Cervical Spine Screening Based on Movement Strategies Improves Shoulder Physical Variables in Neck-Related Shoulder Pain Patients: A Secondary Analysis from an Observational Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
- had prior shoulder surgery, pain at rest that improved with movement, history of trauma, shoulder fractures, systemic diseases, radiculopathy, peripheral neuropathy, or radicular pain (including a score of >11 on the S-LANSS scale);
- had received local analgesic or anti-inflammatory injections in the previous six months or taken those medications on the examination day.
2.3. Variables
2.4. Procedure
2.5. Data Analysis
3. Results
3.1. Shoulder Range of Motion
3.2. Shoulder Isometric Strength
3.3. Shoulder Function (SPADI)
4. Discussion
4.1. Shoulder Range of Motion
4.2. Shoulder Isometric Strength
4.3. Shoulder Function
4.4. Strengths, Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| CSS | Cervical spine screening |
Appendix A
Appendix A.1. Cervical Spine Screening
- Postural adjustment during painful movement: forward head posture and thoracic/lumbar slump were corrected. By maintaining this neutral cervical spine position, the participant performed the painful or restricted movement.
- Repeated end-range movements: cervical retraction, retraction plus extension and flexion were performed 10 times, followed by reassessment of the painful or restricted movement. If the participant was unable to reach the end range, passive overpressure was applied by the examiner. Repeated end-range movements were conducted in all directions in the specified sequence unless a technique resulted in complete symptom resolution.
- Spinal mobilization with shoulder movement:
- a.
- Cervical global traction: with the participant seated, the examiner positioned behind them applied gentle upward traction at the occiput using a bimanual grip. The participant then performed the painful or restricted movement while maintaining this mobilization.
- b.
- C4 lateral glide: with the participant seated, the examiner applied lateral pressure to the C4 spinous process in the direction opposite to the painful shoulder. This mobilization was maintained while the participant performed the painful or restricted movement.
- Passive accessory mobilizations: techniques included posterior–anterior, anteroposterior, and lateral glides. The cervical segment selected for mobilization was the one that elicited symptom reproduction after three posterior–anterior mobilizations. If no symptoms were provoked, the segment associated with the greatest local discomfort was chosen [54]. Each mobilization was performed for 1 min.
- Neck flexor (a) and extensor muscle (b) exercises while performing sets of contractions.
- a.
- Double chin movement (cranio-cervical flexion) was taught to the participant in a supine position. After this, he/she was placed standing with his/her back and head against a wall. A tennis ball was put between his/her head and the wall as feedback to better understand the exercise, and the subject was asked to perform the double chin movement while avoiding the ball falling. This was repeated 10 times.
- b.
- In a seated position, the subject was asked to place a band in his/her occiput and holding it forwards with his hands. Then, he/she was asked to perform a cervical retraction against the resistance of the band. This was repeated 10 times.
- c.
- As the CSS was an accumulation of strategies that could improve or worsen the subject’s baseline status, the possibility of stopping the CSS if the subject experienced an increase of ≥2 points in the NPRS score was contemplated.
