MicroRNAs as Epigenetic Biomarkers of Pathogenetic Mechanisms of the Metabolic Syndrome Induced by Antiseizure Medications: Systematic Review
Abstract
1. Introduction
- measured in liquids (blood, saliva, and urine) or primary types of tissue specimen (fresh, frozen, and fixed with formalin and filled with paraffin);
- helps to identify (diagnose) diseases;
- predicts the severity and outcome of the disease;
- responds to drug and non-drug therapy;
- monitors the response of organs and tissues to non-drug or drug therapy;
- predicts the risk of developing the disease in the future;
- allows simultaneous diagnosis and targeted therapy (teragnosis) [27].
2. Materials and Methods
3. Results
3.1. Epigenetic Biomarkers of ASM-Induced Metabolic Syndrome
3.2. MiRs as Epigenetic Biomarkers of the Main Domains of ASM-Induced Metabolic Syndrome
3.2.1. Oxidative Stress
3.2.2. Systemic Inflammation
3.2.3. Regulation of Adipogenesis and Development of Central Obesity
3.2.4. Changes in Lipid Metabolism
3.2.5. Changes in High-Density Lipoprotein Cholesterol Homeostasis
3.2.6. Changes in Low-Density Lipoprotein Cholesterol Homeostasis
3.2.7. Changes in the Processes of Atherogenesis
3.2.8. The Development of Fatty Hepatosis (Fatty Liver Disease)
3.2.9. Changes in Insulin Responsiveness
3.2.10. Changes in Insulin Expression and Secretion by B Cells of Pancreatic Langerhans Islets
3.2.11. Changes in Glucose Metabolism
3.2.12. Changes in Appetite Regulation
3.2.13. Changes in the Expression of Neuropeptide Y
3.2.14. Changes in Leptin Responsiveness
3.2.15. Changes in Orexin Expression
3.2.16. Changes in Testosterone Expression
3.2.17. Changes in the Expression of Thyroid Hormones
3.2.18. Changes in Parathyroid Hormone Expression
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASMs | Antiseizure medications |
| ADRs | Adverse drug reactions |
| AIMetS | Antiseizure medications-induced metabolic syndrome |
| RNA | Ribonucleic acids |
| NFE2L2/Nrf2 | Nuclear factor 2, related to erythroid factor 2 |
| Keap1 | Kelp-like ECH-associated protein 1 |
| Sirt | Sirtuin |
| NAD | Nicotinamide adenine dinucleotide |
| ATP | Adenosine triphosphate |
| HO-1 | Hemoxidase 1 |
| TNF-α | Tumor necrosis factor alpha |
| IL | Interleukin |
| TGF-β | Transforming growth factor-β |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PI3K | Phosphoinoside kinase type 3 |
| PPARγ | γ-Activated peroxisome proliferator |
| C/EBP | CCAAT/enhancer-binding protein |
| LDL | Low-density lipoprotein |
| NAFLD | Non-alcoholic fatty liver disease |
| SNARE | Protein complexes associated with synaptosomes |
| NPY | Neuropeptide Y |
| IDF | International Diabetes Federation |
Appendix A
| Biomarker | Standard | Change at the MetS | The MetS Symptom |
|---|---|---|---|
| Glucose | >100 mg/dL | High | Insulin resistance |
| Sialic acid | 2.0–2.33 mmol/L | High | CHD Systemic inflammation |
| Uric acid | M: 202.3–416.5 µmol/L F: 142.8–339.2 µmol/L | High | Obesity |
| Adiponectin | 0.6–1.33 g/L | Low | Insulin resistance |
| Aldosterone | 25–315 pg/mL | High | High blood pressure |
| Chimerin | 116–157.5 ng/mL | High | Central obesity Coronary heart disease |
| Ghrelin | 0–100 ng/L | Low | Central obesity |
| Insulin | 2.6–24.9 µU/mL | High | Insulin resistance |
| Leptin | M: 2–5.6 ng/mL F: 3.7–11.1 ng/mL | High | Insulin resistance Leptin resistance |
| Omentin | 5.82−71ng/mL M: 200–960 ng/mL (18–29 years) M: 252–712 ng/mL (30–39 years) M: 272–784 ng/mL (40–49 years) F: 242–764 ng/mL (15–29 years) F: 236–560 ng/mL (30–37 years) F: 220–600 ng/mL (38–49 years) | Low | Central obesity Endothelial dysfunction Coronary heart disease |
| Parathyroid hormone | 15–65 pg/mL | High | Cardiovascular diseases |
| Testosterone | M: 8.64–29.0 nmol/L (18–55 years) F: 0.29–1.67 nmol/L (18–55 years) | Low | Central obesity |
| Thyroid-stimulating hormone | 0.27–4.2 µIU/mL | High | Cardiovascular diseases |
| Total bilirubin | <21 µmol/L | Low | Oxidative stress |
| A protein that binds fatty acids in adipocytes | <6.2 ng/mL | High | Central obesity Cardiometabolic diseases |
| C-peptide | 1.1–4.4 ng/mL | High | Insulin resistance |
| Soluble serum ligand CD40 | <3.5 ng/mL | High | Systemic inflammation Coronary heart disease |
| Cystatin C | 0.5–1.2 mg/L | High | High blood pressure |
| Ferritin | M: 20–250 µg/L F: 10–120 µg/L | Contradictory | Oxidative stress |
| Fibrinogen | 1.8–3.5g/L | High | High blood pressure Coronary heart disease |
| Fibroblast growth factor 21 | M: 3.6–1021.4 pg/mL F: 65.3–1209.8 pg/mL | High | Central obesity Atherosclerosis |
| Monocytic chemotoxic protein-1 | 4.7–300 pg/mL | High | Coronary heart disease |
| Plasminogen activator inhibitor-1 | 5–40 ng/mL | High | Insulin resistance Coronary heart disease |
| Retinol-binding protein 4 | 11–40 µg/mL | High | Central obesity Insulin resistance Cardiovascular diseases |
