Current Trends and Future Challenges in Transcatheter Aortic Valve Replacement: Utility of Cardiac Computed Tomography Angiography
Abstract
1. Introduction
2. Aortic Valve Calcium Score
3. Aortic Valve Assessment
4. Aortic Root Assessment
Aortic Annulus Assessment
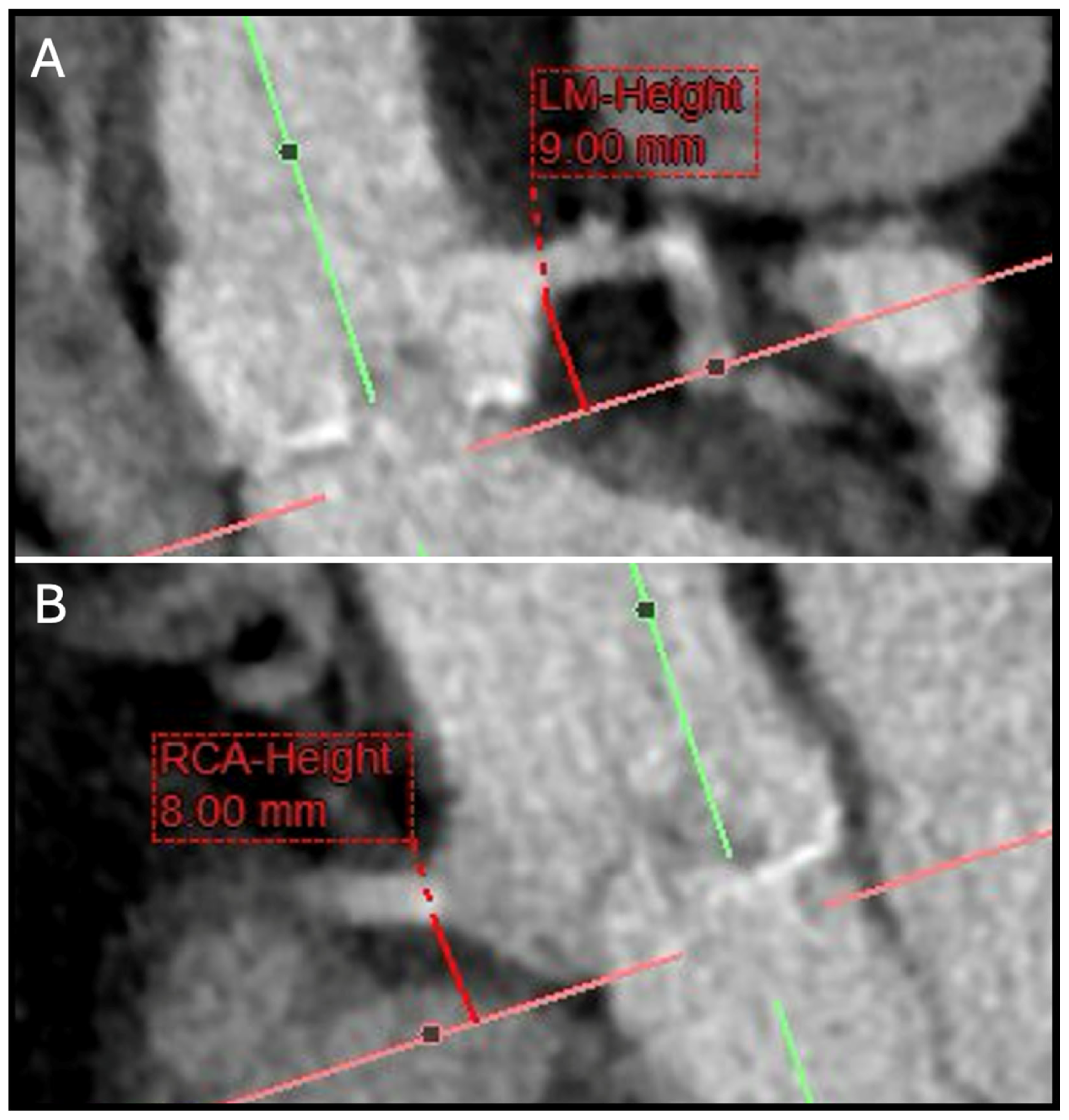
5. Coronary Ostial Height and Sinus of Valsalva Assessment
6. CT Fluoroscopic Angulation
7. Conduction Abnormalities
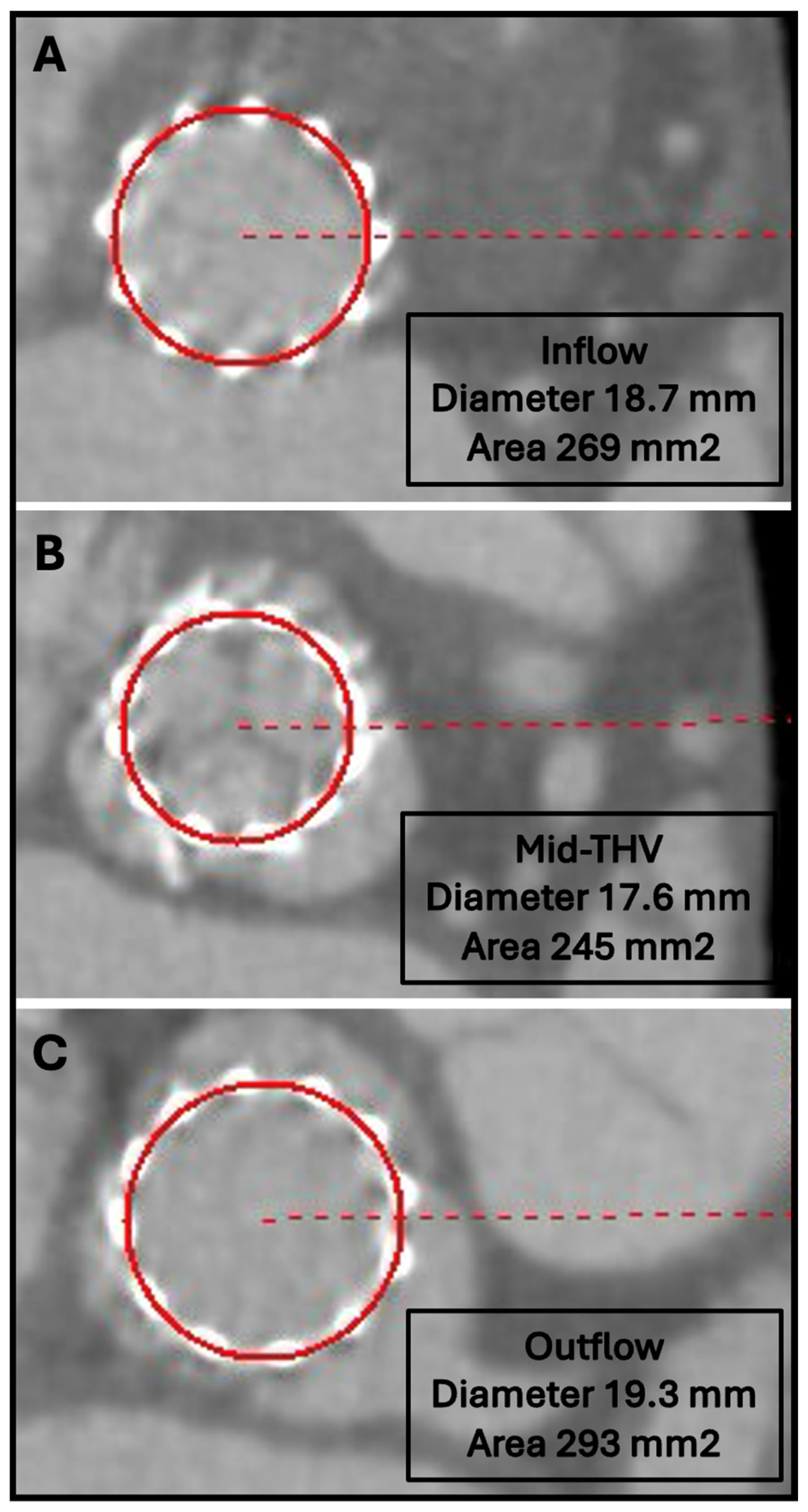
8. THV Sizing
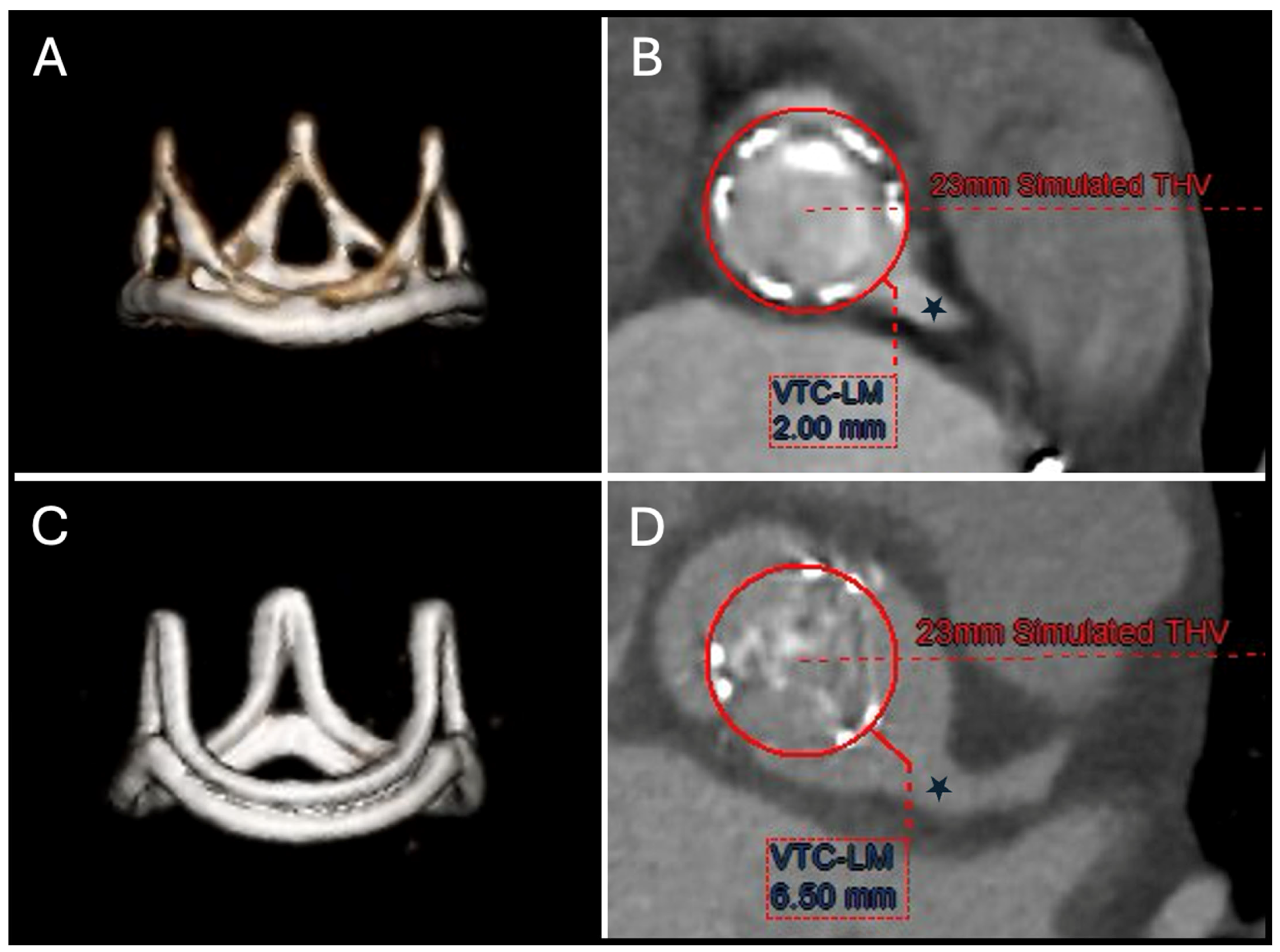
9. Valve-in-Valve TAVR
9.1. TAVR in SHV
9.2. TAVR in TAVR
10. Vascular Access
11. Post-TAVR CT Assessment
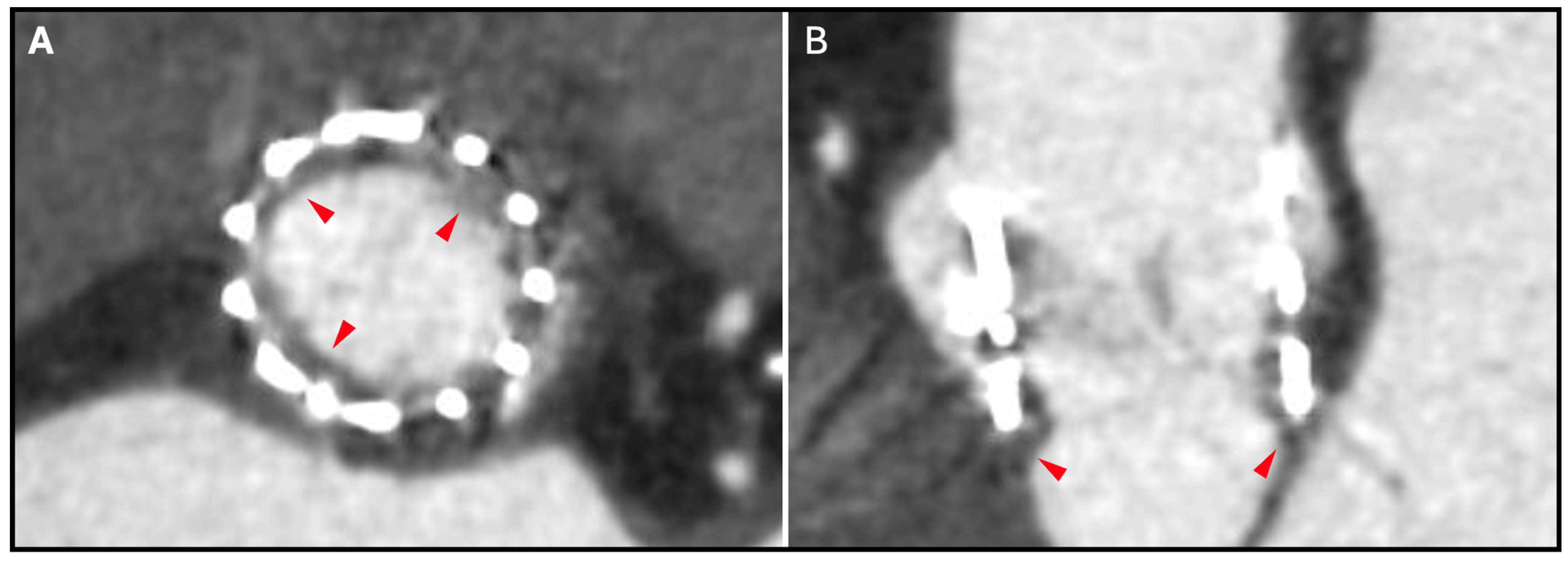
12. Hypoattenuating Leaflet Thickening
13. THV Underexpansion
14. Structural Valve Degeneration
15. Patient–Prosthesis Mismatch
16. Coronary Access
17. Limitations
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CCTA | cardiac computed tomography angiography |
| HALT | hypoattenuating leaflet thickening |
| SHV | surgical heart valve |
| TAVR | transcatheter aortic valve replacement |
| THV | transcatheter heart valve |
| ViV | valve-in-valve |
| VTC | virtual THV-to-coronary distance |
| VTSTJ | virtual THV-to-sinotubular junction distance |
References
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Clavel, M.A.; Pibarot, P.; Messika-Zeitoun, D.; Capoulade, R.; Malouf, J.; Aggarval, S.; Araoz, P.A.; Michelena, H.I.; Cueff, C.; Larose, E.; et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J. Am. Coll. Cardiol. 2014, 64, 1202–1213. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Aggarwal, S.R.; Clavel, M.A.; Messika-Zeitoun, D.; Cueff, C.; Malouf, J.; Araoz, P.A.; Mankad, R.; Michelena, H.; Vahanian, A.; Enriquez-Sarano, M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ. Cardiovasc. Imaging 2013, 6, 40–47. [Google Scholar] [CrossRef]
- Pawade, T.; Sheth, T.; Guzzetti, E.; Dweck, M.R.; Clavel, M.A. Why and How to Measure Aortic Valve Calcification in Patients With Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 1835–1848. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, W.K.; Dhoble, A.; Milhorini Pio, S.; Babaliaros, V.; Jilaihawi, H.; Pilgrim, T.; De Backer, O.; Bleiziffer, S.; Vincent, F.; et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 1018–1030. [Google Scholar] [CrossRef]