References
- Urwin, M.; Symmons, D.; Allison, T.; Brammah, T.; Busby, H.; Roxby, M.; Simmons, A.; Williams, G. Estimating the burden of musculoskeletal disorders in the community: The comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann. Rheum. Dis. 1998, 57, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Falcón, E.; Toledo-Martel, N.d.C.; Sosa-Medina, F.M.; Santana-González, F.; Quintana-de la Fe, M.d.P.; Gallego-Izquierdo, T.; Pecos-Martín, D. Dry needling in a manual physiotherapy and therapeutic exercise protocol for patients with chronic mechanical shoulder pain of unspecific origin: A protocol for a randomized control trial. BMC Musculoskelet. Disord. 2017, 18, 400. [Google Scholar]
- van der Windt, D.A.; Koes, B.W.; de Jong, B.A.; Bouter, L.M. Shoulder disorders in general practice: Incidence, patient characteristics, and management. Ann. Rheum. Dis. 1995, 54, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Greving, K.; Dorrestijn, O.; Winters, J.; Groenhof, F.; van der Meer, K.; Stevens, M.; Diercks, R.L. Incidence, prevalence, and consultation rates of shoulder complaints in general practice. Scand. J. Rheumatol. 2012, 41, 150–155. [Google Scholar]
- Luime, J.J.; Koes, B.W.; Hendriksen, I.J.M.; Burdorf, A.; Verhagen, A.P.; Miedema, H.S.; Verhaar, J.A.N. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand. J. Rheumatol. 2004, 33, 73–81. [Google Scholar] [CrossRef]
- Croft, P.; Pope, D.; Silman, A. The clinical course of shoulder pain: Prospective cohort study in primary care. Prim. Care Rheumatol. Soc. Shoulder Study Group BMJ 1996, 313, 601–602. [Google Scholar]
- Roldán-Ruiz, A.; Bailón-Cerezo, J.; Falla, D.; Torres-Lacomba, M. The prevalence of cervical contribution in patients reporting shoulder pain. An observational study. Musculoskelet. Sci. Pract. 2024, 73, 103158. [Google Scholar]
- Walker, T.; Salt, E.; Lynch, G.; Littlewood, C. Screening of the cervical spine in subacromial shoulder pain: A systematic review. Shoulder Elb. 2018, 11, 305–315. [Google Scholar]
- Heidar Abady, A.; Rosedale, R.; Chesworth, B.M.; Rotondi, M.A.; Overend, T.J. Application of the McKenzie system of Mechanical Diagnosis and Therapy (MDT) in patients with shoulder pain; a prospective longitudinal study. J. Man. Manip. Ther. 2017, 9817, 1–9. [Google Scholar]
- Karel, Y.H.J.M.; Scholten-Peeters, G.G.M.; Thoomes-de Graaf, M.; Duijn, E.; van Broekhoven, J.B.; Koes, B.W.; Verhagen, A.P. Physiotherapy for patients with shoulder pain in primary care: A descriptive study of diagnostic- and therapeutic management. Physiotherapy 2017, 103, 369–378. [Google Scholar]