| Tumor necrosis factor alpha | <8.1 pg/mL | High | Coronary heart disease |
| Oxidized low-density lipoprotein | 26–117 IU/L | High | Oxidative stress Systemic inflammation |
| Apolipoprotein A1 | M: >1.2 g/L F: >1.4 g/L | Low | Insulin resistance Dyslipidemia Central obesity |
| Apolipoprotein B | 0.6–1.33 g/L | High | Insulin resistance Dyslipidemia Central obesity |
| Free fatty acids | M: 8.3–10.9 ng/mL F: 11.4–13.6 ng/mL | High | Insulin resistance |
| High-density lipoproteins | 0.7–1.7 mmol/L | Low | Insulin resistance |
| Low-density lipoprotein cholesterol | <2.6 mmol/L | High | Dyslipidemia Central obesity |
| Triglycerides | <1.7 mmol/L | High | Dyslipidemia Central obesity |
| Type 1 superoxide dismutase (in red blood cells) | 1200–2000 U/g | Low | Oxidative stress Systemic inflammation |
| Gamma-glutamyltransferase | M: 10–71 U/g F: 6–42 U/g | High | Oxidative stress Systemic inflammation |
| Lipoprotein-associated phospholipase A | <200 ng/mL | High | Cardiovascular diseases |
| 25-Hydroxyvitamin D | 30–100 ng/mL | Low | Cardiovascular diseases |
| Vitamin E (tocopherol) | 5–18 µg/mL | Low | Oxidative stress |
| Ethnic Group | Gender | Waist Girth |
|---|---|---|
| Europeoids 1 | Male | ≥94 cm |
| Female | ≥80 cm | |
| People from South Asia 2 | Male | ≥90 cm |
| Female | ≥80 cm | |
| Chinese | Male | ≥90 cm |
| Female | ≥80 cm | |
| Japanese 3 | Male | ≥90 cm |
| Female | ≥80 cm | |
| Ethnic groups from South and Central America 4 | Male | ≥90 cm |
| Female | ≥80 cm | |
| Ethnic groups of the Eastern Mediterranean and the Middle East (Arabs) 5 | Male | ≥94 cm |
| Female | ≥80 cm |
| Component of MetS | Biomarker (Diagnostic Method) |
|---|---|
| Improper distribution of body fat (abdominal obesity) | Total body fat distribution (DEXA). Central distribution of fat (CT/MRI). Biomarkers of adipose tissue: leptin, adiponectin (blood test). Liver fat concentration (MRS). |
| Atherogenic dyslipidemia (besides the high triglyceride levels and low high-density cholesterol lipoproteins in the blood) | Apolipoprotein B or non-high-density cholesterol lipoproteins (blood chemistry). Small particles of low-density cholesterol lipoproteins (blood chemistry). |
| Insulin resistance (except for increased prandial blood glucose) | Prandial insulin/proinsulin levels (blood test). HOMA-IR—the higher the index, the lower the responsiveness of cells to insulin, that is, the more severe the insulin resistance (blood test). Insulin resistance according to the Bergman minimal model is a mathematical modeling of the glycemic regulation system in patients with diabetes—three ordinary differential equations describing changes in the concentration of glucose and insulin in plasma as well as the elimination of insulin in the body without taking into account external influences (blood test). Increased concentration of FFA on an empty stomach and during meals (blood test). Glucose tolerance test (blood test). |
| Vascular dysregulation (besides increased blood pressure) | Detection of markers of endothelial dysfunction (blood test). Microalbuminuria (urine test). |
| Pro-inflammatory status (systemic inflammation) | Elevated levels of highly responsive CRP (blood test). Increased levels of proinflammatory cytokines, including TNF-α, IL-6, etc. (blood test). Decrease in plasma adiponectin levels (blood test). |
| Prothrombotic status | Fibrinolytic factors, including PAI-1, etc. (blood test). Blood clotting factors, including fibrinogen, etc. (blood test). |
| Hormonal status | Pituitary–adrenal system (blood test). |
References
- Magheru, C.; Magheru, S.; Coltau, M.; Hoza, A.; Moldovan, C.; Sachelarie, L.; Gradinaru, I.; Hurjui, L.L.; Marc, F.; Farcas, D.M. Antiepileptic Drugs and Their Dual Mechanism of Action on Carbonic Anhydrase. J. Clin. Med. 2022, 11, 2614. [Google Scholar] [CrossRef] [PubMed]
- Biso, L.; Aringhieri, S.; Carli, M.; Scarselli, M.; Longoni, B. Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals 2024, 17, 642. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Vale, N. Understanding Lamotrigine’s Role in the CNS and Possible Future Evolution. Int. J. Mol. Sci. 2023, 24, 6050. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, D.; Nikolić, M.; Jovanović, V.; Vidonja Uzelac, T.; Nikolić-Kokić, A.; Novaković, E.; Miljević, Č.; Milovanović, M.; Blagojević, D. Influence of Long-Term Anti-Seizure Medications on Redox Parameters in Human Blood. Pharmaceuticals 2024, 17, 130. [Google Scholar] [CrossRef]
- Kaushik, S.; Chopra, D.; Sharma, S.; Aneja, S. Adverse Drug Reactions of Anti-Epileptic Drugs in Children with Epilepsy: A Cross-Sectional Study. Curr. Drug Saf. 2019, 14, 217–224. [Google Scholar] [CrossRef]
- The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Available online: https://idf.org/media/uploads/2023/05/attachments-30.pdf (accessed on 22 December 2024).