- Buellesfeld, L.; Stortecky, S.; Heg, D.; Gloekler, S.; Meier, B.; Wenaweser, P.; Windecker, S. Extent and distribution of calcification of both the aortic annulus and the left ventricular outflow tract predict aortic regurgitation after transcatheter aortic valve replacement. EuroIntervention 2014, 10, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Jilaihawi, H.; Chen, M.; Webb, J.; Himbert, D.; Ruiz, C.E.; Rodes-Cabau, J.; Pache, G.; Colombo, A.; Nickenig, G.; Lee, M.; et al. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC Cardiovasc. Imaging 2016, 9, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Bleiziffer, S.; De Backer, O.; Delgado, V.; Arai, T.; Ziegelmueller, J.; Barbanti, M.; Sharma, R.; Perlman, G.Y.; Khalique, O.K.; et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2017, 69, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Tchetche, D.; de Biase, C.; van Gils, L.; Parma, R.; Ochala, A.; Lefevre, T.; Hovasse, T.; De Backer, O.; Sondergaard, L.; Bleiziffer, S.; et al. Bicuspid Aortic Valve Anatomy and Relationship With Devices: The BAVARD Multicenter Registry. Circ. Cardiovasc. Interv. 2019, 12, e007107. [Google Scholar] [CrossRef]
- Murphy, D.T.; Blanke, P.; Alaamri, S.; Naoum, C.; Rubinshtein, R.; Pache, G.; Precious, B.; Berger, A.; Raju, R.; Dvir, D.; et al. Dynamism of the aortic annulus: Effect of diastolic versus systolic CT annular measurements on device selection in transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 2016, 10, 37–43. [Google Scholar] [CrossRef]
- Sucha, D.; Tuncay, V.; Prakken, N.H.; Leiner, T.; van Ooijen, P.M.; Oudkerk, M.; Budde, R.P. Does the aortic annulus undergo conformational change throughout the cardiac cycle? A systematic review. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1307–1317. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Norgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Ewe, S.H.; Ng, A.C.; Schuijf, J.D.; van der Kley, F.; Colli, A.; Palmen, M.; de Weger, A.; Marsan, N.A.; Holman, E.R.; de Roos, A.; et al. Location and severity of aortic valve calcium and implications for aortic regurgitation after transcatheter aortic valve implantation. Am. J. Cardiol. 2011, 108, 1470–1477. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Nombela-Franco, L.; Urena, M.; Mok, M.; Pasian, S.; Doyle, D.; DeLarochelliere, R.; Cote, M.; Laflamme, L.; DeLarochelliere, H.; et al. Coronary obstruction following transcatheter aortic valve implantation: A systematic review. JACC Cardiovasc. Interv. 2013, 6, 452–461. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Webb, J.G.; Makkar, R.R.; Cohen, M.G.; Kapadia, S.R.; Kodali, S.; Tamburino, C.; Barbanti, M.; Chakravarty, T.; Jilaihawi, H.; et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: Insights from a large multicenter registry. J. Am. Coll. Cardiol. 2013, 62, 1552–1562. