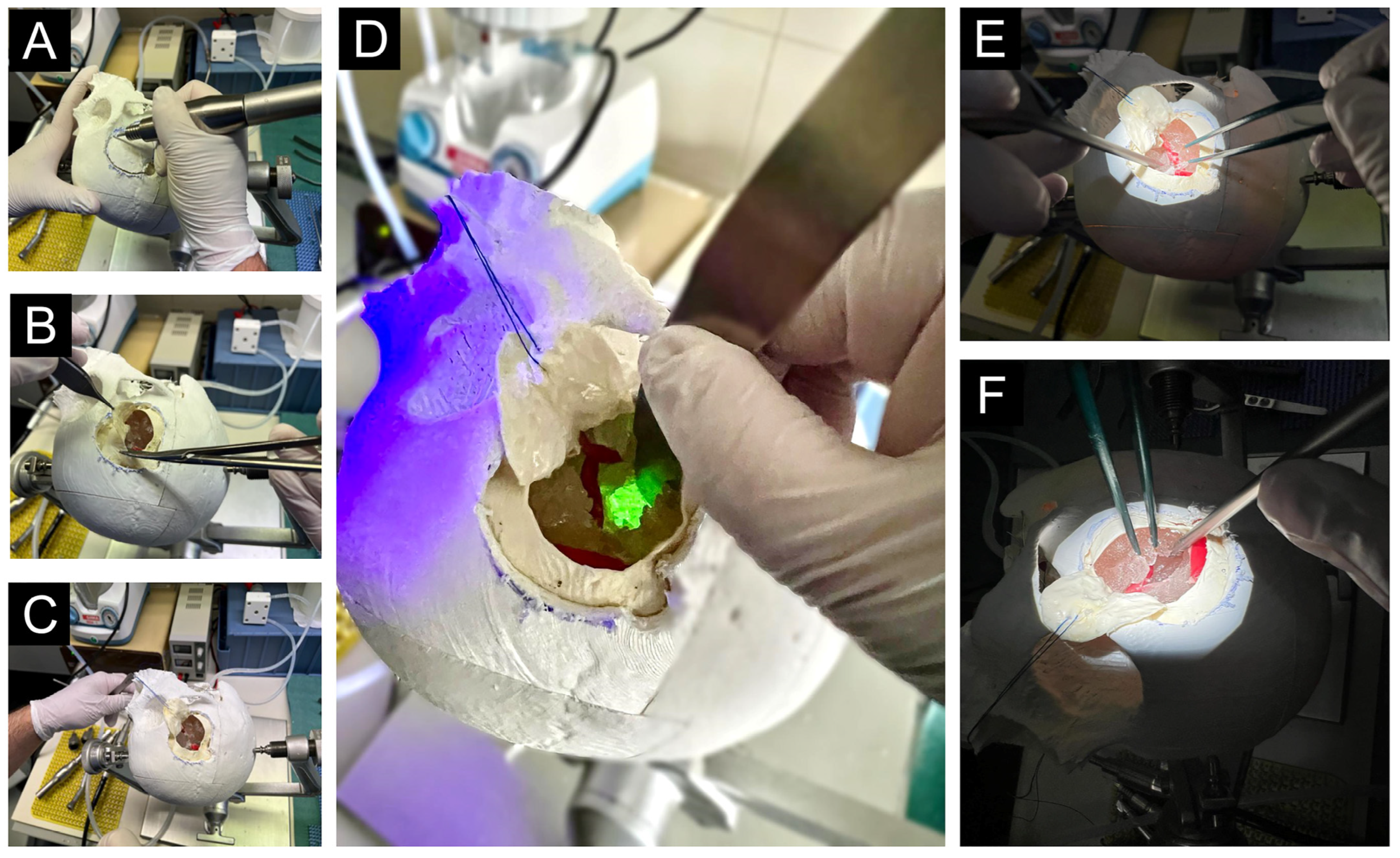
2.2. Construction of the Simulator and Simulation
2.2.1. Simulation Process
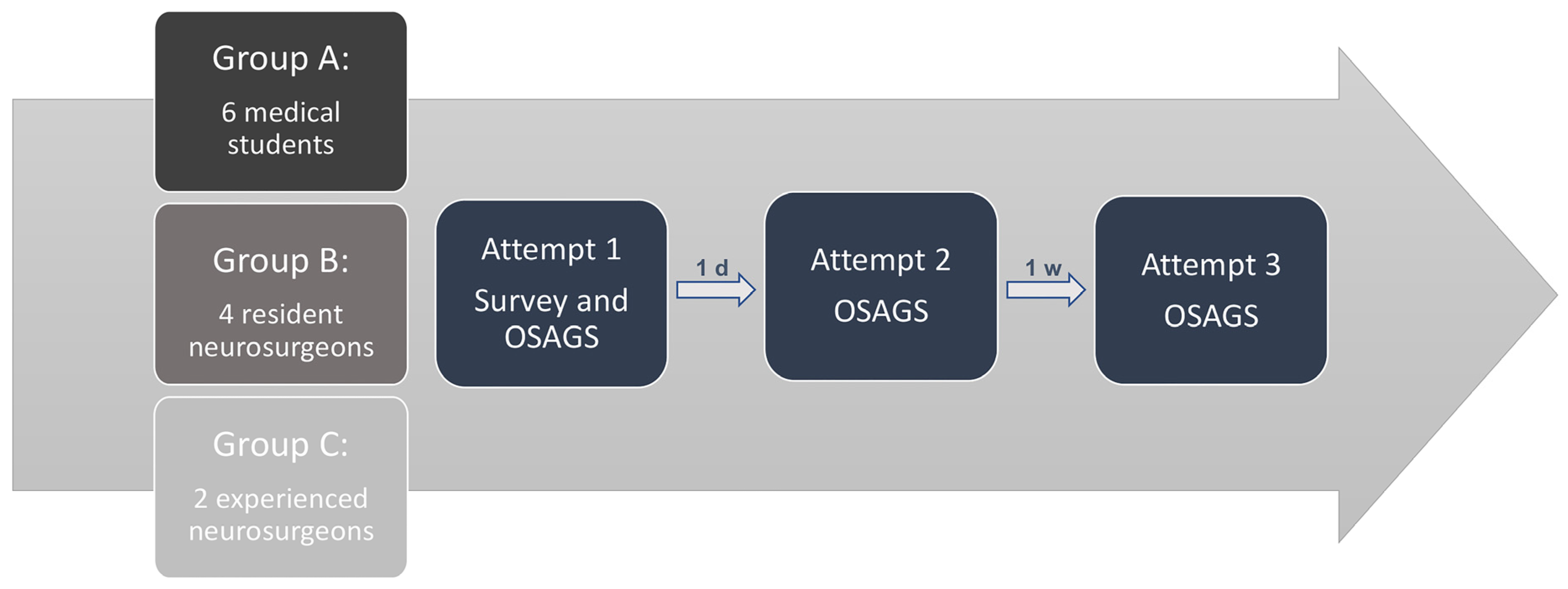
2.2.2. Subjective and Objective Evaluation
3. Results
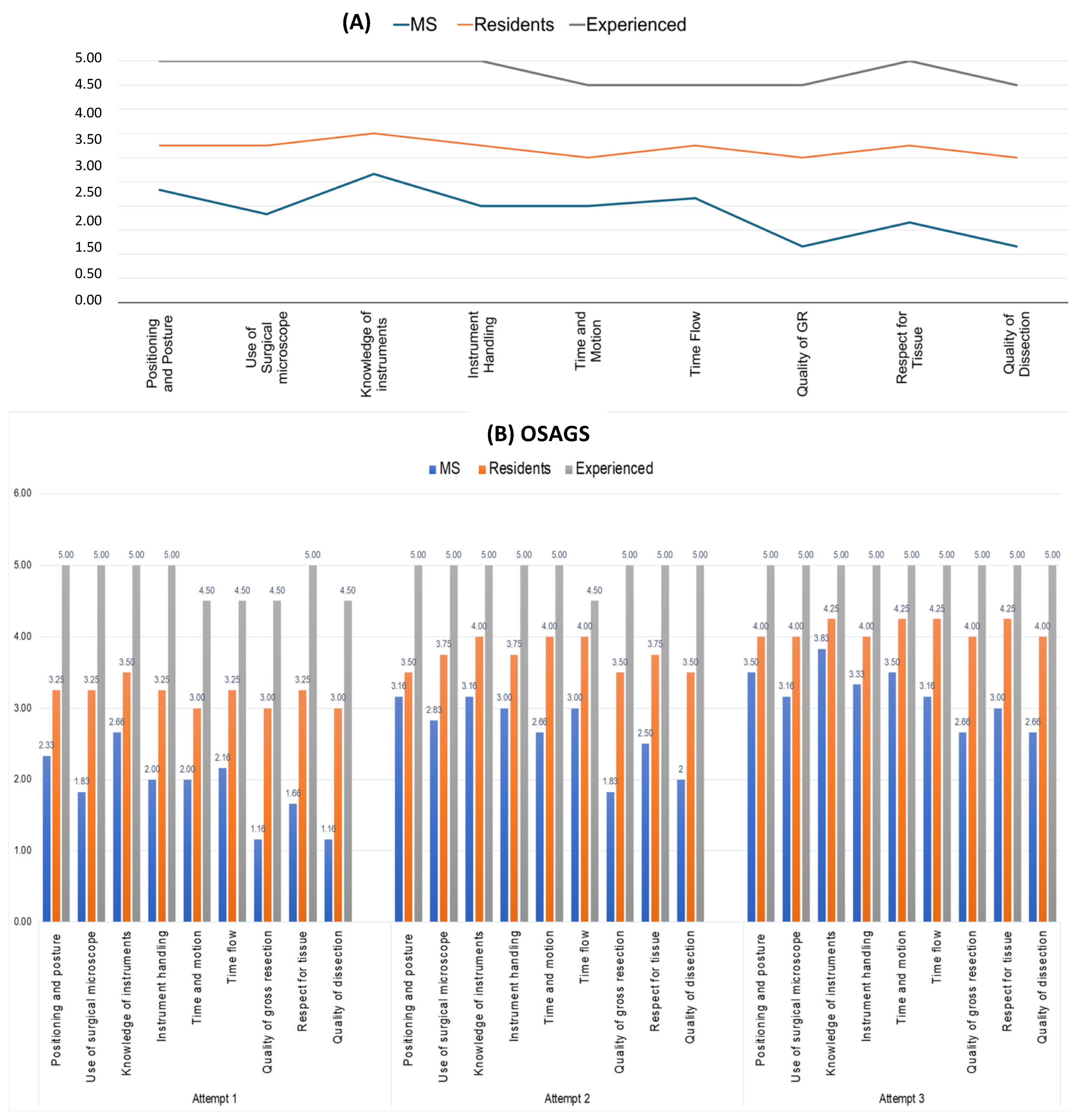
3.1. Face and Content Validity
3.2. Construct Validity
3.3. Cost Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevulinic acid |
| PVA | polyvinyl alcohol |
| PHY | polyhydroxybutyrat |
| PLA | polylactic acid |
| MCA | middle cerebral artery |
| OSAGS | Objective Structured Assessment of Glioma Surgery |
References
- Przybylowski, C.J.; Hervey-Jumper, S.L.; Sanai, N. Surgical Strategy for Insular Glioma. J. Neurooncol. 2021, 151, 491–497. [Google Scholar] [CrossRef]
- Benet, A.; Hervey-Jumper, S.L.; Sánchez, J.J.G.; Lawton, M.T.; Berger, M.S. Surgical Assessment of the Insula. Part 1: Surgical Anatomy and Morphometric Analysis of the Transsylvian and Transcortical Approaches to the Insula. J. Neurosurg. 2016, 124, 469–481. [Google Scholar] [CrossRef]
- Safaee, M.M.; Englot, D.J.; Han, S.J.; Lawton, M.T.; Berger, M.S. The Transsylvian Approach for Resection of Insular Gliomas: Technical Nuances of Splitting the Sylvian Fissure. J. Neurooncol. 2016, 130, 283–287. [Google Scholar] [CrossRef]
- Barbosa, B.J.A.P.; Dimostheni, A.; Teixeira, M.J.; Tatagiba, M.; Lepski, G. Insular Gliomas and the Role of Intraoperative Assistive Technologies: Results from a Volumetry-Based Retrospective Cohort. Clin. Neurol. Neurosurg. 2016, 149, 104–110. [Google Scholar] [CrossRef]