- Li, W.; Qiu, X.; Ma, H.; Geng, Q. Incidence and Long-Term Specific Mortality Trends of Metabolic Syndrome in the United States. Front. Endocrinol. 2023, 13, 1029736. [Google Scholar] [CrossRef]
- Chong, K.S.; Chang, Y.H.; Yang, C.T.; Chou, C.K.; Ou, H.T.; Kuo, S. Longitudinal Economic Burden of Incident Complications Among Metabolic Syndrome Populations. Cardiovasc. Diabetol. 2024, 23, 246. [Google Scholar] [CrossRef]
- Nazish, S. Obesity And Metabolic Syndrome in Patients with Epilepsy, Their Relation with Epilepsy Control. Ann. Afr. Med. 2023, 22, 136–144. [Google Scholar] [CrossRef]
- Beyene Kassaw, A.; Tezera Endale, H.; Hunie Tesfa, K.; Derbew Molla, M. Metabolic Syndrome and Its Associated Factors Among Epileptic Patients at Dessie Comprehensive S pecialized Hospital, Northeast Ethiopia; A Hospital-Based Comparative Cross-Sectional Study. PLoS ONE 2022, 17, e0279580. [Google Scholar] [CrossRef]
- Nair, S.S.; Harikrishnan, S.; Sarma, P.S.; Thomas, S.V. Metabolic Syndrome in Young Adults with Epilepsy. Seizure 2016, 37, 61–64. [Google Scholar] [CrossRef]
- Chen, B.; Choi, H.; Hirsch, L.J.; Moeller, J.; Javed, A.; Kato, K.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Cosmetic Side Effects of Antiepileptic Drugs in Adults with Epilepsy. Epilepsy Behav. 2015, 42, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Grechkina, V.V.; Trefilova, V.V.; Efremov, I.S.; Dontceva, E.A.; Narodova, E.A.; Petrova, M.M.; Soloveva, I.A.; Tepnadze, L.E.; Reznichenko, P.A.; et al. Valproate-Induced Metabolic Syndrome. Biomedicines 2023, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.; Wu, T.-Y.; Lin, C.-L.; Chu, F.-Y.; Wang, C.C.N.; Hsu, C.Y.; Tsai, F.-J.; Fang, Y.-J.; Lim, Y.-P. Association of Epilepsy, Anti-Epileptic Drugs (Aeds), and Type 2 Diabetes Mellitus (T2dm): A Population-Based Cohort Retrospective Study, Impact of Aeds on T2dm-Related Molecular Pathway, and Via Peroxisome Proliferator-Activated Receptor γ Transactivation. Front. Endocrinol. 2023, 14, 1156952. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, A.; Kõks, S.; Haldre, S. Metabolic Syndrome and Anticonvulsants: A Comparative Study of Valproic Acid and Carbamazepine. Seizure 2016, 38, 11–16. [Google Scholar] [CrossRef]
- Meenakshi-Sundaram, S.; Sankaranarayanan, M. Epilepsy, Phenytoin, and Atherogenic Risk—Current Perspectives. Neurol. India 2021, 69, 962–963. [Google Scholar] [CrossRef]
- Li, Y.X.; Guo, W.; Chen, R.X.; Lv, X.R.; Li, Y. The Relationships Between Obesity and Epilepsy: A Systematic Review with Meta-Analysis. PLoS ONE 2024, 19, e0306175. [Google Scholar] [CrossRef]
- Dehury, S.; Patro, P.; Sahu, L.; Nayak, L.; Mallik, A.K. Evaluation of Metabolic Parameters on Use of Newer Antiepileptics Versus Conventional Antiepileptics in Patients of Generalised Tonic-Clonic Seizure: An Observational Study. Cureus 2023, 15, e35181. [Google Scholar] [CrossRef]
- Stols-Gonçalves, D.; Tristão, L.S.; Henneman, P.; Nieuwdorp, M. Epigenetic Markers and Microbiota/Metabolite-Induced Epigenetic Modifications in the Pathogenesis of Obesity, Metabolic Syndrome, Type 2 Diabetes, and Non-alcoholic Fatty Liver Disease. Curr. Diabetes Rep. 2019, 19, 31. [Google Scholar] [CrossRef]
- Pant, R.; Firmal, P.; Shah, V.K.; Alam, A.; Chattopadhyay, S. Epigenetic Regulation of Adipogenesis in Development of Metabolic Syndrome. Front. Cell Dev. Biol. 2021, 8, 619888. [Google Scholar] [CrossRef]
- Bethesda, M. Biomarkers Definitions Working Group. Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Lee, S.Y. Useful Biomarkers of Metabolic Syndrome. Int. J. Environ. Res. Public. Health. 2022, 19, 15003. [Google Scholar] [CrossRef] [PubMed]
- Lauschke, V.M.; Zhou, Y.; Ingelman-Sundberg, M. Novel Genetic and Epigenetic Factors of Importance for Inter-Individual Differences in Drug Disposition, Response and Toxicity. Pharmacol. Ther. 2019, 197, 122–152. [Google Scholar] [CrossRef]
- Reynolds, E.H. Antiepileptic Drugs, Folate One-Carbon Metabolism, Genetics, and Epigenetics: Congenital, Developmental, and Neuropsychological Risks and Antiepileptic Action. Epilepsia 2024, 65, 3469–3473. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; Seco-Cervera, M.; Tollefsbol, T.O.; Romá-Mateo, C.; Peiró-Chova, L.; Lapunzina, P.; Pallardó, F.V. Epigenetic Biomarkers: Current Strategies and Future Challenges for Their Use in the Clinical Laboratory. Crit. Rev. Clin. Lab. Sci. 2017, 54, 529–550. [Google Scholar] [CrossRef]
- Mironova, O.I.; Berdysheva, M.V.; Elfimova, E.M. Microrna: A Clinician’s View of the State of the Problem. Part 2. MicroRNA as a Biomarker. Eurasian Heart J. 2023, 2, 64–71. (In Russian) [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Pozniak, T.; Shcharbin, D.; Bryszewska, M. Circulating microRNAs in Medicine. Int. J. Mol. Sci. 2022, 23, 3996. [Google Scholar] [CrossRef]
