The Impact of Intraoperative Respiratory Patterns on Morbidity and Mortality in Patients with COPD Undergoing Elective Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Selection Criteria
2.3. Data Extraction
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Key Findings
4.2. Relationship with Previous Studies
4.3. Significance of Study Findings
4.4. Strengths and Limitations
4.5. Future Studies and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 January 2025).
- Wang, H.; Ye, X.; Zhang, Y.; Ling, S. Global, Regional, and National Burden of Chronic Obstructive Pulmonary Disease from 1990 to 2019. Front. Physiol. 2022, 13, 925132. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Gao, T.-Y.; Fang, W.; Xian-Yu, C.-Y.; Deng, N.-J.; Zhang, C.; Niu, Y.-M. Global, Regional and National Burden of Chronic Obstructive Pulmonary Disease over a 30-Year Period: Estimates from the 1990 to 2019 Global Burden of Disease Study. Respirology 2023, 28, 29–36. [Google Scholar] [CrossRef] [PubMed]
- AL Wachami, N.; Guennouni, M.; Iderdar, Y.; Boumendil, K.; Arraji, M.; Mourajid, Y.; Bouchachi, F.Z.; Barkaoui, M.; Louerdi, M.L.; Hilali, A.; et al. Estimating the Global Prevalence of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review and Meta-Analysis. BMC Public Health 2024, 24, 297. [Google Scholar] [CrossRef]
- Gupta, H.; Ramanan, B.; Gupta, P.K.; Fang, X.; Polich, A.; Modrykamien, A.; Schuller, D.; Morrow, L.E. Impact of COPD on Postoperative Outcomes: Results from a National Database. Chest 2013, 143, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, C.R.; Tee, V.S.; Carson-Chahhoud, K.V.; Picot, J.; Wedzicha, J.A.; Smith, B.J. Non-Invasive Ventilation for the Management of Acute Hypercapnic Respiratory Failure Due to Exacerbation of Chronic Obstructive Pulmonary Disease. Cochrane Database Syst. Rev. 2017, 7, CD004104. [Google Scholar] [CrossRef] [PubMed]
- Khrapov, K.N.; Kovalev, M.G.; Sedov, S.S. Preparation for Anesthesia of Patients with Concomitant Lung Pathology and a High Risk of Developing Postoperative Pulmonary Complications. Messenger Anesthesiol. Resusc. 2020, 17, 20–28. [Google Scholar] [CrossRef]