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Rodes-Cabau, J.; Blanke, P.; Leipsic, J.; Kwan Park, J.; Bapat, V.; Makkar, R.; Simonato, M.; Barbanti, M.; Schofer, J.; et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Insights from the VIVID registry. Eur. Heart J. 2018, 39, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Kamioka, N.; Lisko, J.C.; Perdoncin, E.; Zhang, C.; Maini, A.; Chen, M.; Li, Y.; Ludwig, S.; Westermann, D.; et al. Coronary Obstruction From TAVR in Native Aortic Stenosis: Development and Validation of Multivariate Prediction Model. JACC Cardiovasc. Interv. 2023, 16, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shoshan, J.; Alosaimi, H.; Lauzier, P.T.; Pighi, M.; Talmor-Barkan, Y.; Overtchouk, P.; Martucci, G.; Spaziano, M.; Finkelstein, A.; Gada, H.; et al. Double S-Curve Versus Cusp-Overlap Technique: Defining the Optimal Fluoroscopic Projection for TAVR With a Self-Expanding Device. JACC Cardiovasc. Interv. 2021, 14, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Blanke, P.; Nestelberger, T.; Alosail, A.; Chatfield, A.G.; Chuang, M.A.; Leipsic, J.A.; Tzimas, G.; Lounes, Y.; Meier, D.; et al. Hybrid Approach Using the Cusp-Overlap Technique for Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve. JACC Cardiovasc. Interv. 2022, 15, 2387–2395. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Zaid, S.; Michev, I.; Ahmad, H.; Kaple, R.; Undemir, C.; Cohen, M.; Lansman, S.L. “Cusp-Overlap” View Simplifies Fluoroscopy-Guided Implantation of Self-Expanding Valve in Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 1663–1665. [Google Scholar] [CrossRef]
- Maeno, Y.; Abramowitz, Y.; Kawamori, H.; Kazuno, Y.; Kubo, S.; Takahashi, N.; Mangat, G.; Okuyama, K.; Kashif, M.; Chakravarty, T.; et al. A Highly Predictive Risk Model for Pacemaker Implantation After TAVR. JACC Cardiovasc. Imaging 2017, 10, 1139–1147. [Google Scholar] [CrossRef]
- Fujita, B.; Kutting, M.; Seiffert, M.; Scholtz, S.; Egron, S.; Prashovikj, E.; Borgermann, J.; Schafer, T.; Scholtz, W.; Preuss, R.; et al. Calcium distribution patterns of the aortic valve as a risk factor for the need of permanent pacemaker implantation after transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1385–1393. [Google Scholar] [CrossRef]
- Franzoni, I.; Latib, A.; Maisano, F.; Costopoulos, C.; Testa, L.; Figini, F.; Giannini, F.; Basavarajaiah, S.; Mussardo, M.; Slavich, M.; et al. Comparison of incidence and predictors of left bundle branch block after transcatheter aortic valve implantation using the CoreValve versus the Edwards valve. Am. J. Cardiol. 2013, 112, 554–559. [Google Scholar] [CrossRef]
- Urena, M.; Mok, M.; Serra, V.; Dumont, E.; Nombela-Franco, L.; DeLarochelliere, R.; Doyle, D.; Igual, A.; Larose, E.; Amat-Santos, I.; et al. Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon-expandable valve. J. Am. Coll. Cardiol. 2012, 60, 1743–1752. [Google Scholar] [CrossRef]