- Brandão-Lima, P.N.; de Carvalho, G.B.; Payolla, T.B.; Sarti, F.M.; Fisberg, R.M.; Malcomson, F.C.; Mathers, J.C.; Rogero, M.M. Circulating microRNAs Showed Specific Responses according to Metabolic Syndrome Components and Sex of Adults from a Population-Based Study. Metabolites 2023, 13, 2. [Google Scholar] [CrossRef]
- Solís-Toro, D.; Mosquera Escudero, M.; García-Perdomo, H.A. Association Between Circulating microRNAs and the Metabolic Syndrome In Adult Populations: A Systematic Review. Diabetes Metab. Syndr. 2022, 16, 102376. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Hussen, B.M.; Abak, A.; Taheri, M.; Jalili Khoshnoud, R. Aberrant Expression of miRNAs in Epilepsy. Mol. Biol. Rep. 2022, 49, 5057–5074. [Google Scholar] [CrossRef] [PubMed]
- Soler-Botija, C.; Gálvez-Montón, C.; Bayés-Genís, A. Epigenetic Biomarkers in Cardiovascular Diseases. Front. Genet. 2019, 10, 950. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Saha, S. Role of microRNA in Oxidative Stress. Stresses 2024, 4, 269–281. [Google Scholar] [CrossRef]
- Włodarski, A.; Strycharz, J.; Wróblewski, A.; Kasznicki, J.; Drzewoski, J.; Śliwińska, A. The Role of microRNAs in Metabolic Syndrome-Related Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 6902. [Google Scholar] [CrossRef]
- Carvalho, G.B.; Brandão-Lima, P.N.; Payolla, T.B.; Lucena, S.E.F.; Sarti, F.M.; Fisberg, R.M.; Rogero, M.M. Circulating miRNAs Are Associated with Low-grade Systemic Inflammation and Leptin Levels in Older Adults. Inflammation 2023, 46, 2132–2146. [Google Scholar] [CrossRef]
- Das, K.; Rao, L.V.M. The Role of microRNAs in Inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A. Adipogenesis-related microRNAs in Obesity. ExRNA 2022, 4, 16. [Google Scholar] [CrossRef]
- Agbu, P.; Carthew, R.W. MicroRNA-mediated Regulation of Glucose and Lipid Metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Ye, Y.; Chen, Z.; Xiao, T.; Liu, W.; Hu, F. MicroRNA 182 is a Novel Negative Regulator of Adipogenesis by Targeting CCAAT/Enhancer-Binding Protein α. Obesity 2020, 28, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Wagschal, A.; Najafi-Shoushtari, S.H.; Wang, L.; Goedeke, L.; Sinha, S.; deLemos, A.S.; Black, J.C.; Ramírez, C.M.; Li, Y.; Tewhey, R.; et al. Genome-wide Identification of microRNAs Regulating Cholesterol and Triglyceride Homeostasis. Nat. Med. 2015, 21, 1290–1297. [Google Scholar] [CrossRef]
- Cheng, J.; Cheng, A.; Clifford, B.L.; Wu, X.; Hedin, U.; Maegdefessel, L.; Pamir, N.; Sallam, T.; Tarling, E.J.; de Aguiar Vallim, T.Q. MicroRNA-144 Silencing Protects Against Atherosclerosis in Male, but Not Female Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 412–425. [Google Scholar] [CrossRef]
- Irani, S.; Iqbal, J.; Antoni, W.J.; Ijaz, L.; Hussain, M.M. MicroRNA-30c Reduces Plasma Cholesterol in Homozygous Familial Hypercholesterolemic and Type 2 Diabetic Mouse Models. J. Lipid Res. 2018, 59, 144–154. [Google Scholar] [CrossRef]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 Regulate Insulin Sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Liu, W.; Cao, H.; Ye, C.; Chang, C.; Lu, M.; Jing, Y.; Zhang, D.; Yao, X.; Duan, Z.; Xia, H.; et al. Hepatic miR-378 Targets P110α and Controls Glucose and Lipid Homeostasis by Modulating Hepatic Insulin Signalling. Nat. Commun. 2014, 5, 5684. [Google Scholar] [CrossRef]
- Kornfeld, J.W.; Baitzel, C.; Könner, A.C.; Kornfeld, J.W.; Baitzel, C.; Könner, A.C.; Nicholls, H.T.; Vogt, M.C.; Herrmanns, K.; Scheja, L.; et al. Obesity-induced Overexpression of miR-802 Impairs Glucose Metabolism through Silencing of Hnf1b. Nature 2013, 494, 111–115. [Google Scholar] [CrossRef]
- Xu, H.; Du, X.; Xu, J.; Zhang, Y.; Tian, Y.; Liu, G.; Wang, X.; Ma, M.; Du, W.; Liu, Y.; et al. Pancreatic β Cell microRNA-26a Alleviates Type 2 Diabetes by Improving Peripheral Insulin Sensitivity and Preserving β Cell Function. PLoS Biol. 2020, 18, e3000603. [Google Scholar] [CrossRef]
- Ofori, J.K.; Salunkhe, V.A.; Bagge, A.; Vishnu, N.; Nagao, M.; Mulder, H.; Wollheim, C.B.; Eliasson, L.; Esguerra, J.L. Elevated miR-130a/miR130b/miR-152 Expression Reduces Intracellular ATP Levels in the Pancreatic Beta Cell. Sci. Rep. 2017, 7, 44986. [Google Scholar] [CrossRef]
- Belgardt, B.F.; Ahmed, K.; Spranger, M.; Latreille, M.; Denzler, R.; Kondratiuk, N.; von Meyenn, F.; Villena, F.N.; Herrmanns, K.; Bosco, D.; et al. The microRNA-200 Family Regulates Pancreatic Beta Cell Survival in Type 2 Diabetes. Nat. Med. 2015, 21, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, D.; Zhao, W.; Wang, D.; Liu, T.; Liu, Y.; Yang, Y.; Liu, Y.; Mu, J.; Li, B.; et al. Obesity-induced overexpression of miR-802 Impairs Insulin Transcription and Secretion. Nat. Commun. 2020, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Melkman-Zehavi, T.; Oren, R.; Kredo-Russo, S.; Shapira, T.; Mandelbaum, A.D.; Rivkin, N.; Nir, T.; Lennox, K.A.; Behlke, M.A.; Dor, Y.; et al. MiRNAs Control Insulin Content in Pancreatic β-Cells Via Downregulation of Transcriptional Repressors. EMBO J. 2011, 30, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Fernández-Tussy, P.; Varela, L.; Cardelo, M.P.; Shanabrough, M.; Aryal, B.; de Cabo, R.; Suárez, Y.; Horvath, T.L.; Fernández-Hernando, C. MicroRNA-33 Controls Hunger Signaling in Hypothalamic AgRP Neurons. Nat. Commun. 2024, 15, 2131. [Google Scholar] [CrossRef]