- Mussi, E.; Mussa, F.; Santarelli, C.; Scagnet, M.; Uccheddu, F.; Furferi, R.; Volpe, Y.; Genitori, L. Current Practice in Preoperative Virtual and Physical Simulation in Neurosurgery. Bioengineering 2020, 7, 7. [Google Scholar] [CrossRef]
- Forte, A.E.; Galvan, S.; Manieri, F.; Rodriguez Y Baena, F.; Dini, D. A Composite Hydrogel for Brain Tissue Phantoms. Mater. Des. 2016, 112, 227–238. [Google Scholar] [CrossRef]
- Leibinger, A.; Forte, A.E.; Tan, Z.; Oldfield, M.J.; Beyrau, F.; Dini, D.; Rodriguez Y Baena, F. Erratum to: Soft Tissue Phantoms for Realistic Needle Insertion: A Comparative Study. Ann. Biomed. Eng. 2016, 44, 3750. [Google Scholar] [CrossRef]
- Li, W.; Shepherd, D.E.T.; Espino, D.M. Investigation of the Compressive Viscoelastic Properties of Brain Tissue Under Time and Frequency Dependent Loading Conditions. Ann. Biomed. Eng. 2021, 49, 3737–3747. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Fenollosa-Artés, F.; Achaerandio, I.; Rey-Vinolas, S.; Buj-Corral, I.; Mateos-Timoneda, M.Á.; Engel, E. Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms. Gels 2022, 8, 40. [Google Scholar] [CrossRef]
- Amini, A.; Zeller, Y.; Stein, K.-P.; Hartmann, K.; Wartmann, T.; Wex, C.; Mirzaee, E.; Swiatek, V.M.; Saalfeld, S.; Haghikia, A.; et al. Overcoming Barriers in Neurosurgical Education: A Novel Approach to Practical Ventriculostomy Simulation. Oper. Neurosurg. 2022, 23, 225–234. [Google Scholar] [CrossRef]
- Fromageau, J.; Gennisson, J.-L.; Schmitt, C.; Maurice, R.L.; Mongrain, R.; Cloutier, G. Estimation of Polyvinyl Alcohol Cryogel Mechanical Properties with Four Ultrasound Elastography Methods and Comparison with Gold Standard Testings. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2007, 54, 498–509. [Google Scholar] [CrossRef]