- Zabolotskikh, I.B.; Gritsan, A.I.; Kirov, M.Y.; Kuzovlev, A.N.; Lebedinskii, K.M.; Mazurok, V.A.; Protsenko, D.N.; Trembach, N.V.; Shadrin, R.V.; Yaroshetskiy, A.I. Perioperative Management of Patients with Respiratory Failure: Methodological Recommendations of the All-Russian Public Organization “Federation of Anesthesiologists and Reanimatologists”. Ann. Crit. Care 2022, 28, 7–23. [Google Scholar] [CrossRef]
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.J.; Johnson, A.E.W.; Raffa, J.D.; Celi, L.A.; Mark, R.G.; Badawi, O. The EICU Collaborative Research Database, a Freely Available Multi-Center Database for Critical Care Research. Sci. Data 2018, 5, 180178. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69. [Google Scholar] [PubMed]
- Sikora, J.P.; Karawani, J.; Sobczak, J. Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS). Int. J. Mol. Sci. 2023, 24, 13469. [Google Scholar] [CrossRef] [PubMed]
- Kriplani, A.; Pandit, S.; Chawla, A.; de la Rosette, J.J.M.C.H.; Laguna, P.; Jayadeva Reddy, S.; Somani, B.K. Neutrophil-Lymphocyte Ratio (NLR), Platelet-Lymphocyte Ratio (PLR) and Lymphocyte-Monocyte Ratio (LMR) in Predicting Systemic Inflammatory Response Syndrome (SIRS) and Sepsis after Percutaneous Nephrolithotomy (PNL). Urolithiasis 2022, 50, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Doğan, N.Ö.; Özturan, İ.U.; Pekdemir, M.; Yaka, E.; Yılmaz, S. Prognostic Value of Early Warning Scores in Patients Presenting to the Emergency Department with Exacerbation of COPD. Med. Klin. Intensivmed. Notfallmedizin 2024, 119, 129–135. [Google Scholar] [CrossRef]
- Futier, E.; Constantin, J.-M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.-Y.; et al. A Trial of Intraoperative Low-Tidal-Volume Ventilation in Abdominal Surgery. N. Engl. J. Med. 2013, 369, 428–437. [Google Scholar] [CrossRef] [PubMed]
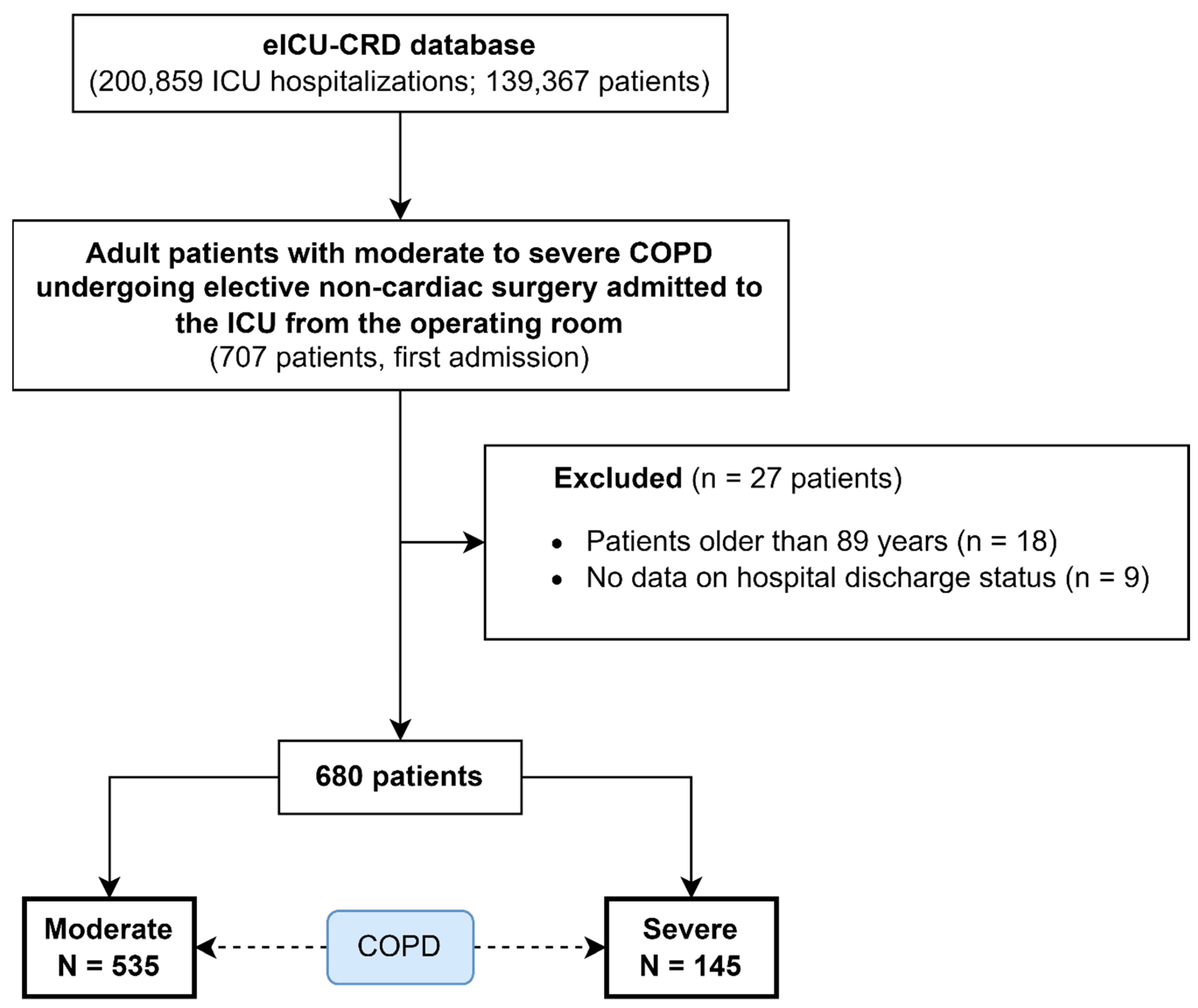
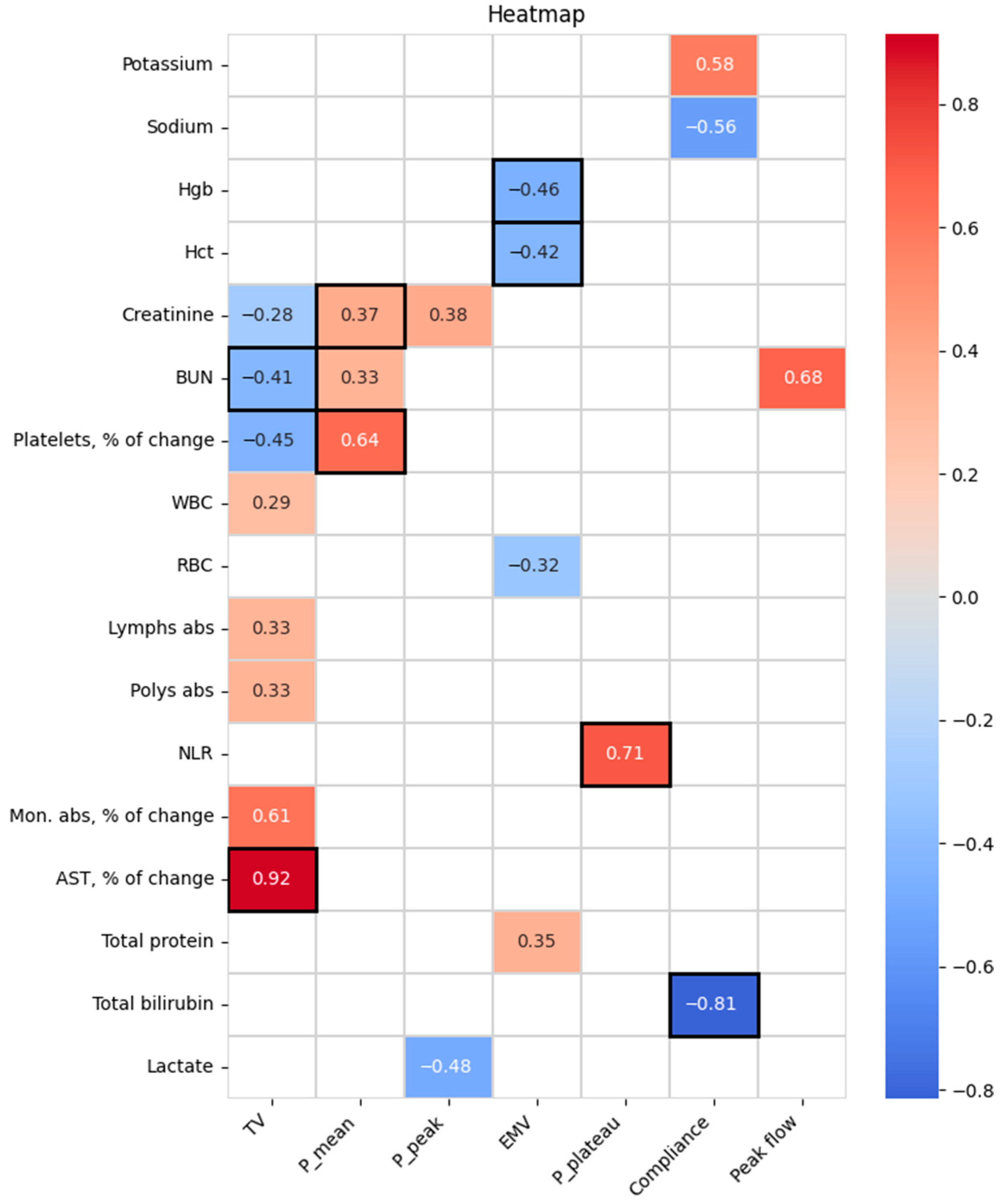