- Aranda, A. MicroRNAs and Thyroid Hormone Action. Mol. Cell. Endocrinol. 2021, 525, 111175. [Google Scholar] [CrossRef]
- Taouis, M. MicroRNAs in the Hypothalamus. Best. Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 641–651. [Google Scholar] [CrossRef]
- Zhang, D.; Yamaguchi, S.; Zhang, X.; Yang, B.; Kurooka, N.; Sugawara, R.; Albuayjan, H.H.H.; Nakatsuka, A.; Eguchi, J.; Hiyama, T.Y.; et al. Upregulation of Mir342 in Diet-Induced Obesity Mouse and the Hypothalamic Appetite Control. Front. Endocrinol. 2021, 12, 727915, Erratum in Front. Endocrinol. 2021, 12, 811189. https://doi.org/10.3389/fendo.2021.811189; Erratum in Front. Endocrinol. 2021, 12, 811765. https://doi.org/10.3389/fendo.2021.811765. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Pena-Bello, L.; Manfredi-Lozano, M.; Tena-Sempere, M.; Cordido, F. Perturbation of Hypothalamic Microrna Expression Patterns in Male Rats after Metabolic Distress: Impact of Obesity and Conditions of Negative Energy Balance. Endocrinology 2014, 155, 1838–1850. [Google Scholar] [CrossRef]
- Derghal, A.; Djelloul, M.; Azzarelli, M.; Degonon, S.; Tourniaire, F.; Landrier, J.F.; Mounien, L. MicroRNAs are Involved in the Hypothalamic Leptin Sensitivity. Epigenetics 2018, 13, 1127–1140. [Google Scholar] [CrossRef]
- Mak, K.W.Y.; He, W.; Loganathan, N.; Belsham, D.D. Bisphenol A Alters the Levels of miRNAs That Directly and/or Indirectly Target Neuropeptide Y in Murine Hypothalamic Neurons. Genes 2023, 14, 1773. [Google Scholar] [CrossRef]
- Holm, A.; Possovre, M.L.; Bandarabadi, M.; Moseholm, K.F.; Justinussen, J.L.; Bozic, I.; Lemcke, R.; Arribat, Y.; Amati, F.; Silahtaroglu, A.; et al. The Evolutionarily Conserved miRNA-137 Targets the Neuropeptide Hypocretin/Orexin and Modulates the Wake to Sleep Ratio. Proc. Natl. Acad. Sci. USA 2022, 119, e2112225119. [Google Scholar] [CrossRef] [PubMed]
- Azhar, S.; Dong, D.; Shen, W.J.; Hu, Z.; Kraemer, F.B. The Role of miRNAs in Regulating Adrenal and Gonadal Steroidogenesis. J. Mol. Endocrinol. 2020, 64, R21–R43. [Google Scholar] [CrossRef] [PubMed]
- Vaira, V.; Verdelli, C.; Forno, I.; Corbetta, S. MicroRNAs in Parathyroid Physiopathology. Mol. Cell. Endocrinol. 2017, 456, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Martinc, B.; Grabnar, I.; Milosheska, D.; Lorber, B.; Vovk, T. A Cross-Sectional Study Comparing Oxidative Stress in Patients with Epilepsy Treated with Old and New Generation Antiseizure Medications. Medicina 2024, 60, 1299. [Google Scholar] [CrossRef]
- Swathi, B.; Aruna, D. Evaluation of Antioxidant Effects of Antiepileptic Drugs in Adult Epileptic Patients: An Open Label, Non Randomised Interventional Study. J. Clin. Diagn. Res. 2022, 16, 10–14. [Google Scholar] [CrossRef]
- Morimoto, M.; Satomura, S.; Hashimoto, T.; Kyotani, S. A Study of oxidative Stress and the Newer Antiepileptic Drugs in Epilepsy Associated with Severe Motor and Intellectual Disabilities. J. Chin. Med. Assoc. 2017, 80, 19–28. [Google Scholar] [CrossRef][Green Version]
- Tkachev, V.O.; Menshchikova, E.B.; Zenkov, N.K. Mechanism of the NRF2/KEAP1/ARE Signaling System. Biochemistry 2011, 76, 407–422. (In Russian) [Google Scholar]
- Zhao, M.; Li, G.; Zhao, L. The Role of SIRT1-FXR Signaling Pathway in Valproic Acid Induced Liver Injury: A Quantitative Targeted Metabolomic Evaluation in Epileptic Children. Front. Pharmacol. 2024, 15, 1477619. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, S.; Yang, Y.; Wei, J.; Li, X.; Luo, P.; Jiang, X. Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System. Biomolecules 2024, 13, 735. [Google Scholar] [CrossRef]
- Yang, W.; Nagasawa, K.; Munch, C.; Xu, Y.; Satterstrom, K.; Jeong, S.; Hayes, S.D.; Jedrychowski, M.P.; Vyas, F.S.; Zaganjor, E.; et al. Mitochondrial Sirtuin Network Reveals Dynamic Sirt3-Dependent Deacetylation in Response to Membrane Depolarization. Cell 2016, 167, 985–1000.e21. [Google Scholar] [CrossRef]
- Tang, P.; Dang, H.; Huang, J.; Xu, T.; Yuan, P.; Hu, J.; Sheng, J.F. NADPH Oxidase NOX4 is a Glycolytic Regulator through mROS-HIF1α Axis in Thyroid Carcinomas. Sci. Rep. 2018, 8, 15897. [Google Scholar] [CrossRef]
- Palsamy, P.; Bidasee, K.R.; Shinohara, T. Valproic Acid Suppresses Nrf2/Keap1 Dependent Antioxidant Protection through Induction of Endoplasmic Reticulum Stress and Keap1 Promoter DNA Demethylation in Human Lens Epithelial Cells. Exp. Eye Res. 2014, 121, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Chen, S.D.; Lin, K.J.; Chuang, Y.C. Seizure-Induced Oxidative Stress in Status Epilepticus: Is Antioxidant Beneficial? Antioxidants 2020, 9, 1029. [Google Scholar] [CrossRef]