- Sueki, D.G.; Cleland, J.A.; Wainner, R.S. A regional interdependence model of musculoskeletal dysfunction: Research, mechanisms, and clinical implications. J. Man. Manip. Ther. 2013, 21, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Wainner, R.S.; Whitman, J.M.; Cleland, J.A.; Flynn, T.W. Regional Interdependence: A Musculoskeletal Examination Model Whose Time Has Come. J. Orthop. Sports Phys. Ther. 2007, 37, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.; Aprill, C.; Bogduk, N. Cervical zygapophyseal joint pain patterns. I: A study in normal volunteers. Spine 1990, 15, 453–457. [Google Scholar]
- Aprill, C.; Dwyer, A.; Bogduk, N. Cervical zygapophyseal joint pain patterns. II: A clinical evaluation. Spine 1990, 15, 458–461. [Google Scholar] [CrossRef]
- Fukui, S.; Ohseto, K.; Shiotani, M.; Ohno, K.; Karasawa, H.; Naganuma, Y.; Yuda, Y. Referred pain distribution of the cervical zygapophyseal joints and cervical dorsal rami. Pain 1996, 68, 79–83. [Google Scholar] [CrossRef]
- Cooper, G.; Bailey, B.; Bogduk, N. Cervical zygapophysial joint pain maps. Pain Med. 2007, 8, 344–353. [Google Scholar] [CrossRef]
- Friedenberg, Z.B.; Miller, W.T. Degenerative Disc Disease of the Cervical Spine. J. Bone Jt. Surg. Am. 1963, 45, 1171–1178. [Google Scholar] [CrossRef]
- Bogduk, N.; Simons, D.G. Neck pain: Joint pain or trigger points. In Progress in Fibromyalgia and Myofascial Pain; Vaeroy, H., Merskey, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 267–273. [Google Scholar]
- Katsuura, Y.; Bruce, J.; Taylor, S.; Gullota, L.; Kim, H.J. Overlapping, Masquerading, and Causative Cervical Spine and Shoulder Pathology: A Systematic Review. Glob. Spine J. 2020, 10, 195–208. [Google Scholar] [CrossRef]
- Bokshan, S.L.; DePasse, J.M.; Eltorai, A.E.M.; Paxton, E.S.; Green, A.; Daniels, A.H. An Evidence-Based Approach to Differentiating the Cause of Shoulder and Cervical Spine Pain. Am. J. Med. 2016, 129, 913–918. [Google Scholar] [CrossRef]
- Holmes, R.E.; Barfield, W.R.; Woolf, S.K. Clinical evaluation of nonarthritic shoulder pain: Diagnosis and treatment. Phys. Sportsmed. 2015, 43, 262–268. [Google Scholar] [CrossRef]
- Throckmorton, T.Q.; Kraemer, P.; Kuhn, J.E.; Sasso, R.C. Differentiating cervical spine and shoulder pathology: Common disorders and key points of evaluation and treatment. Instr. Course Lect. 2014, 63, 401–408. [Google Scholar] [PubMed]
- Heidar Abady, A.; Rosedale, R.; Chesworth, B.M.; Rotondi, M.A.; Overend, T.J. Consistency of commonly used orthopedic special tests of the shoulder when used with the McKenzie system of mechanical diagnosis and therapy. Musculoskelet. Sci. Pract. 2018, 33, 11–17. [Google Scholar] [PubMed]
- Rosedale, R.; Rastogi, R.; Kidd, J.; Lynch, G.; Supp, G.; Robbins, S.M. A study exploring the prevalence of Extremity Pain of Spinal Source (EXPOSS). J. Man. Manip. Ther. 2020, 28, 222–230. [Google Scholar]