| Parameters | All Patients, N = 680 | Moderate COPD, N = 535 | Severe COPD, N = 145 | p-Value | |
|---|---|---|---|---|---|
| Sex | Male | 361; 53% | 274; 51% | 87; 60% | 0.060 1 |
| Female | 319; 47% | 261; 49% | 58; 40% | ||
| Age, years | 69 (61; 76) | 69 (61; 77) | 69 (61; 75) | 0.704 2 | |
| BMI, kg/m2 | N = 660; 26.6 (22.5; 32.5) | N = 521; 26.7 (22.7; 32.3) | N = 139; 26.5 (21.7; 33.2) | 0.816 2 | |
| APACHE IV, score | N = 623; 51 (39; 67) | N = 494; 50 (38; 65) | N = 129; 53 (39.5; 70) | 0.186 2 | |
| Surgery types | |||||
| Abdominal surgery | 226; 33% | 173; 32% | 53; 37% | 0.339 1 | |
| Cesarean section | 1; 0.1% | 1; 0.2% | 0; 0% | >0.9 3 | |
| Thrombectomy under general anesthesia | 9; 1.3% | 5; 0.9% | 4; 2.8% | 0.103 3 | |
| Head and neck surgery | 19; 3% | 18; 3% | 1; 0.7% | >0.9 3 | |
| Mastectomy | 2; 0.3% | 2; 0.4% | 0; 0% | >0.9 3 | |
| Neurological surgery | 48; 7% | 41; 8% | 7; 4.8% | 0.237 1 | |
| Orthopedic surgery | 18; 3% | 15; 3% | 3; 2.1% | 0.777 3 | |
| Skin surgery | 14; 2% | 11; 2% | 3; 2.1% | >0.9 3 | |
| Thoracic surgery | 149; 22% | 106; 20% | 43; 30% | 0.011 1 | |
| Trauma surgery | 23; 3% | 19; 4% | 4; 2.8% | 0.799 3 | |
| Urogenital surgery | 23; 3% | 17; 3% | 6; 4.1% | 0.604 3 | |
| Vascular surgery | 132; 19% | 115; 22% | 17; 12% | 0.008 1 | |
| Other | 16; 2% | 12; 2% | 4; 2.8% | 0.757 3 | |
| Comorbidities | |||||
| HIV | 3; 0.4% | 2; 0.4% | 1; 0.7% | 0.514 3 | |
| AIDS | 2; 0.3% | 2; 0.4% | 0; 0% | >0.9 3 | |
| Anemia | 1; 0.1% | 0; 0% | 1; 0.7% | 0.213 3 | |
| Angina | 24; 4% | 21; 4% | 3; 2.1% | 0.283 1 | |
| Arrhythmia | 81; 12% | 59; 11% | 22; 15% | 0.172 1 | |
| Arterial hypertension | 412; 61% | 335; 63% | 77; 53% | 0.038 1 | |
| Coronary artery bypass grafting | 46; 7% | 35; 7% | 11; 8% | 0.657 1 | |
| Procedural coronary intervention | 64; 9% | 50; 9% | 14; 10% | >0.9 1 | |
| Chronic heart failure | 108; 16% | 82; 15% | 26; 18% | 0.447 1 | |
| Heart transplant | 1; 0.1% | 1; 0.2% | 0; 0% | >0.9 3 | |
| Heart valve disease | 26; 4% | 22; 4% | 4; 2.8% | 0.451 1 | |