- Ignacio-Mejía, I.; Contreras-García, I.J.; Mendoza-Torreblanca, J.G.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; García-Cruz, M.E.; Romo-Mancillas, A.; Gómez-Manzo, S.; Bandala, C.; Sánchez-Mendoza, M.E.; et al. Evaluation of the Antioxidant Activity of Levetiracetam in a Temporal Lobe Epilepsy Model. Biomedicines 2023, 11, 848. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Budziszewska, B.; Lasoń, W. Wpływ Leków Przeciwpadaczkowych na Układ Odpornościowy [Effects of Antiepileptic Drugs on Immune System]. Przegl Lek. 2008, 65, 799–802. [Google Scholar] [PubMed]
- Beghi, E.; Shorvon, S. Antiepileptic Drugs and the Immune System. Epilepsia 2011, 52 (Suppl. 3), 40–44. [Google Scholar] [CrossRef]
- Dambach, H.; Hinkerohe, D.; Prochnow, N.; Stienen, M.N.; Moinfar, Z.; Haase, C.G.; Hufnagel, A.; Faustmann, P.M. Glia and Epilepsy: Experimental Investigation of Antiepileptic Drugs in an Astroglia/Microglia Co-Culture Model of Inflammation. Epilepsia 2014, 55, 184–192. [Google Scholar] [CrossRef]
- Godhwani, N.; Bahna, S.L. Antiepilepsy Drugs and the Immune System. Ann. Allergy Asthma Immunol. 2016, 117, 634–640. [Google Scholar] [CrossRef]
- Sun, H.; Ma, D.; Cheng, Y.; Li, J.; Zhang, W.; Jiang, T.; Li, Z.; Li, X.; Meng, H. The JAK-STAT Signaling Pathway in Epilepsy. Curr. Neuropharmacol. 2023, 21, 2049–2069. [Google Scholar] [CrossRef]
- Cai, M.; Lin, W. The Function of NF-Kappa B During Epilepsy, a Potential Therapeutic Target. Front. Neurosci. 2022, 16, 851394. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Bai, S.; Yu, H.; He, H.; Fan, C.; Hao, Y.; Guan, Y. A PIK3R2 Mutation in Familial Temporal Lobe Epilepsy as a Possible Pathogenic Variant. Front. Genet. 2021, 12, 596709. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; Li, X.; Cheng, H.; Gan, J.; Liu, Z. The TLR4 Mediated Inflammatory Signal Pathway Might Be Involved in Drug Resistance in Drug-Resistant Epileptic Rats. J. Neuroimmunol. 2022, 365, 577802. [Google Scholar] [CrossRef] [PubMed]
- Rafi, S.K.; Goering, J.P.; Olm-Shipman, A.J.; Hipp, L.A.; Ernst, N.J.; Wilson, N.R.; Hall, E.G.; Gunewardena, S.; Saadi, I. Anti-Epileptic Drug Topiramate Upregulates TGFβ1 and SOX9 Expression in Primary Embryonic Palatal Mesenchyme Cells: Implications for Teratogenicity. PLoS ONE 2021, 16, e0246989. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Cabral, J.L.; Beas-Zárate, C.; Rocha-Arrieta, L.L.; Orozco-Suárez, S.A.; Alonso-Vanegas, M.; Guevara-Guzmán, R.; Ureña-Guerrero, M.E. Increased Protein Expression of VEGF-A, VEGF-B, VEGF-C and Their Receptors in the Temporal Neocortex of Pharmacoresistant Temporal Lobe Epilepsy Patients. J. Neuroimmunol. 2019, 328, 68–72. [Google Scholar] [CrossRef]
- Cheng, L.; Xia, F.; Li, Z.; Shen, C.; Yang, Z.; Hou, H.; Sun, S.; Feng, Y.; Yong, X.; Tian, X.; et al. Structure, Function and Drug Discovery of GPCR Signaling. Mol. Biomed. 2023, 4, 46. [Google Scholar] [CrossRef]
- Wang, N.; Han, X.; Liu, H.; Zhao, T.; Li, J.; Feng, Y.; Mi, X.; Zhang, Y.; Chen, Y.; Wang, X. Myeloid Differentiation Factor 88 is Up-Regulated in Epileptic Brain and Contributes to Experimental Seizures in Rats. Exp. Neurol. 2017, 295, 23–35. [Google Scholar] [CrossRef]
- Poonaki, E.; Kahlert, U.D.; Meuth, S.G.; Gorji, A. The Role of the ZEB1-Neuroinflammation Axis in CNS Disorders. J. Neuroinflamm. 2022, 19, 275. [Google Scholar] [CrossRef]
- Porretti, J.; Dalton, G.N.; Massillo, C.; Scalise, G.D.; Farré, P.L.; Elble, R.; Gerez, E.N.; Accialini, P.; Cabanillas, A.M.; Gardner, K.; et al. CLCA2 Epigenetic Regulation by CTBP1, HDACs, ZEB1, EP300 and miR-196b-5p Impacts Prostate Cancer Cell Adhesion and EMT in Metabolic Syndrome Disease. Int. J. Cancer 2018, 143, 897–906. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J.; Teo, J.C.M.; Abou-Kheir, W.; Al-Sayegh, M. Modeling Adipogenesis: Current and Future Perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef]
- Ghesmati, Z.; Rashid, M.; Fayezi, S.; Gieseler, F.; Alizadeh, E.; Darabi, M. An Update on the Secretory Functions Of Brown, White, and Beige Adipose Tissue: Towards Therapeutic Applications. Rev. Endocr. Metab. Disord. 2024, 25, 279–308. [Google Scholar] [CrossRef]
- Im, D.U.; Kim, S.C.; Chau, G.C.; Um, S.H. Carbamazepine Enhances Adipogenesis by Inhibiting Wnt/β-catenin Expression. Cells 2019, 8, 1460. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Choo, K.B.; Chen, C.F. The microRNA-Signaling-Peroxisome Proliferator-Activated Receptor Gamma Connection in the Modulation of Adipogenesis: Bioinformatics Projection on Chicken. Poult. Sci. 2022, 101, 101950. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Nishina, M.; Koizumi, K.; Katayama, M.; Inoue, S.; Suga, S. Impact of Enzyme-Inducing Anti-Epilepsy Drugs on Lipid Levels in Elderly Patients with Epilepsy. Epilepsy Res. 2020, 166, 106428. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.L.; Diaz-Arias, L.; Cervenka, M.C.; McDonald, T.J.W. The Effect of Anti-Seizure Medications on Lipid Values in Adults with Epilepsy. Epilepsy Behav. 2023, 144, 109260. [Google Scholar] [CrossRef]