- Rastogi, R.; Rosedale, R.; Kidd, J.; Lynch, G.; Supp, G.; Robbins, S.M. Exploring indicators of extremity pain of spinal source as identified by Mechanical Diagnosis and Therapy (MDT): A secondary analysis of a prospective cohort study. J. Man. Manip. Ther. 2022, 30, 172–179. [Google Scholar]
- Norlander, S.; Aste-Norlander, U.; Nordgren, B.; Sahlstedt, B. Mobility in the cervico-thoracic motion segment: An indicative factor of musculo-skeletal neck-shoulder pain. Scand. J. Rehabil. Med. 1996, 28, 183–192. [Google Scholar]
- Roldán-Ruiz, A.; Bailón-Cerezo, J.; Torres-Lacomba, M. The prevalence of subclassification-based diagnoses when considering cervical contribution in shoulder pain patients: A secondary analysis from a previous research. J. Man. Manip. Ther. 2024, 1–9. [Google Scholar] [CrossRef]
- Maccio, J.R.; Carlton, L.; Levesque, K.; Maccio, J.G.; Egan, L. Directional preference of the extremity: A preliminary investigation. J. Man. Manip. Ther. 2018, 26, 272–280. [Google Scholar]
- Khan, S.; Hameed, N.; Mazar, S.; Hashmi, I.A.; Rafi, M.S.; Shah, M.I.; Baloch, N.A.; Shah, M. Persistent Shoulder Pain After Anterior Cervical Discectomy and Fusion (ACDF): Another Dual Pathology. Cureus 2021, 13, e13709. [Google Scholar]
- Kholinne, E.; Kwak, J.; Sun, Y.; Lee, H.; Koh, K.H.; Jeon, I. Risk Factors for Persistent Shoulder Pain After Cervical Spine Surgery. Orthop. Surg. 2019, 11, 845–849. [Google Scholar]
- Hauswirth, J.; Ernst, M.J.; Preusser, M.L.; Meichtry, A.; Kool, J.; Crawford, R.J. Immediate effects of cervical unilateral anterior-posterior mobilisation on shoulder pain and impairment in post-operative arthroscopy patients. J. Back Musculoskelet. Rehabil. 2017, 30, 615–623. [Google Scholar]
- Wang, S.S.; Meadows, J. Immediate and Carryover Changes of C5-6 Joint Mobilization on Shoulder External Rotator Muscle Strength. J. Manip. Physiol. Ther. 2010, 33, 102–108. [Google Scholar]
- Suter, E.; McMorland, G. Decrease in elbow flexor inhibition after cervical spine manipulation in patients with chronic neck pain. Clin. Biomech. 2002, 17, 541–544. [Google Scholar]
- Mintken, P. Shoulder Pain and Regional Interdependence: Contributions of the Cervicothoracic Spine. J. Yoga Phys. Ther. 2014, 05, 1–2. [Google Scholar]
- McClatchie, L.; Laprade, J.; Martin, S.; Jaglal, S.B.; Richardson, D.; Agur, A. Mobilizations of the asymptomatic cervical spine can reduce signs of shoulder dysfunction in adults. Man. Ther. 2009, 14, 369–374. [Google Scholar]
- Bergman, G.J.D.; Winters, J.C.; Groenier, H.; Pool, J.J.M.; Meyboom-de Jong, B. Article Manipulative Therapy in Addition to Usual Medical Care for Patients with Shoulder Dysfunction and Pain. Am. Coll. Physicians 2004, 21, 432–440. [Google Scholar]