- Sinning, J.M.; Petronio, A.S.; Van Mieghem, N.; Zucchelli, G.; Nickenig, G.; Bekeredjian, R.; Bosmans, J.; Bedogni, F.; Branny, M.; Stangl, K.; et al. Relation Between Clinical Best Practices and 6-Month Outcomes After Transcatheter Aortic Valve Implantation With CoreValve (from the ADVANCE II Study). Am. J. Cardiol. 2017, 119, 84–90. [Google Scholar] [CrossRef]
- Gurvitch, R.; Webb, J.G.; Yuan, R.; Johnson, M.; Hague, C.; Willson, A.B.; Toggweiler, S.; Wood, D.A.; Ye, J.; Moss, R.; et al. Aortic annulus diameter determination by multidetector computed tomography: Reproducibility, applicability, and implications for transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2011, 4, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Willson, A.B.; Webb, J.G.; Achenbach, S.; Piazza, N.; Min, J.K.; Pache, G.; Leipsic, J. Oversizing in transcatheter aortic valve replacement, a commonly used term but a poorly understood one: Dependency on definition and geometrical measurements. J. Cardiovasc. Comput. Tomogr. 2014, 8, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Yang, T.H.; Rodes Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013, 128, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Hansson, N.C.; Norgaard, B.L.; Barbanti, M.; Nielsen, N.E.; Yang, T.H.; Tamburino, C.; Dvir, D.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; et al. The impact of calcium volume and distribution in aortic root injury related to balloon-expandable transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2015, 9, 382–392. [Google Scholar] [CrossRef]
- Sucha, D.; Daans, C.G.; Symersky, P.; Planken, R.N.; Mali, W.P.; van Herwerden, L.A.; Budde, R.P. Reliability, Agreement, and Presentation of a Reference Standard for Assessing Implanted Heart Valve Sizes by Multidetector-Row Computed Tomography. Am. J. Cardiol. 2015, 116, 112–120. [Google Scholar] [CrossRef]
- Anastasius, M.; Godoy, M.; Weir-McCall, J.R.; Bapat, V.; Sathananthan, J.; Hensey, M.; Sellers, S.L.; Cheung, A.; Ye, J.; Wood, D.A.; et al. Reference dimensions of stented surgical aortic bioprostheses for valve size determination. EuroIntervention 2020, 16, e502–e506. [Google Scholar] [CrossRef]
- Bapat, V.N.; Attia, R.; Thomas, M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: A concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc. Interv. 2014, 7, 115–127. [Google Scholar] [CrossRef]
- Khan, J.M.; Babaliaros, V.C.; Greenbaum, A.B.; Spies, C.; Daniels, D.; Depta, J.P.; Oldemeyer, J.B.; Whisenant, B.; McCabe, J.M.; Muhammad, K.I.; et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: Results From the Multicenter International BASILICA Registry. JACC Cardiovasc. Interv. 2021, 14, 941–948. [Google Scholar] [CrossRef]