| Myocardial infarction | 68; 10% | 56; 11% | 12; 8% | 0.435 1 | |
| Stroke | 75; 11% | 58; 11% | 17; 12% | 0.763 1 | |
| Pulmonary embolism | 15; 2% | 9; 1.7% | 6; 4.1% | 0.104 3 | |
| Deep vein thrombosis | 29; 4% | 19; 4% | 10; 7% | 0.077 1 | |
| Peripheral vascular disease | 77; 11% | 63; 12% | 14; 10% | 0.475 1 | |
| Oncology | 114; 17% | 93; 17% | 21; 15% | 0.407 1 | |
| Lung cancer | 48; 7% | 43; 8% | 5; 3.4% | 0.056 1 | |
| Respiratory failure | 10; 2% | 3; 0.6% | 7; 4.8% | 0.001 3 | |
| Home oxygen | 82; 12% | 37; 7% | 45; 31% | <0.001 1 | |
| Asthma | 98; 14% | 80; 15% | 18; 12% | 0.440 1 | |
| Restrictive pulmonary disease | 12; 2% | 7; 1.3% | 5; 3.4% | 0.144 3 | |
| Lung transplant | 1; 0.1% | 0; 0% | 1; 0.7% | 0.213 3 | |
| Chronic kidney disease | 74; 11% | 61; 11% | 13; 9% | 0.403 1 | |
| Renal transplant | 1; 0.1% | 1; 0.2% | 0; 0% | >0.9 3 | |
| Liver cirrhosis | 9; 1.3% | 8; 1.5% | 1; 0.7% | 0.692 3 | |
| Liver transplant | 1; 0.1% | 1; 0.2% | 0; 0% | >0.9 3 | |
| Peptic ulcer disease | 22; 3% | 19; 4% | 3; 2.1% | 0.595 3 | |
| Hypothyroidism | 58; 9% | 44; 8% | 14; 10% | 0.584 1 | |
| Insulin-dependent diabetes | 80; 12% | 64; 12% | 16; 11% | 0.758 1 | |
| Sarcoidosis | 2; 0.3% | 1; 0.2% | 1; 0.7% | 0.381 3 | |
| Neuromuscular disease | 1; 0.1% | 1; 0.2% | 0; 0% | >0.9 3 | |
| Seizures | 28; 4% | 25; 5% | 3; 2.1% | 0.162 1 | |
| Parameter | Preoperative | Postoperative | % of Change | p-Value (Pre-Post) |
|---|---|---|---|---|
| Glucose, mg/dL | N = 194; 116 (99; 141) | N = 643; 138 (114; 168) | N = 190; 12.3 (−8.7; 42.3) | <0.001 |
| Potassium, mmol/L | N = 200; 4.1 (3.7; 4.4) | N = 654; 4.1 (3.8; 4.5) | N = 196; 2.4 (−9.3; 13.1) | 0.288 |
| Sodium, mmol/L | N = 199; 138 (135; 140) | N = 655; 138 (135; 140) | N = 195; 0.0 (−1.5; 2.2) | 0.112 |
| Hgb, g/dL | N = 198; 11.5 (9.8; 13.0) | N = 655; 10.9 (9.7; 12.3) | N = 193; −7.0 (−15.0; 0.9) | <0.001 |
| Hct, % | N = 198; 35.5 (30.4; 39.9) | N = 656; 33.8 (29.5; 37.8) | N = 194; −7.1 (−14.8; 0.6) | <0.001 |