- Schneider, G. Restricted Shoulder Movement: Capsular Contracture or Cervical Referral—A Clinical Study. Aust. J. Physiother. 1989, 35, 97–100. [Google Scholar]
- Hathcock, J.A.; Boyer, C.W.; Morris, J.B. Shoulder Pain of Spinal Source in the Military: A Case Series. Mil. Med. 2022, 187, e1240–e1246. [Google Scholar]
- Pheasant, S. Cervical Contribution to Functional Shoulder Impingement: Two Case Reports. Int. J. Sports Phys. Ther. 2016, 11, 980–991. [Google Scholar]
- Seo, Y.; Lee, J.; Han, D. The effects of spinal mobilization with arm movements on shoulder muscle strengthening. J. Phys. Ther. Sci. 2015, 27, 11–13. [Google Scholar]
- Requejo-Salinas, N.; Fernández-Matías, R.; Cadogan, A.; Chester, R.; Roy, J.-S.; Struyf, F.; Bateman, M.; Balster, S.; Haik, M.N.; Seitz, A.L.; et al. Neck or Shoulder? Establishing Consensus for Spine Screening in Patients with Shoulder Pain: An International Modified Delphi Study. Phys. Ther. 2024, 105, pzae133. [Google Scholar]
- Cuesta-Vargas, A.I.; Roldan-Jimenez, C.; Neblett, R.; Gatchel, R.J. Cross-cultural adaptation and validity of the Spanish central sensitization inventory. Springerplus 2016, 5, 1837. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; McFarland, C.A. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 1993, 55, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Karoly, P.; Braver, S. The measurement of clinical pain intensity: A comparison of six methods. Pain 1986, 27, 117–126. [Google Scholar] [CrossRef]
- Bahat, H.S.; Kerner, O. The Shoulder Symptom Modification Procedure (SSMP): A Reliability Study. J. Nov. Physiother. 2016, S3, 1–6. [Google Scholar] [CrossRef]
- Lewis, J.S. Rotator cuff tendinopathy/subacromial impingement syndrome: Is it time for a new method of assessment? Br. J. Sports Med. 2009, 43, 259–264. [Google Scholar] [CrossRef]
- Werner, B.C.; Holzgrefe, R.E.; Griffin, J.W.; Lyons, M.L.; Cosgrove, C.T.; Hart, J.M.; Brockmeier, S.F. Validation of an innovative method of shoulder range-of-motion measurement using a smartphone clinometer application. J. Shoulder Elb. Surg. 2014, 23, e275–e282. [Google Scholar] [CrossRef]
- McLaine, S.J.; Ginn, K.A.; Kitic, C.M.; Fell, J.W.; Bird, M.-L. The Reliability of Strength Tests Performed in Elevated Shoulder Positions Using a Handheld Dynamometer. J. Sport Rehabil. 2016, 25, jsr.2015-0034. [Google Scholar] [CrossRef]
- Holt, K.L.; Raper, D.P.; Boettcher, C.E.; Waddington, G.S.; Drew, M.K. Hand-held dynamometry strength measures for internal and external rotation demonstrate superior reliability, lower minimal detectable change and higher correlation to isokinetic dynamometry than externally-fixed dynamometry of the shoulder. Phys. Ther. Sport. 2016, 21, 75–81. [Google Scholar] [CrossRef]