- Ochiai, T.; Oakley, L.; Sekhon, N.; Komatsu, I.; Flint, N.; Kaewkes, D.; Yoon, S.H.; Raschpichler, M.; Patel, V.; Tiwana, R.; et al. Risk of Coronary Obstruction Due to Sinus Sequestration in Redo Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 2617–2627. [Google Scholar] [CrossRef]
- Jabbour, R.J.; Tanaka, A.; Finkelstein, A.; Mack, M.; Tamburino, C.; Van Mieghem, N.; de Backer, O.; Testa, L.; Gatto, P.; Purita, P.; et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1513–1524. [Google Scholar] [CrossRef]
- Tzimas, G.; Akodad, M.; Meier, D.; Duchscherer, J.; Kalk, K.; Everett, R.J.; Haidari, O.; Chuang, M.A.; Sellers, S.L.; Dvir, D.; et al. Predicted vs Observed Valve to Coronary Distance in Valve-in-Valve TAVR: A Computed Tomography Study. JACC Cardiovasc. Interv. 2023, 16, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Heitkemper, M.; Hatoum, H.; Azimian, A.; Yeats, B.; Dollery, J.; Whitson, B.; Rushing, G.; Crestanello, J.; Lilly, S.M.; Dasi, L.P. Modeling risk of coronary obstruction during transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2020, 159, 829–838.e3. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Akodad, M.; Landes, U.; Barlow, A.M.; Chatfield, A.G.; Lai, A.; Tzimas, G.; Tang, G.H.L.; Puehler, T.; Lutter, G.; et al. Coronary Access Following Redo TAVR: Impact of THV Design, Implant Technique, and Cell Misalignment. JACC Cardiovasc. Interv. 2022, 15, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Sellers, S.; Gulsin, G.S.; Tzimas, G.; Landes, U.; Chatfield, A.G.; Chuang, A.; Meier, D.; Leipsic, J.; Blanke, P.; et al. Leaflet and Neoskirt Height in Transcatheter Heart Valves: Implications for Repeat Procedures and Coronary Access. JACC Cardiovasc. Interv. 2021, 14, 2298–2300. [Google Scholar] [CrossRef]
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and avoidance. Radiographics 2004, 24, 1679–1691. [Google Scholar] [CrossRef]
- Bonnet, M.; Maxo, L.; Lohse, T.; Mangin, L.; Courand, P.Y.; Ricard, C.; Bouali, A.; Boussel, L.; Aktaa, S.; Ali, N.; et al. Association Between Aortic Wall Thrombus and Thromboembolic Events After Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2024, 17, 1680–1690. [Google Scholar] [CrossRef]
- Sawaya, F.J.; Bajoras, V.; Vanhaverbeke, M.; Wang, C.; Bieliauskas, G.; Sondergaard, L.; De Backer, O. Intravascular Lithotripsy-Assisted Transfemoral TAVI: The Copenhagen Experience and Literature Review. Front. Cardiovasc. Med. 2021, 8, 739750. [Google Scholar] [CrossRef]