| Creatinine, mg/dL | N = 200; 0.9 (0.7; 1.5) | N = 654; 0.9 (0.7; 1.3) | N = 196; −4.4 (−16.9; 12.5) | 0.118 |
| BUN, mg/dL | N = 200; 18.0 (11.3; 29.8) | N = 655; 17.0 (11.0; 24.0) | N = 196; 0.0 (−20.5; 21.3) | 0.702 |
| Platelets, K/mcL | N = 199; 232 (173; 310) | N = 650; 201 (156; 268) | N = 195; −3.9 (−16.7; 10.1) | 0.002 |
| WBC, K/mcL | N = 198; 9.7 (7.8; 13.7) | N = 648; 11.9 (8.8; 15.5) | N = 194; 19.5 (−6.5; 68.2) | <0.001 |
| RBC, M/mcL | N = 198; 3.9 (3.4; 4.4) | N = 651; 3.7 (3.2; 4.2) | N = 194; −8.1 (−15.1; 0.3) | <0.001 |
| Lymphs abs, K/mcL | N = 149; 1.2 (0.8; 1.8) | N = 487; 0.9 (0.6; 1.4) | N = 149; −28.1 (−58.1; 1.5) | <0.001 |
| Polys abs, K/mcL | N = 139; 7.5 (4.8; 11.1) | N = 431; 10.0 (6.7; 13.4) | N = 123; 46.0 (−4.2; 101.1) | <0.001 |
| NLR | N = 149; 5.9 (3.0; 11.9) | N = 484; 9.2 (5.1; 17.8) | N = 125; 64.8 (7.9; 211.6) | <0.001 |
| Eos. abs, K/mcL | N = 150; 0.1 (0.0; 0.2) | N = 474; 0.0 (0.0; 0.1) | N = 90; −100.0 (−100.0; −30.3) | <0.001 |
| Mon. abs, K/mcL | N = 149; 0.7 (0.5; 1.1) | N = 484; 0.7 (0.4; 1.1) | N = 133; −3.7 (−30.2; 56.6) | 0.499 |
| Baso abs, K/mcL | N = 141; 0.0 (0.0; 4.8) | N = 453; 0.0 (0.0; 2.3) | N = 58; −48.6 (−100.0; 25.1) | 0.042 |
| Albumin, g/dL | N = 133; 3.1 (2.4; 3.5) | N = 385; 2.6 (2.2; 3.1) | N = 106; −13.0 (−25.2; −3.5) | <0.001 |
| AST, Units/L | N = 126; 20.0 (14.0; 28.3) | N = 355; 26.0 (17.0; 44.0) | N = 91; 4.3 (−18.9; 53.8) | 0.228 |
| ALT, Units/L | N = 125; 23.0 (13.5; 36.5) | N = 353; 21.0 (14.5; 36.0) | N = 91; −6.3 (−30.8; 20.0) | 0.097 |
| Total protein, g/dL | N = 126; 6.3 (5.6; 7.2) | N = 350; 5.4 (4.8; 6.0) | N = 92; −13.5 (−24.0; −5.4) | <0.001 |
| Total bilirubin, mg/dL | N = 124; 0.6 (0.3; 0.8) | N = 352; 0.6 (0.4; 1.0) | N = 90; 31.0 (−20.0; 100.0) | 0.002 |
| Lactate, mmol/L | N = 41; 1.3 (1.0; 2.1) | N = 170; 1.6 (1.1; 2.6) | N = 21; 0.0 (−38.3; 97.2) | 0.765 |
| Troponin I, ng/mL | N = 24; 0.0 (0.0; 0.1) | N = 79; 0.1 (0.0; 0.2) | N = 9; 0.0 (−40.2; 200.0) | 0.779 |
| pH | N = 19; 7.4 (7.4; 7.4) | N = 332; 7.3 (7.3; 7.4) | N = 16; 0.1 (−1.3; 0.4) | 0.798 |