- Roy, J.S.; MacDermid, J.C.; Orton, B.; Tran, T.; Faber, K.J.; Drosdowech, D.; Athwal, G.S. The Concurrent Validity of a Hand-held versus a Stationary Dynamometer in Testing Isometric Shoulder Strength. J Hand Ther. 2009, 22, 320–327. [Google Scholar] [CrossRef]
- Breckenridge, J.D.; McAuley, J.H. Shoulder Pain and Disability Index (SPADI). J. Physiother. 2011, 57, 197. [Google Scholar] [CrossRef]
- Beaton, D.E.; Richards, R.R. Measuring function of the shoulder. A cross-sectional comparison of five questionnaires. J. Bone Jt. Surg. Am. 1996, 78, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences (2. Auflage); Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Cook, C.; Learman, K.; Houghton, S.; Showalter, C.; O’Halloran, B. The addition of cervical unilateral posterior-anterior mobilisation in the treatment of patients with shoulder impingement syndrome: A randomised clinical trial. Man. Ther. 2014, 19, 18–24. [Google Scholar] [PubMed]
- Menon, A.; May, S. Shoulder pain: Differential diagnosis with mechanical diagnosis and therapy extremity assessment—A case report. Man. Ther. 2013, 18, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Shelke, A.; Kumaran, S.D. Immediate effect of craniocervical flexion exercise and Mulligan mobilisation in patients with mechanical neck pain—A randomised clinical trial. Hong Kong Physiother. J. 2023, 43, 137–147. [Google Scholar]
- Paul, A.; Lewis, M.; Shadforth, M.F.; Croft, P.R.; Van Der Windt, D.A.W.M.; Hay, E.M. A comparison of four shoulder-specific questionnaires in primary care. Ann. Rheum. Dis. 2004, 63, 1293–1299. [Google Scholar] [CrossRef]
- Schibany, N.; Zehetgruber, H.; Kainberger, F.; Wurnig, C.; Ba-Ssalamah, A.; Herneth, A.M.; Lang, T.; Gruber, D.; Breitenseher, M.J. Rotator cuff tears in asymptomatic individuals: A clinical and ultrasonographic screening study. Eur. J. Radiol. 2004, 51, 263–268. [Google Scholar] [CrossRef]
- Somerville, K.; Walston, Z.; Marr, T.; Yake, D. Treatment of shoulder pathologies based on irritability: A case series. Physiother. Theory Pract. 2020, 36, 1266–1274. [Google Scholar]
- Beaudreuil, J.; Nizard, R.; Thomas, T.; Peyre, M.; Liotard, J.P.; Boileau, P.; Marc, T.; Dromard, C.; Steyer, E.; Bardin, T.; et al. Contribution of clinical tests to the diagnosis of rotator cuff disease: A systematic literature review. Jt. Bone Spine 2009, 76, 15–19. [Google Scholar] [CrossRef]
- Walton, D.M.; Sadi, J. Identifying SLAP lesions: A meta-analysis of clinical tests and exercise in clinical reasoning. Phys. Ther. Sport 2008, 9, 167–176. [Google Scholar]