- Abellan, C.; Antiochos, P.; Fournier, S.; Skali, H.; Shah, P.; Maurizi, N.; Eeckhout, E.; Roguelov, C.; Monney, P.; Tzimas, G.; et al. Extrathoracic Against Intrathoracic Vascular Accesses for Transcatheter Aortic Valve Replacement: A Systematic Review With Meta-Analysis. Am. J. Cardiol. 2023, 203, 473–483. [Google Scholar] [CrossRef]
- Wee, I.J.Y.; Stonier, T.; Harrison, M.; Choong, A. Transcarotid transcatheter aortic valve implantation: A systematic review. J. Cardiol. 2018, 71, 525–533. [Google Scholar] [CrossRef]
- Salihu, A.; Ferlay, C.; Kirsch, M.; Shah, P.B.; Skali, H.; Fournier, S.; Meier, D.; Muller, O.; Hugelshofer, S.; Skalidis, I.; et al. Outcomes and Safety of Transcaval Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2024, 40, 2054–2062. [Google Scholar] [CrossRef]
- Antiochos, P.; Kirsch, M.; Monney, P.; Tzimas, G.; Meier, D.; Fournier, S.; Ferlay, C.; Nowacka, A.; Rancati, V.; Abellan, C.; et al. Transcaval versus Supra-Aortic Vascular Accesses for Transcatheter Aortic Valve Replacement: A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 455. [Google Scholar] [CrossRef]
- Makkar, R.R.; Blanke, P.; Leipsic, J.; Thourani, V.; Chakravarty, T.; Brown, D.; Trento, A.; Guyton, R.; Babaliaros, V.; Williams, M.; et al. Subclinical Leaflet Thrombosis in Transcatheter and Surgical Bioprosthetic Valves: PARTNER 3 Cardiac Computed Tomography Substudy. J. Am. Coll. Cardiol. 2020, 75, 3003–3015. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Leipsic, J.A.; Popma, J.J.; Yakubov, S.J.; Deeb, G.M.; Gada, H.; Mumtaz, M.; Ramlawi, B.; Kleiman, N.S.; Sorajja, P.; et al. Bioprosthetic Aortic Valve Leaflet Thickening in the Evolut Low Risk Sub-Study. J. Am. Coll. Cardiol. 2020, 75, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, T.; Sondergaard, L.; Friedman, J.; De Backer, O.; Berman, D.; Kofoed, K.F.; Jilaihawi, H.; Shiota, T.; Abramowitz, Y.; Jorgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef]
- Hansson, N.C.; Grove, E.L.; Andersen, H.R.; Leipsic, J.; Mathiassen, O.N.; Jensen, J.M.; Jensen, K.T.; Blanke, P.; Leetmaa, T.; Tang, M.; et al. Transcatheter Aortic Valve Thrombosis: Incidence, Predisposing Factors, and Clinical Implications. J. Am. Coll. Cardiol. 2016, 68, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Fukui, M.; Dworak, M.W.; Okeson, B.K.; Garberich, R.; Hashimoto, G.; Sato, H.; Cavalcante, J.L.; Bapat, V.N.; Lesser, J.; et al. Clinical Impact of Hypoattenuating Leaflet Thickening After Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2022, 15, e011480. [Google Scholar] [CrossRef]
- Fukui, M.; Bapat, V.N.; Garcia, S.; Dworak, M.W.; Hashimoto, G.; Sato, H.; Gossl, M.; Enriquez-Sarano, M.; Lesser, J.R.; Cavalcante, J.L.; et al. Deformation of Transcatheter Aortic Valve Prostheses: Implications for Hypoattenuating Leaflet Thickening and Clinical Outcomes. Circulation 2022, 146, 480–493. [Google Scholar] [CrossRef]