- Madsen, E.L.; Hobson, M.A.; Shi, H.; Varghese, T.; Frank, G.R. Tissue-Mimicking Agar/Gelatin Materials for Use in Heterogeneous Elastography Phantoms. Phys. Med. Biol. 2005, 50, 5597–5618. [Google Scholar] [CrossRef]
- Taghizadeh, S.; Labuda, C.; Mobley, J. Development of a Tissue-Mimicking Phantom of the Brain for Ultrasonic Studies. Ultrasound Med. Biol. 2018, 44, 2813–2820. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.M.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging. 2012, 30, 1323–1341. [Google Scholar]
- Bunevicius, A.; Schregel, K.; Sinkus, R.; Golby, A.; Patz, S. REVIEW: MR Elastography of Brain Tumors. NeuroImage Clin. 2020, 25, 102109. [Google Scholar] [CrossRef]
- Amini, A.; Allgaier, M.; Saalfeld, S.; Stein, K.-P.; Rashidi, A.; Swiatek, V.M.; Sandalcioglu, I.E.; Neyazi, B. Virtual Reality vs Phantom Model: Benefits and Drawbacks of Simulation Training in Neurosurgery. Oper. Neurosurg. 2024, 27, 618–631. [Google Scholar] [CrossRef]
- Reiser, J.; Amini, A.; Swiatek, V. How Good Is Neurosurgical Training?—Validation of a Perfused Microsurgical Aneurysm Training Simulator (MATS) Using a Modified OSAACS Score and Indocyanine-Green Angiography. Oper. Neurosurg. 2025. [Google Scholar] [CrossRef]
- Belykh, E.; Miller, E.J.; Lei, T.; Chapple, K.; Byvaltsev, V.A.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Face, Content, and Construct Validity of an Aneurysm Clipping Model Using Human Placenta. World Neurosurg. 2017, 105, 952–960.e2. [Google Scholar] [CrossRef]