- Knebel, B.; Hartwig, S.; Jacob, S.; Kettel, U.; Schiller, M.; Passlack, W.; Koellmer, C.; Lehr, S.; Müller-Wieland, D.; Kotzka, J. Inactivation of SREBP-1a Phosphorylation Prevents Fatty Liver Disease in Mice: Identification of Related Signaling Pathways by Gene Expression Profiles in Liver and Proteomes of Peroxisomes. Int. J. Mol. Sci. 2018, 19, 980. [Google Scholar] [CrossRef]
- Babu, B.S.; Varghese, C.P.; Gilvaz, P.C. Effects of Long Term Antiseizure Medications on Atherosclerosis. J. Med. Sci. Res. 2023, 11, 329–335. [Google Scholar] [CrossRef]
- Farinelli, E.; Giampaoli, D.; Cenciarini, A.; Cercado, E.; Verrotti, A. Valproic Acid and Nonalcoholic Fatty Liver Disease: A Possible Association? World J. Hepatol. 2015, 7, 1251–1257. [Google Scholar] [CrossRef]
- Rehman, T.; Sachan, D.; Chitkara, A. Serum Insulin and Leptin Levels in Children with Epilepsy on Valproate-associated Obesity. J. Pediatr. Neurosci. 2017, 12, 135–137. [Google Scholar] [CrossRef]
- Shlobin, N.A.; Sander, J.W. Drivers for the Comorbidity of Type 2 Diabetes Mellitus and Epilepsy: A Scoping Review. Epilepsy Behav. 2020, 106, 107043. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Petrosino, M.I.; Chiarelli, F. Diabetes and Epilepsy in Children and Adolescents. Curr. Diabetes Rep. 2015, 15, 21. [Google Scholar] [CrossRef]
- Li, Y.Z.; Di Cristofano, A.; Woo, M. Metabolic Role of PTEN in Insulin Signaling and Resistance. Cold Spring Harb. Perspect. Med. 2020, 10, a036137. [Google Scholar] [CrossRef] [PubMed]
- Ramasubbu, K.; Devi Rajeswari, V. Impairment of Insulin Signaling Pathway PI3K/Akt/mTOR and Insulin Resistance Induced AGEs on Diabetes Mellitus and Neurodegenerative Diseases: A Perspective Review. Mol. Cell Biochem. 2023, 478, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, S.C.; Pattnaik, S.S.; Dash, Y.; Tripathi, M.; Velpandian, T. Is There Any Concern of Insulin Resistance and Metabolic Dysfunctions with Antiseizure Medications? A Prospective Comparative Study of Valproate Vs. Levetiracetam. Seizure 2024, 121, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Buraniqi, E.; Dabaja, H.; Wirrell, E.C. Impact of Antiseizure Medications on Appetite and Weight in Children. Paediatr. Drugs 2022, 24, 335–363. [Google Scholar] [CrossRef]
- Sonmez, F.M.; Zaman, D.; Aksoy, A.; Deger, O.; Aliyazicioglu, R.; Karaguzel, G.; Fazlioglu, K. The Effects of Topiramate and Valproate Therapy on Insulin, C-Peptide, Leptin, Neuropeptide Y, Adiponectin, Visfatin, and Resistin Levels in Children with Epilepsy. Seizure 2013, 22, 856–861. [Google Scholar] [CrossRef]
- Shan, Y.; Chen, Y.; Gu, H.; Wang, Y.; Sun, Y. Regulatory Basis of Adipokines Leptin and Adiponectin in Epilepsy: From Signaling Pathways to Glucose Metabolism. Neurochem. Res. 2023, 48, 2017–2028. [Google Scholar] [CrossRef]
- Arslan, G.A.; Saygi, S.; Bodur, E.; Cicek, C.; Tezer, F.I. Relation Between Orexin A and Epileptic Seizures. Epilepsy Res. 2022, 184, 106972. [Google Scholar] [CrossRef]
- Burakgazi Dalkilic, E. Effects of Antiepileptic Drugs on Hormones. Neurosci. Lett. 2021, 754, 135800. [Google Scholar] [CrossRef]
- Adhimoolam, M.; Arulmozhi, R. Effect of Antiepileptic Drug Therapy on Thyroid Hormones Among Adult Epileptic Patients: An Analytical Cross-Sectional Study. J. Res. Pharm. Pract. 2016, 5, 171–174. [Google Scholar] [CrossRef]
- Su, X.; Chen, X.; Peng, H.; Song, J.; Wang, B.; Wu, X. Novel Insights into the Pathological Development of Dyslipidemia in Patients with Hypothyroidism. Bosn. J. Basic. Med. Sci. 2022, 22, 326–339. [Google Scholar] [CrossRef]
- Dontseva, E.A.; Pilipenko, P.I.; Shnayder, N.A.; Petrova, M.M.; Nasyrova, R.F. Prevalence of Anticonvulsant-Induced Vitamin D Deficiency. Epilepsy Paroxysmal Cond. 2022, 14, 304–315. [Google Scholar] [CrossRef]
- Tombini, M.; Palermo, A.; Assenza, G.; Pellegrino, G.; Benvenga, A.; Campana, C.; Naciu, A.M.; Assenza, F.; Lazzaro, V.D. Calcium Metabolism Serum Markers in Adult Patients with Epilepsy and the Effect of Vitamin D Supplementation on Seizure Control. Seizure 2018, 58, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.N.; Kufareva, I.; Josephs, T.M.; Diao, J.; Mai, V.T.; Conigrave, A.D.; Christopoulos, A.; Gregory, K.J.; Leach, K. Identification of Global and Ligand-Specific Calcium Sensing Receptor Activation Mechanisms. Mol. Pharmacol. 2018, 93, 619–630. [Google Scholar] [CrossRef]
- Anghel, S.A.; Dinu-Pirvu, C.E.; Costache, M.A.; Voiculescu, A.M.; Ghica, M.V.; Anuța, V.; Popa, L. Receptor Pharmacogenomics: Deciphering Genetic Influence on Drug Response. Int. J. Mol. Sci. 2024, 25, 9371. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Grechkina, V.V.; Kissin, M.Y.; Dmitrenko, D.V.; Nasyrova, R.F. Role of Neuropeptide Y in Development of Valproate-Induced Eating Behaviour Disorder. Epilepsy Paroxysmal Cond. 2024, 16, 349–361. [Google Scholar] [CrossRef]