- Collet, J.P.; Van Belle, E.; Thiele, H.; Berti, S.; Lhermusier, T.; Manigold, T.; Neumann, F.J.; Gilard, M.; Attias, D.; Beygui, F.; et al. Apixaban vs. standard of care after transcatheter aortic valve implantation: The ATLANTIS trial. Eur. Heart J. 2022, 43, 2783–2797. [Google Scholar] [CrossRef]
- De Backer, O.; Dangas, G.D.; Jilaihawi, H.; Leipsic, J.A.; Terkelsen, C.J.; Makkar, R.; Kini, A.S.; Veien, K.T.; Abdel-Wahab, M.; Kim, W.K.; et al. Reduced Leaflet Motion after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 130–139. [Google Scholar] [CrossRef]
- Dangas, G.D.; Tijssen, J.G.P.; Wohrle, J.; Sondergaard, L.; Gilard, M.; Mollmann, H.; Makkar, R.R.; Herrmann, H.C.; Giustino, G.; Baldus, S.; et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 120–129. [Google Scholar] [CrossRef]
- Khodaee, F.; Barakat, M.; Abbasi, M.; Dvir, D.; Azadani, A.N. Incomplete expansion of transcatheter aortic valves is associated with propensity for valve thrombosis. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Sun, W. Transcatheter Valve Underexpansion Limits Leaflet Durability: Implications for Valve-in-Valve Procedures. Ann. Biomed. Eng. 2017, 45, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; De Backer, O.; Brooks, M.; de Knegt, M.C.; Bieliauskas, G.; Yamamoto, M.; Yanagisawa, R.; Hayashida, K.; Sondergaard, L.; Kofoed, K.F. Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention 2017, 13, e1067–e1075. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Blanke, P.; Chuang, M.A.; Duchscherer, J.; Sellers, S.L.; Chatfield, A.G.; Gulsin, G.G.; Lauck, S.; Leipsic, J.A.; Meier, D.; et al. Late Balloon Valvuloplasty for Transcatheter Heart Valve Dysfunction. J. Am. Coll. Cardiol. 2022, 79, 1340–1351. [Google Scholar] [CrossRef]
- Teshima, H.; Hayashida, N.; Fukunaga, S.; Tayama, E.; Kawara, T.; Aoyagi, S.; Uchida, M. Usefulness of a multidetector-row computed tomography scanner for detecting pannus formation. Ann. Thorac. Surg. 2004, 77, 523–526. [Google Scholar] [CrossRef]
- Rogers, T.; Greenspun, B.C.; Weissman, G.; Torguson, R.; Craig, P.; Shults, C.; Gordon, P.; Ehsan, A.; Wilson, S.R.; Goncalves, J.; et al. Feasibility of Coronary Access and Aortic Valve Reintervention in Low-Risk TAVR Patients. JACC Cardiovasc. Interv. 2020, 13, 726–735. [Google Scholar] [CrossRef]
- Ochiai, T.; Chakravarty, T.; Yoon, S.H.; Kaewkes, D.; Flint, N.; Patel, V.; Mahani, S.; Tiwana, R.; Sekhon, N.; Nakamura, M.; et al. Coronary Access After TAVR. JACC Cardiovasc. Interv. 2020, 13, 693–705. [Google Scholar] [CrossRef]