| CPK, Units/L | N = 13; 86.0 (46.0; 167.5) | N = 72; 178.0 (63.0; 529.0) | N = 4; 81.5 (−51.1; 223.6) | >0.9 |
| BNP | N = 18; 502.5 (58.4; 1160.0) | N = 53; 560.0 (146.0; 1196.0) | N = 6; 22.1 (−4.5; 63.3) | 0.249 |
| PaCO2, mm Hg | N = 19; 41.6 (35.0; 49.3) | N = 337; 43.0 (37.7; 51.0) | N = 16; 7.6 (−11.0; 22.4) | 0.605 |
| O2 saturation, % | N = 10; 90.5 (87.6; 95.9) | N = 266; 97.6 (95.0; 99.0) | ND | 0.012 |
| Outcomes | Hospital Mortality | ICU Mortality | Duration of Mechanical Ventilation | Use of Mechanical Ventilation | Use of Vasopressors and Inotropes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ventilation Parameters | Alive, N = 621 | Expired, N = 59 | p-Value | Alive, N = 650 | Expired, N = 30 | p-Value | 1 Day, N = 91 | >1 Days, N = 189 | p-Value | Yes (MV), N = 280 | No (Spont), N = 400 | p-Value | Yes, N = 86 | No, N = 594 | p-Value |
| PEEP, cm H2O | N = 64; 5 (5; 5) | N = 16; 5 (5; 5) | 0.844 | N = 73; 5 (5; 5) | N = 7; 5 (5; 5) | 0.452 | N = 30; 5 (5; 8) | N = 37; 5 (5; 5) | 0.404 | N = 67; 5 (5; 5) | N = 13; 5 (5; 5) | 0.372 | N = 15; 5 (5; 5) | N = 65; 5 (5; 5) | 0.893 |
| TV, mL/kg | N = 58; 8.0 (7.3; 10.0) | N = 12; 7.5 (7.3; 8.2) | 0.115 | N = 64; 7.9 (7.3; 9.8) | N = 6; 7.5 (7.2; 8.4) | 0.288 | N = 24; 7.8 (7.1; 8.8) | N = 34; 7.9 (7.3; 10.2) | 0.622 | N = 58; 7.9 (7.3; 9.9) | N = 12; 7.9 (7.4; 9.5) | 0.945 | N = 15; 7.6 (7.0; 8.8) | N = 55; 8.0 (7.3; 9.9) | 0.200 |
| Pmean, cm H2O | N = 38; 9.0 (8.0; 11.0) | N = 12; 9.7 (8.1; 12.0) | 0.537 | N = 45; 9.7 (8.0; 11.5) | N = 5; 8.5 (7.0; 9.2) | 0.115 | N = 9; 9.1 (8.4; 11.0) | N = 32; 9.6 (8.0; 12.0) | 0.889 | N = 41; 9.4 (8.0; 11.5) | N = 9; 9.0 (7.3; 10.4) | 0.383 | N = 14; 9.6 (8.4; 10.5) | N = 36; 9.0 (8.0; 11.0) | 0.693 |
| Ppeak, cm H2O | N = 28; 24.0 (19.0; 26.8) | N = 10; 22.5 (19.5; 25.8) | 0.961 | N = 33; 24.0 (19.5; 27.0) | N = 5; 21.0 (16.0; 24.5) | 0.310 | N = 6; 20.0 (18.3; 22.0) | N = 23; 25.0 (19.0; 28.0) | 0.278 | N = 29; 24.0 (19.0; 27.5) | N = 9; 24.0 (17.5; 25.6) | 0.866 | N = 12; 25.0 (21.8; 30.0) | N = 26; 22.0 (18.8; 26.0) | 0.129 |