- Cadogan, A.; Laslett, M.; Hing, W.; McNair, P.; Williams, M. Interexaminer reliability of orthopaedic special tests used in the assessment of shoulder pain. Man. Ther. 2011, 16, 131–135. [Google Scholar] [CrossRef]
- Tuttle, N. Do changes within a manual therapy treatment session predict between-session changes for patients with cervical spine pain? Aust. J. Physiother. 2005, 51, 43–48. [Google Scholar]
- Cook, C.; Lawrence, J.; Michalak, K.; Dhiraprasiddhi, S.; Donaldson, M.; Petersen, S.; Learman, K. Is there preliminary value to a within- and/or between-session change for determining short-term outcomes of manual therapy on mechanical neck pain? J. Man. Manip. Ther. 2014, 22, 173–180. [Google Scholar]
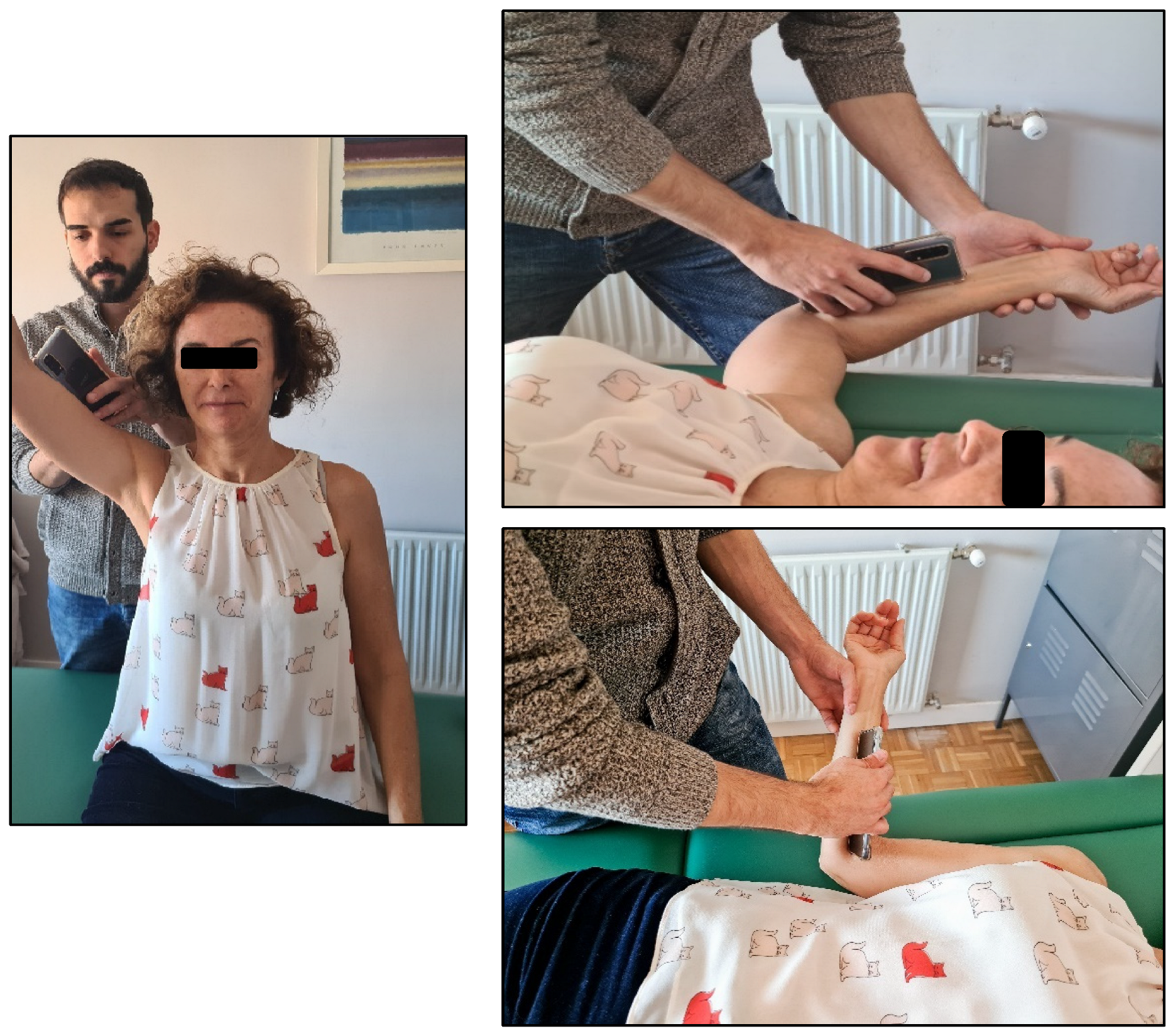
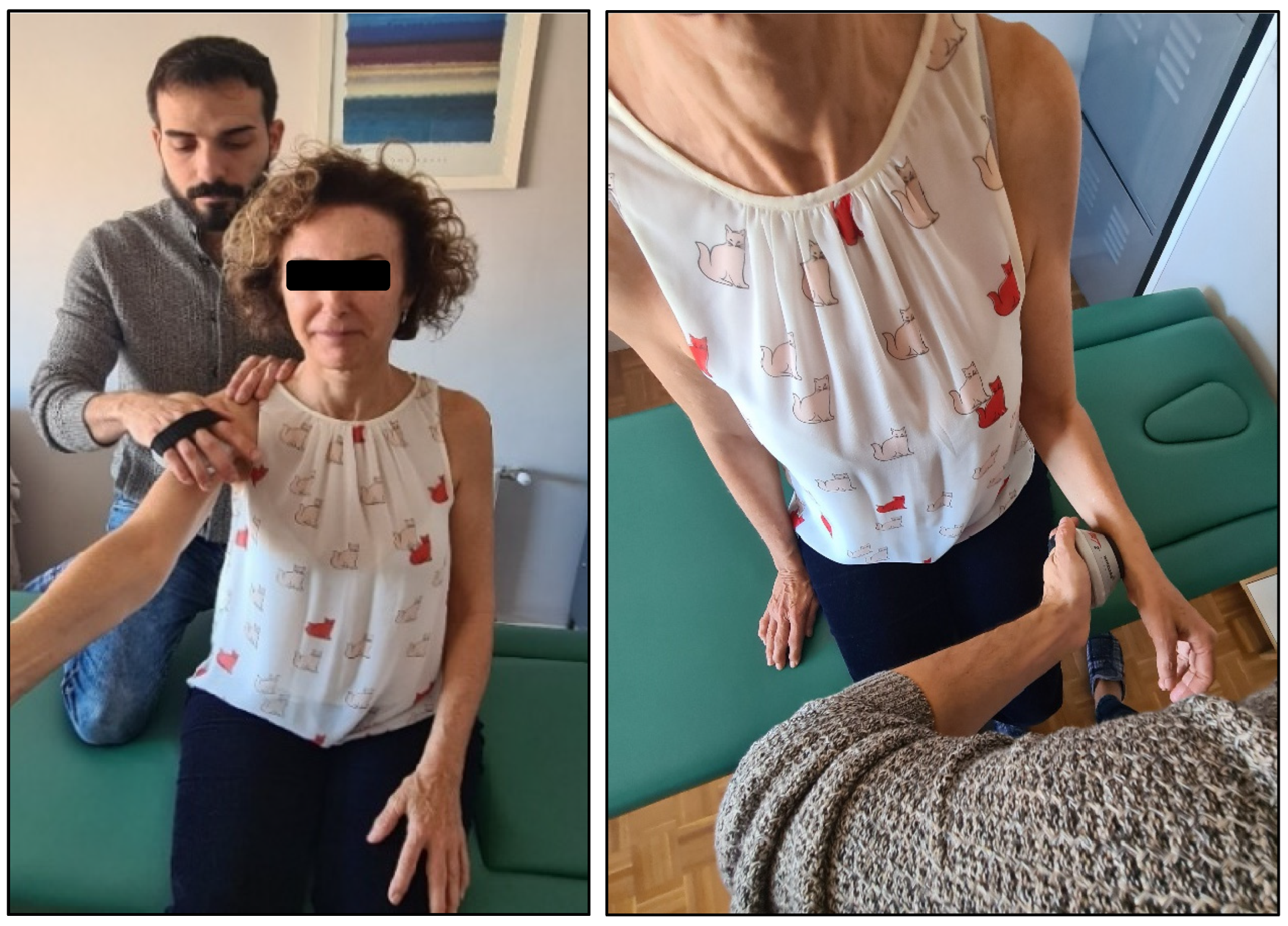
| Intervention | Included Variants |
|---|---|
| Postural change while performing painful movement | - |
| Repeated end-range movements | Cervical retraction, retraction plus extension and flexion |
| Spinal accessory mobilization with painful shoulder movement | Cervical global traction and lateral glide of C4 spinous process |
| Passive accessory mobilization | Posterior–anterior and anterior–posterior mobilizations and lateral glides |
| Neck flexor and extensor muscle activation | - |
| Variables | Descriptive Data |
|---|---|
| Clinical characteristics. Absolute frequency—n (%) | |
| Painful shoulder (left) | 28 (46.7) |
| Upper limb referred pain | 27 (45) |
| Unilateral pain | 49 (81.7) |
| Previous neck pain | 22 (36.7) |
| Concomitant neck pain | 23 (38.3) |
| Symptoms duration | |
| <3 months | 16 (26.7) |
| 3–6 months | 9 (15) |
| >6 months | 35 (58.3) |
| Clinimetrics | |
| Central sensitization (CSI), >40 points | 12 (20) |
| Shoulder function (SPADI)—median [IQR] | 38.57 [28] |
| Pain intensity (NPRS)—median [IQR] | 6 [2] |
| V0 Median (IQR) | V1 Median (IQR) | p | Effect Size (r) * | ||
|---|---|---|---|---|---|
| Flexion | Whole sample (n = 60) | 150.5 (37.25) | 156.5 (34.25) | <0.001 | 0.46 |
| Cervical contribution (n = 30) | 153.50 (30.50) | 165 (27.75) | <0.001 | 0.55 | |
| Non-cervical contribution (n = 30) | 150 (42.75) | 151 (46.25) | 0.007 | 0.35 | |
| Abduction | Whole sample (n = 60) | 155 (30) | 160 (40.75) | <0.001 | 0.37 |
| Cervical contribution (n = 30) | 155 (35.25) | 170 (36.25) | <0.001 | 0.55 | |
| Non-cervical contribution (n = 30) | 155 (35) | 155 (39.25) | 0.833 | 0.03 | |
| External rotation at 0° abduction | Whole sample (n = 60) | 62.43 (21.95) M (SD) | 65.48 (20.29) M (SD) | 0.010 | 0.23 |
| Cervical contribution (n = 30) | 63.90 (18.76) M (SD) | 69.43 (15.15) M (SD) | 0.008 | 0.34 | |