- Abdelghani, M.; Landt, M.; Traboulsi, H.; Becker, B.; Richardt, G. Coronary Access After TAVR With a Self-Expanding Bioprosthesis: Insights From Computed Tomography. JACC Cardiovasc. Interv. 2020, 13, 709–722. [Google Scholar] [CrossRef]
- Nai Fovino, L.; Scotti, A.; Massussi, M.; Fabris, T.; Cardaioli, F.; Rodino, G.; Matsuda, Y.; Frigo, F.; Fraccaro, C.; Tarantini, G. Incidence and feasibility of coronary access after transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2020, 96, E535–E541. [Google Scholar] [CrossRef]
- Barbanti, M.; Costa, G.; Picci, A.; Criscione, E.; Reddavid, C.; Valvo, R.; Todaro, D.; Deste, W.; Condorelli, A.; Scalia, M.; et al. Coronary Cannulation After Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc. Interv. 2020, 13, 2542–2555. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Amat-Santos, I.J.; De Backer, O.; Avvedimento, M.; Redondo, A.; Barbanti, M.; Costa, G.; Tchetche, D.; Eltchaninoff, H.; Kim, W.K.; et al. Rationale, Definitions, Techniques, and Outcomes of Commissural Alignment in TAVR: From the ALIGN-TAVR Consortium. JACC Cardiovasc. Interv. 2022, 15, 1497–1518. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzimas, G.; Meier, D.; Beneki, E.; Antiochos, P.; Auberson, D.; Leboube, S.; Lu, H.; Liabot, Q.; Zimmerli, A.; Salihu, A.; et al. Current Trends and Future Challenges in Transcatheter Aortic Valve Replacement: Utility of Cardiac Computed Tomography Angiography. J. Clin. Med. 2025, 14, 2474. https://doi.org/10.3390/jcm14072474
Tzimas G, Meier D, Beneki E, Antiochos P, Auberson D, Leboube S, Lu H, Liabot Q, Zimmerli A, Salihu A, et al. Current Trends and Future Challenges in Transcatheter Aortic Valve Replacement: Utility of Cardiac Computed Tomography Angiography. Journal of Clinical Medicine. 2025; 14(7):2474. https://doi.org/10.3390/jcm14072474
Chicago/Turabian StyleTzimas, Georgios, David Meier, Eirini Beneki, Panagiotis Antiochos, Denise Auberson, Simon Leboube, Henri Lu, Quentin Liabot, Aurelia Zimmerli, Adil Salihu, and et al. 2025. "Current Trends and Future Challenges in Transcatheter Aortic Valve Replacement: Utility of Cardiac Computed Tomography Angiography" Journal of Clinical Medicine 14, no. 7: 2474. https://doi.org/10.3390/jcm14072474
APA StyleTzimas, G., Meier, D., Beneki, E., Antiochos, P., Auberson, D., Leboube, S., Lu, H., Liabot, Q., Zimmerli, A., Salihu, A., Monney, P., Muller, O., Akodad, M., & Fournier, S. (2025). Current Trends and Future Challenges in Transcatheter Aortic Valve Replacement: Utility of Cardiac Computed Tomography Angiography. Journal of Clinical Medicine, 14(7), 2474. https://doi.org/10.3390/jcm14072474