| EMV, L/min | N = 33; 7.2 (6.0; 8.9) | N = 11; 7.7 (7.0; 10.1) | 0.334 | N = 39; 7.3 (6.0; 9.4) | N = 5; 7.6 (4.5; 11.2) | 0.886 | N = 9; 7.3 (6.5; 9.7) | N = 26; 7.5 (6.0; 9.7) | 0.810 | N = 35; 7.4 (6.0; 9.6) | N = 9; 6.7 (5.4; 9.6) | 0.474 | N = 12; 7.6 (6.1; 9.3) | N = 32; 7.3 (6.0; 9.8) | 0.948 |
| SaO2, % | N = 5; 96.0 (92.0; 98.5) | ND | <0.001 | N = 5; 96.0 (92.0; 98.5) | ND | <0.001 | N = 2; 98.5 (98.0; 0.0) | ND | <0.001 | N = 2; 98.5 (98.0; 0.0) | N = 3; 94.0 (90.0; 0.0) | 0.200 | ND | N = 5; 96.0 (92.0; 98.5) | <0.001 |
| Pplateau, cm H2O | N = 23; 18.0 (13.0; 20.0) | N = 5; 17.0 (12.5; 19.5) | 0.6 | N = 26; 18.5 (13.8; 20.0) | N = 2; 12.5 (12.0; 0.0) | 0.106 | N = 7; 19.0 (13.0; 20.0) | N = 14; 16.5 (12.8; 20.0) | 0.585 | N = 21; 17.0 (13.0; 20.0) | N = 7; 19.0 (14.0; 21.0) | 0.376 | N = 5; 13.0 (11.5; 15.0) | N = 23; 19.0 (15.0; 20.0) | 0.016 |
| Compliance, mL/cm H2O | N = 11; 66.0 (40.0; 88.0) | N = 5; 43.2 (21.6; 51.0) | 0.115 | N = 14; 47.5 (39.9; 83.6) | N = 2; 44.5 (31.0; 0.0) | 0.5 | N = 4; 41.6 (39.6; 76.8) | N = 7; 58.0 (31.0; 91.0) | 0.648 | N = 11; 44.0 (39.4; 88.0) | N = 5; 51.0 (37.7; 67.5) | 0.913 | N = 5; 58.0 (28.1; 80.0) | N = 11; 43.2 (39.4; 82.1) | 0.743 |
| FiO2, % | N = 110; 50.0 (40.0; 65.0) | N = 21; 60.0 (50.0; 100.0) | 0.015 | N = 120; 50.0 (40.0; 77.5) | N = 11; 80.0 (50.0; 100.0) | 0.012 | N = 34; 55.0 (40.0; 100.0) | N = 59; 50.0 (40.0; 99.0) | 0.482 | N = 93; 50.0 (40.0; 100.0) | N = 38; 40.0 (40.0; 60.0) | 0.008 | N = 28; 50.0 (42.5; 75.0) | N = 103; 50.0 (40.0; 100.0) | 0.598 |
| Outcomes | Hospital LoS 6.6 (IQR 3.2 to 10.5) | ICU LoS 1.9 (IQR 1.0 to 3.6) | ||
|---|---|---|---|---|
| Ventilation Parameters | R [95% CI] | p-Value | R [95% CI] | p-Value |
| PEEP, N = 80 | 0.142 [−0.087; 0.357] | 0.208 | −0.076 [−0.296; 0.153] | 0.505 |
| Tidal volume, N = 70 | −0.23 [−0.447; 0.013] | 0.056 | −0.036 [−0.276; 0.207] | 0.765 |
| Pmean, N = 50 | 0.373 [0.097; 0.595] | 0.008 | 0.115 [−0.177; 0.389] | 0.426 |