| Non-cervical contribution (n = 30) | 61.06 (24.98) M (SD) | 61.53 (23.99) M (SD) | 0.461 | 0.10 | |
| External rotation at 90° abduction | Whole sample (n = 60) | 84 (24.25) | 85 (20.75) | 0.363 | 0.08 |
| Cervical contribution (n = 30) | 83 (24.25) | 86 (19.25) | 0.201 | 0.17 | |
| Non-cervical contribution (n = 30) | 84.5 (27.75) | 83 (27.25) | 0.843 | 0.03 | |
| Internal rotation at 90° | Whole sample (n = 60) | 63.9 (18.73) M (SD) | 66.95 (16.23) M (SD) | 0.014 | 0.22 |
| Cervical contribution (n = 30) | 64.97 (17.86) M (SD) | 70.47 (13.63) M (SD) | 0.020 | 0.30 | |
| Non-cervical contribution (n = 30) | 62.83 (19.81) M (SD) | 63.43 (18.01) M (SD) | 0.529 | 0.08 | |
| V0 Median (IQR) | V1 Median (IQR) | p | Effect Size (r) * | |
|---|---|---|---|---|
| Whole sample (n = 60) | 90 (77.87) | 89 (61.75) | 0.40 | 0.08 |
| Cervical contribution (n = 30) | 78.20 (44.93) | 80.25 (46.25) | 0.094 | 0.22 |
| Non-cervical contribution (n = 30) | 112 (67.75) | 105 (66) | 0.51 | 0.08 |
| V0 Median (IQR) | V1 Median (IQR) | p | Effect Size (r) * | |
|---|---|---|---|---|
| Whole sample (n = 60) | 38.57 (27.95) | 39 (33.77) | 0.868 | 0.02 |
| Cervical contribution (n = 30) | 37.7 (29.97) | 29.63 (33.01) | 0.088 | 0.22 |
| Non-cervical contribution (n = 30) | 39.35 (31.21) | 43.5 (33.57) | 0.002 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roldán-Ruiz, A.; Bailón-Cerezo, J.; Falla, D.; Torres-Lacomba, M. Cervical Spine Screening Based on Movement Strategies Improves Shoulder Physical Variables in Neck-Related Shoulder Pain Patients: A Secondary Analysis from an Observational Study. J. Clin. Med. 2025, 14, 2433. https://doi.org/10.3390/jcm14072433
Roldán-Ruiz A, Bailón-Cerezo J, Falla D, Torres-Lacomba M. Cervical Spine Screening Based on Movement Strategies Improves Shoulder Physical Variables in Neck-Related Shoulder Pain Patients: A Secondary Analysis from an Observational Study. Journal of Clinical Medicine. 2025; 14(7):2433. https://doi.org/10.3390/jcm14072433
Chicago/Turabian StyleRoldán-Ruiz, Alberto, Javier Bailón-Cerezo, Deborah Falla, and María Torres-Lacomba. 2025. "Cervical Spine Screening Based on Movement Strategies Improves Shoulder Physical Variables in Neck-Related Shoulder Pain Patients: A Secondary Analysis from an Observational Study" Journal of Clinical Medicine 14, no. 7: 2433. https://doi.org/10.3390/jcm14072433
APA StyleRoldán-Ruiz, A., Bailón-Cerezo, J., Falla, D., & Torres-Lacomba, M. (2025). Cervical Spine Screening Based on Movement Strategies Improves Shoulder Physical Variables in Neck-Related Shoulder Pain Patients: A Secondary Analysis from an Observational Study. Journal of Clinical Medicine, 14(7), 2433. https://doi.org/10.3390/jcm14072433