- Cameron, J.L. William Stewart Halsted. Our Surgical Heritage. Ann. Surg. 1997, 225, 445–458. [Google Scholar] [CrossRef]
- Rolston, J.D.; Zygourakis, C.C.; Han, S.J.; Lau, C.Y.; Berger, M.S.; Parsa, A.T. Medical Errors in Neurosurgery. Surg. Neurol. Int. 2014, 5, S435–S440. [Google Scholar] [CrossRef]
- Rolston, J.D.; Bernstein, M. Errors in Neurosurgery. Neurosurg. Clin. N. Am. 2015, 26, 149–155, vii. [Google Scholar] [CrossRef]
- Stone, S.; Bernstein, M. Prospective Error Recording in Surgery: An Analysis of 1108 Elective Neurosurgical Cases. Neurosurgery 2007, 60, 1075–1080; discussion 1080–1082. [Google Scholar] [CrossRef]
- Benet, A.; Plata-Bello, J.; Abla, A.A.; Acevedo-Bolton, G.; Saloner, D.; Lawton, M.T. Implantation of 3D-Printed Patient-Specific Aneurysm Models into Cadaveric Specimens: A New Training Paradigm to Allow for Improvements in Cerebrovascular Surgery and Research. Biomed. Res. Int. 2015, 2015, 939387. [Google Scholar] [CrossRef]
- Budday, S.; Sommer, G.; Haybaeck, J.; Steinmann, P.; Holzapfel, G.A.; Kuhl, E. Rheological Characterization of Human Brain Tissue. Acta Biomater. 2017, 60, 315–329. [Google Scholar] [CrossRef]
- Thiong’o, G.M.; Bernstein, M.; Drake, J.M. 3D Printing in Neurosurgery Education: A Review. 3D Print. Med. 2021, 7, 9. [Google Scholar] [CrossRef]
- Thawani, J.P.; Singh, N.; Pisapia, J.M.; Abdullah, K.G.; Parker, D.; Pukenas, B.A.; Zager, E.L.; Verma, R.; Brem, S. Three-Dimensional Printed Modeling of Diffuse Low-Grade Gliomas and Associated White Matter Tract Anatomy. Neurosurgery 2017, 80, 635–645. [Google Scholar] [CrossRef]