| Ppeak, N = 38 | 0.223 [−0.114; 0.514] | 0.179 | 0.193 [−0.144; 0.49] | 0.245 |
| EMV, N = 44 | 0.098 [−0.213; 0.392] | 0.526 | −0.012 [−0.316; 0.294] | 0.936 |
| SaO2, N = 5 | 0.7 [−0.508; 0.98] | 0.188 | 0.600 [−0.625; 0.972] | 0.285 |
| Pplateau, N = 28 | 0.217 [−0.181; 0.554] | 0.266 | −0.042 [−0.419; 0.346] | 0.831 |
| Compliance, N = 16 | 0.241 [−0.304; 0.667] | 0.368 | 0.006 [−0.503; 0.512] | 0.983 |
| Peak flow, N = 10 | −0.4 [−0.83; 0.326] | 0.252 | −0.113 [−0.704; 0.571] | 0.757 |
| FiO2, N = 131 | 0.08 [−0.097; 0.253] | 0.361 | 0.086 [−0.092; 0.258] | 0.331 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shemetova, M.M.; Berikashvili, L.B.; Yadgarov, M.Y.; Korolenok, E.M.; Kuznetsov, I.V.; Yakovlev, A.A.; Likhvantsev, V.V. The Impact of Intraoperative Respiratory Patterns on Morbidity and Mortality in Patients with COPD Undergoing Elective Surgery. J. Clin. Med. 2025, 14, 2438. https://doi.org/10.3390/jcm14072438
Shemetova MM, Berikashvili LB, Yadgarov MY, Korolenok EM, Kuznetsov IV, Yakovlev AA, Likhvantsev VV. The Impact of Intraoperative Respiratory Patterns on Morbidity and Mortality in Patients with COPD Undergoing Elective Surgery. Journal of Clinical Medicine. 2025; 14(7):2438. https://doi.org/10.3390/jcm14072438
Chicago/Turabian StyleShemetova, Mariya M., Levan B. Berikashvili, Mikhail Ya. Yadgarov, Elizaveta M. Korolenok, Ivan V. Kuznetsov, Alexey A. Yakovlev, and Valery V. Likhvantsev. 2025. "The Impact of Intraoperative Respiratory Patterns on Morbidity and Mortality in Patients with COPD Undergoing Elective Surgery" Journal of Clinical Medicine 14, no. 7: 2438. https://doi.org/10.3390/jcm14072438
APA StyleShemetova, M. M., Berikashvili, L. B., Yadgarov, M. Y., Korolenok, E. M., Kuznetsov, I. V., Yakovlev, A. A., & Likhvantsev, V. V. (2025). The Impact of Intraoperative Respiratory Patterns on Morbidity and Mortality in Patients with COPD Undergoing Elective Surgery. Journal of Clinical Medicine, 14(7), 2438. https://doi.org/10.3390/jcm14072438