- Mashiko, T.; Oguma, H.; Konno, T.; Gomi, A.; Yamaguchi, T.; Nagayama, R.; Sato, M.; Iwase, R.; Kawai, K. Training of Intra-Axial Brain Tumor Resection Using a Self-Made Simple Device with Agar and Gelatin. World Neurosurg. 2018, 109, e298–e304. [Google Scholar] [CrossRef]
- Mashiko, T.; Konno, T.; Kaneko, N.; Watanabe, E. Training in Brain Retraction Using a Self-Made Three-Dimensional Model. World Neurosurg. 2015, 84, 585–590. [Google Scholar] [CrossRef]
- Regelsberger, J.; Eicker, S.; Siasios, I.; Hänggi, D.; Kirsch, M.; Horn, P.; Winkler, P.; Signoretti, S.; Fountas, K.; Dufour, H.; et al. In Vivo Porcine Training Model for Cranial Neurosurgery. Neurosurg. Rev. 2015, 38, 157–163. [Google Scholar] [CrossRef]
- Valli, D.; Belykh, E.; Zhao, X.; Gandhi, S.; Cavallo, C.; Martirosyan, N.L.; Nakaji, P.; Lawton, M.T.; Preul, M.C. Development of a Simulation Model for Fluorescence-Guided Brain Tumor Surgery. Front. Oncol. 2019, 9, 748. [Google Scholar] [CrossRef]
- Kamp, M.A.; Knipps, J.; Steiger, H.-J.; Rapp, M.; Cornelius, J.F.; Folke-Sabel, S.; Sabel, M. Training for Brain Tumour Resection: A Realistic Model with Easy Accessibility. Acta Neurochir. 2015, 157, 1975–1981. [Google Scholar] [CrossRef] [PubMed]




| Material | Brand Name/Source | Concentrations of the Hydrogels (in %) |
|---|---|---|
| Polyvinyl alcohol–BF-04 (PVA) | COLLTEC© GmbH & Co. KG data (Bielefeld, Germany) | 20 |
| 22.5 | ||
| 25 | ||
| 27.5 | ||
| 30 | ||
| Gel wax/Candle gel 1 kg colorless (Candle gel) | KerzenKiste (Bullenkuhlen, Germany) | 100 |
| Gelatin Bloom 260 (Gelatin) | Local pig slaughterhouse | 7.5 |
| 12.5 | ||
| 17.5 | ||
| Agar-Agar (Agar) | Mr. P Ingredients (North Newbald, England) | 2 |
| 4 | ||
| 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamid, S.; Swiatek, V.M.; Reiser, J.; Taskaya, F.; Amini, A.; Stein, K.-P.; Rashidi, A.; Sandalcioglu, I.E.; Neyazi, B. Overcoming Barriers in Neurosurgical Education: Introducing a Simulator for Insular Glioma Resection with Fluorescence Imaging (SIGMA). J. Clin. Med. 2025, 14, 2479. https://doi.org/10.3390/jcm14072479
Al-Hamid S, Swiatek VM, Reiser J, Taskaya F, Amini A, Stein K-P, Rashidi A, Sandalcioglu IE, Neyazi B. Overcoming Barriers in Neurosurgical Education: Introducing a Simulator for Insular Glioma Resection with Fluorescence Imaging (SIGMA). Journal of Clinical Medicine. 2025; 14(7):2479. https://doi.org/10.3390/jcm14072479
Chicago/Turabian StyleAl-Hamid, Sifian, Vanessa Magdalena Swiatek, Julius Reiser, Firat Taskaya, Amir Amini, Klaus-Peter Stein, Ali Rashidi, I. Erol Sandalcioglu, and Belal Neyazi. 2025. "Overcoming Barriers in Neurosurgical Education: Introducing a Simulator for Insular Glioma Resection with Fluorescence Imaging (SIGMA)" Journal of Clinical Medicine 14, no. 7: 2479. https://doi.org/10.3390/jcm14072479
APA StyleAl-Hamid, S., Swiatek, V. M., Reiser, J., Taskaya, F., Amini, A., Stein, K.-P., Rashidi, A., Sandalcioglu, I. E., & Neyazi, B. (2025). Overcoming Barriers in Neurosurgical Education: Introducing a Simulator for Insular Glioma Resection with Fluorescence Imaging (SIGMA). Journal of Clinical Medicine, 14(7), 2479. https://doi.org/10.3390/jcm14072479