- Neznanov, N.G. A Paradigm Shift To Treat Psychoneurological Disorders. Pers. Psychiatry Neurol. 2021, 1, 1–2. [Google Scholar]
- Ashurov, Z.S. The Evolution of Personalized Psychiatry. Pers. Psychiatry Neurol. 2023, 3, 1–2. [Google Scholar]
- Martinez, B.; Peplow, P.V. MicroRNAs as Potential Biomarkers in Temporal Lobe Epilepsy and Mesial Temporal Lobe Epilepsy. Neural Regen. Res. 2023, 18, 716–726. [Google Scholar] [CrossRef]
- De Benedittis, S.; Fortunato, F.; Cava, C.; Gallivanone, F.; Iaccino, E.; Caligiuri, M.E.; Castiglioni, I.; Bertoli, G.; Manna, I.; Labate, A.; et al. Circulating microRNAs as Potential Novel Diagnostic Biomarkers to Predict Drug Resistance in Temporal Lobe Epilepsy: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 702. [Google Scholar] [CrossRef]
- Rzepka-Migut, B.; Paprocka, J. Prospects and Limitations Related to the Use of MicroRNA as a Biomarker of Epilepsy in Children: A Systematic Review. Life 2021, 11, 26. [Google Scholar] [CrossRef]
- Iacomino, G. miRNAs: The Road from Bench to Bedside. Genes 2023, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Wang, E.; Zhao, Y.; Marincola, F.; Landi, M.T. Strengths and Limitations of Laboratory Procedures for microRNA Detection. Cancer Epidemiol. Biomark. Prev. 2010, 19, 907–911. [Google Scholar] [CrossRef]
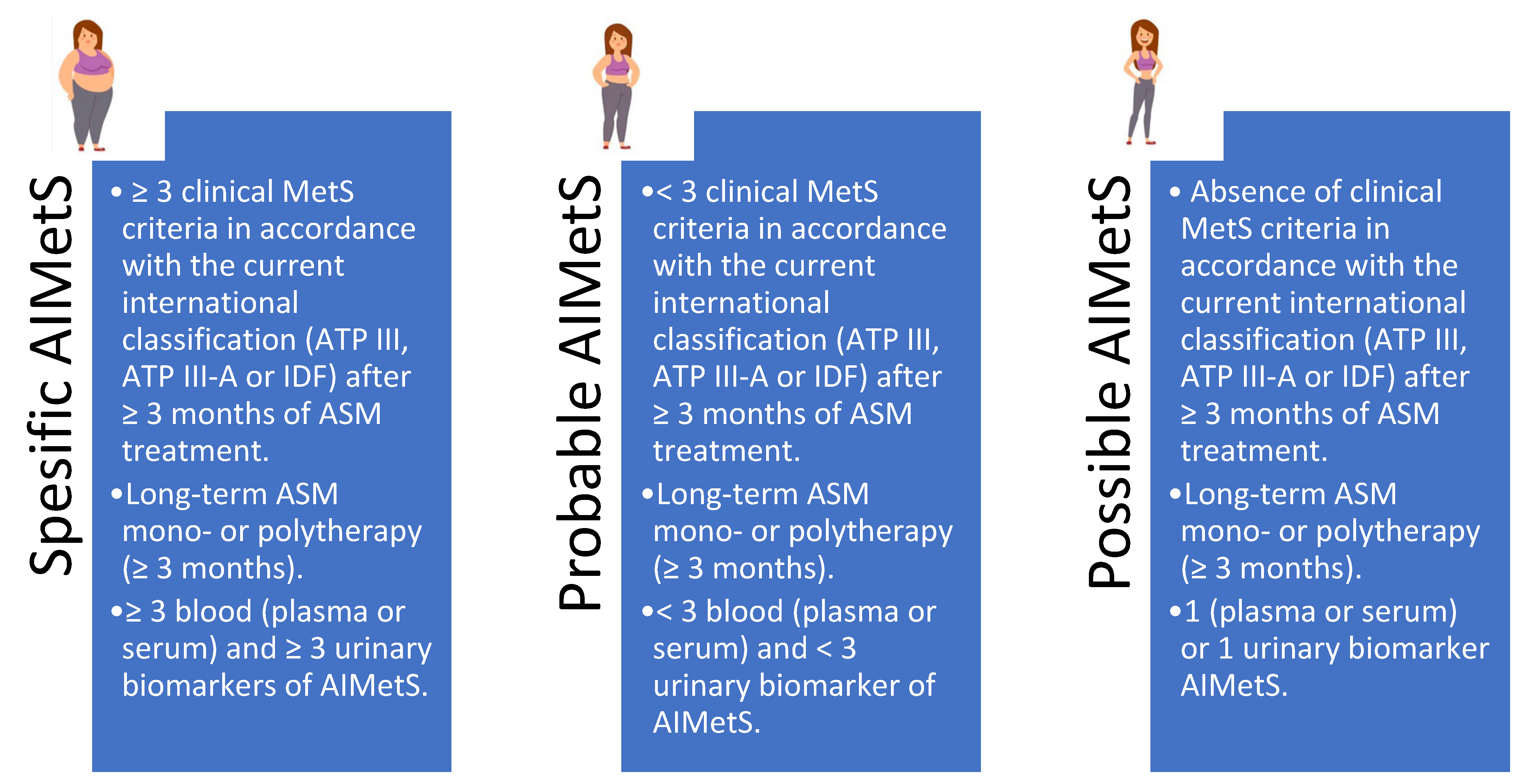
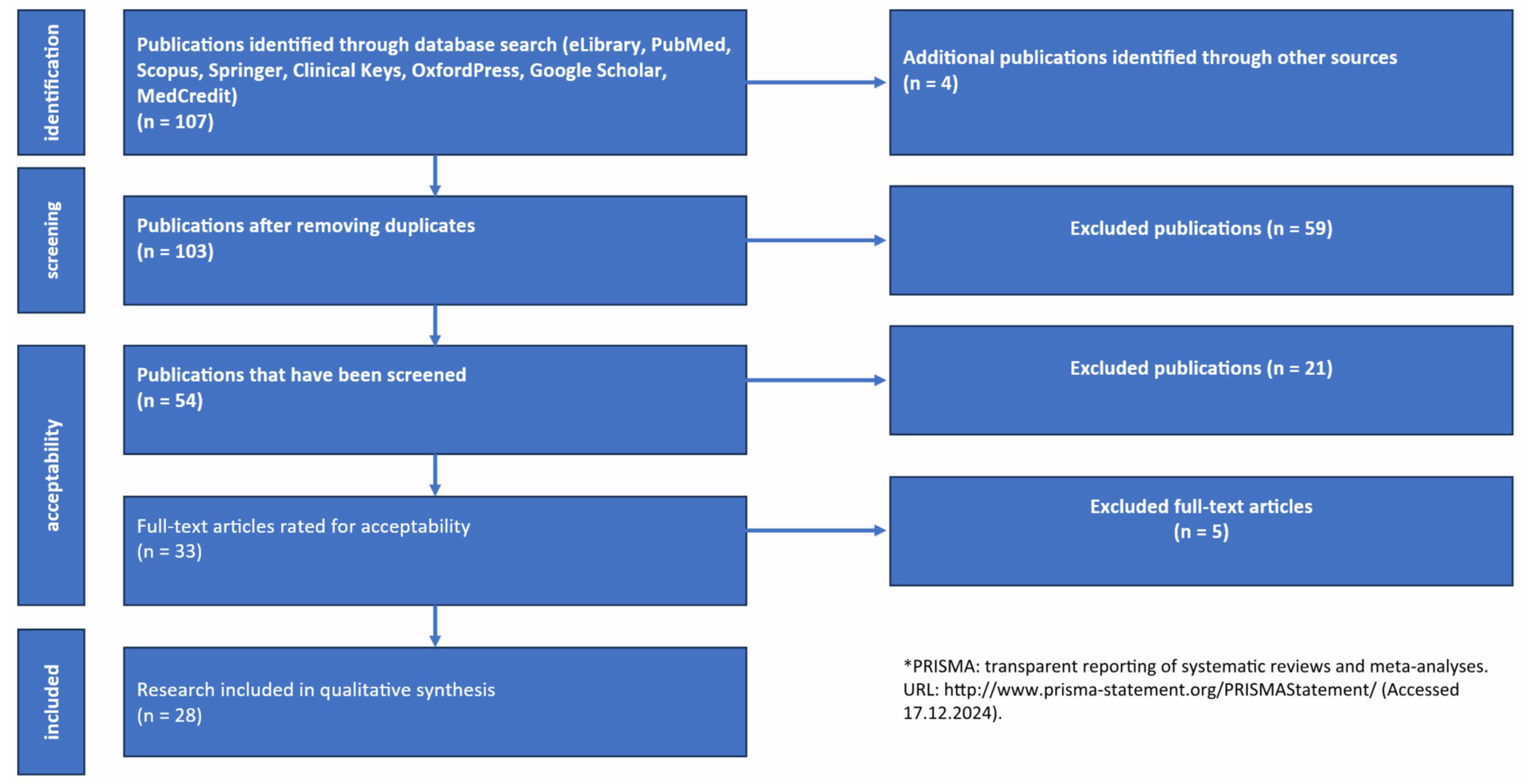
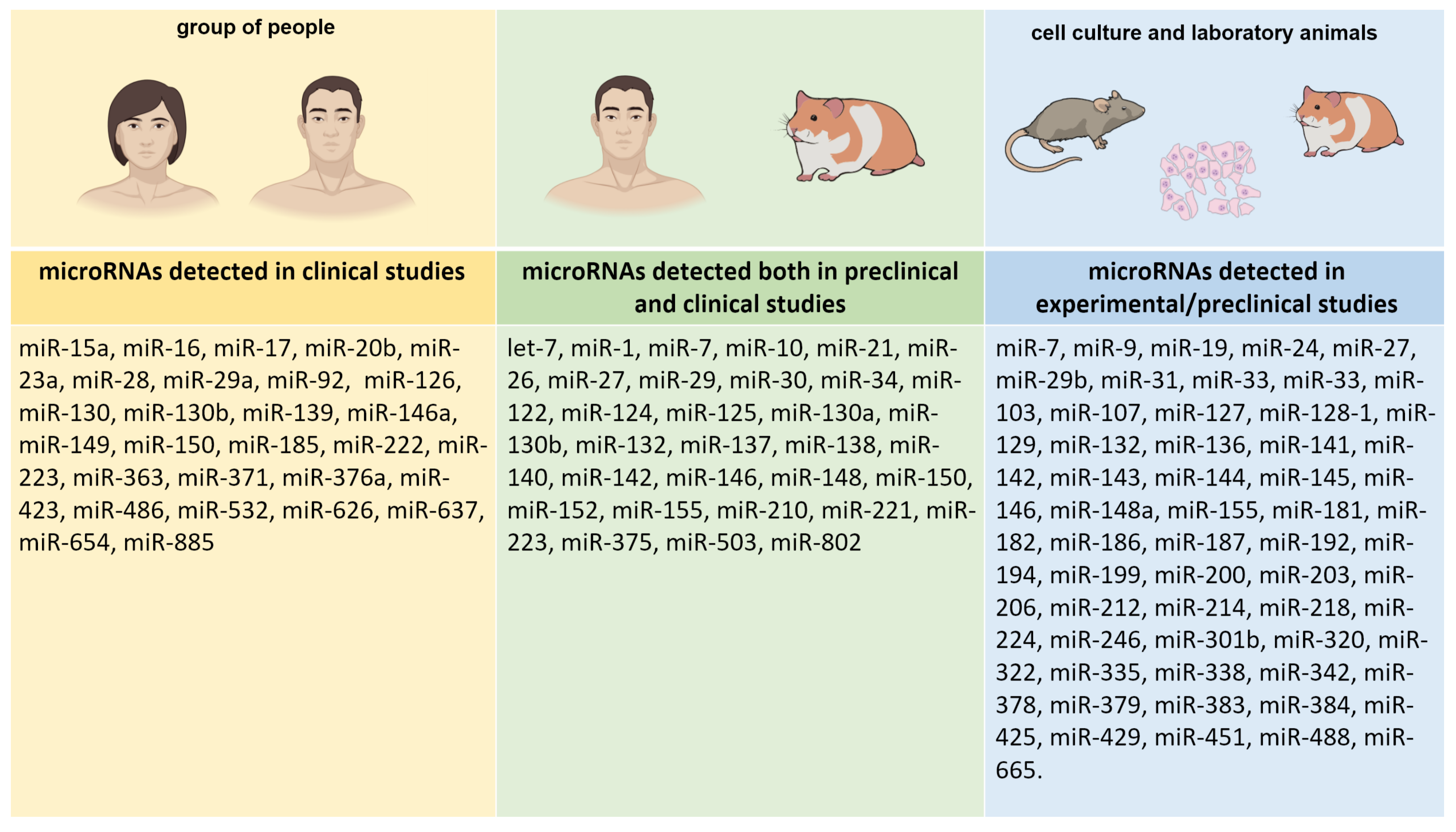
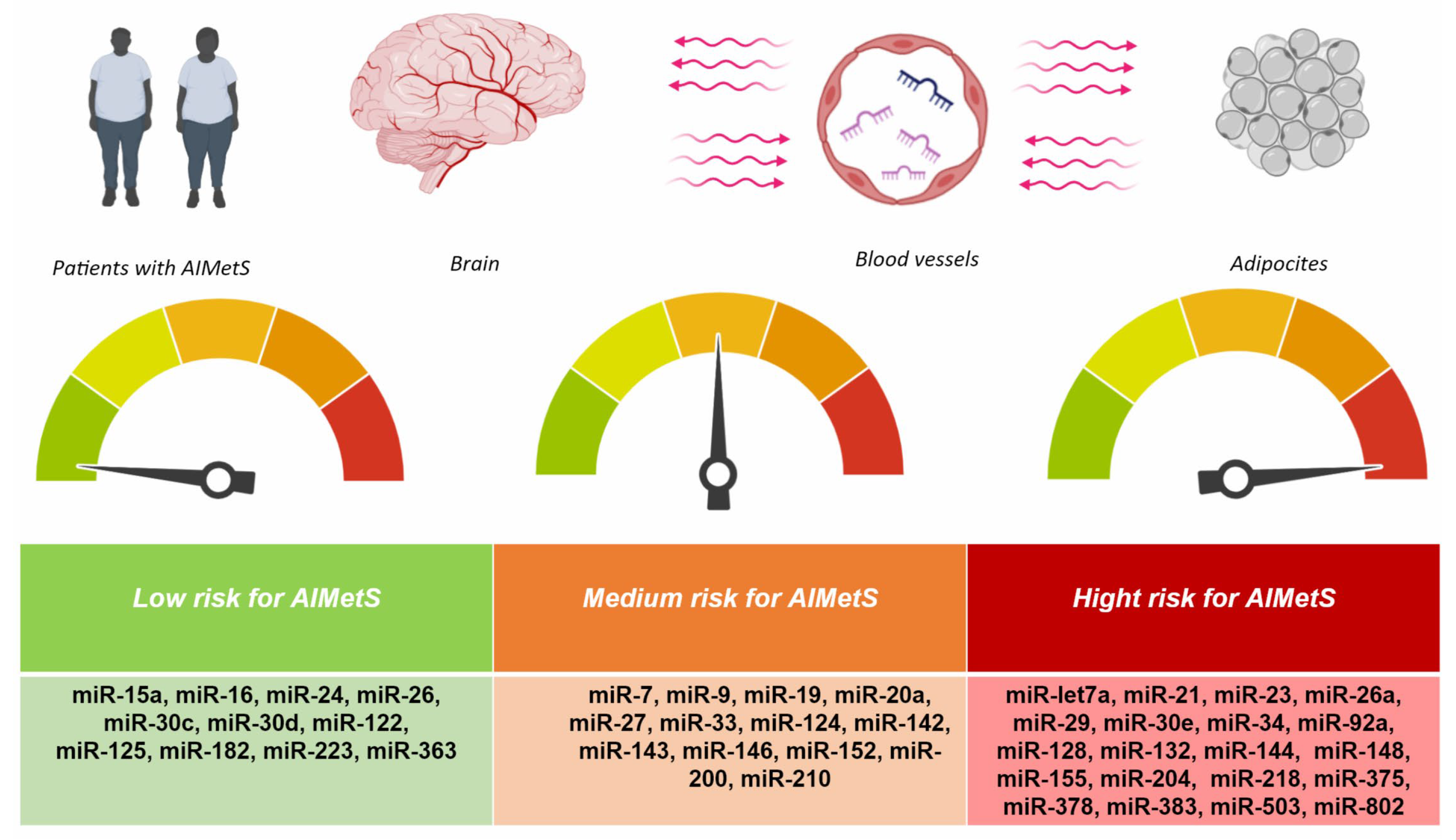
| Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Type of publication | Article, case report, systematic review, meta-analysis, or Cochrane review | Abstract, poster, or thesis |
| Access to the publication | Full version is available | Full version is not available |
| Language of publication | English, Russian | Other languages |
| Database | PubMed, Scopus, Clinical Keys, Oxford Press, Google Scholar, eLibrary | Other databases |
| Search Depth | 2014–2024 (10 years) | Before November 2024 |
| References | Mechanism of Pathogenesis | Pathophysiological Role of miRs | ||
|---|---|---|---|---|
| MiRs that Decrease the Risk of Developing a Pathogenetic Mechanism | MiRs that Increase the Risk of Developing a Pathogenetic Mechanism | |||
| [37,38] | Oxidative stress | miR-19b, miR-20a, miR-24, miR-99a, miR-125b, miR-141, miR-152, miR-200a, miR-200c, miR-210, miR-221, miR-455, miR-601, miR-626 | miR-1, miR-21, miR-23b, miR-27a, miR-28, miR-29, miR-34a, miR-92a, miR-93, miR-101, miR-106b, miR-128, miR-129, miR-140, miR-142, miR-144, miR-146, miR-148, miR-153, miR-155, miR-181c, miR-193b, miR-320, miR-365, miR-375, miR-383, miR-495, miR-503, miR-802 | |
| [39,40] | Systemic inflammation | miR-7, miR-9, miR-10a, miR-15a, miR-16, miR-24, miR-31, miR-124, miR-125, miR-126, miR-142, miR-143, miR-146, miR-149, miR-150, miR-210, miR-223, miR-363 | miR-21, miR-23a, miR-27a, miR-29a, miR-34a, miR-34c, miR-92a, miR-132, miR-138, miR-155, miR-200, let-7a | |
| [39,41,42,43] | Adipogenesis and development of central obesity | miR-27, miR-27a, miR-30c, miR-33a, miR-33b, miR-130, miR-145, miR-146a, miR-155, miR-181, miR-182, miR-200b, miR-236, miR-363, miR-344, miR-448, miR-4429 | miR-17, miR-20a, miR-21, miR-103, miR-128-1, miR-143, miR-144, miR-146b, miR-148a, miR-194, miR-210, miR-322, miR-375, intronic miR-378 | |
| [42,56] | Lipid metabolism | miR-30c, miR-33a, miR-33b, miR-34a, miR-128-1, miR-144, miR-148a, miR-223, miR-246b | miR-7, miR-27a, miR-27b, miR-122 | |
| [42,44,45] | Level of high- density lipoprotein cholesterol homeostasis | miR-33a, miR-33b, miR-128-1, miR-144, miR-148b | N/D | |
| [44,46] | Level of low- density lipoprotein cholesterol homeostasis | miR-128-1, miR-148a | miR-30c | |
| [42,45,46] | Atherogenesis | miR-30c | miR-33,miR-144 | |
| [42] | Fatty hepatosis (fatty liver disease) | miR-27a, miR-122, miR-223 | miR-34a | |
| [39,42,47,48,49] | Insulin resistance | N/D | miR-let7 (muscle tissue), miR-15b, miR-19, miR-29, miR-33a/b (liver), miR-103 (adipose tissue), miR-107 (adipose tissue), miR-143, miR-155, miR-223 miR-378 (liver), miR-451-1, miR-802 (liver) | |
| [42,50,51,52,53,54] | Insulin expression and secretion by B cells of pancreatic Langerhans islets | miR-7a, miR-26a, miR-29, miR-124a, miR-130a, miR-130b, miR-152, miR-187, miR-200, miR-204, miR-375, miR-802 | miR-24, miR-26, miR-30d, miR-148, miR-182 | |
| [42,47,48,49,50,51,52,53,54] | Glucose metabolism | miR-7a, miR-26a, miR-27, miR-29, miR-33b, miR-103, miR-107, miR-124, miR-130a, miR-130b, miR-143, miR-152, miR-155, miR-187, miR-200, miR-204, miR-336, miR-375, miR-378, miR-451-1, miR-466b, miR-802 | miR-19, miR-24, miR-26, miR-27a, miR-30d, miR-33, miR-148, miR-182 | |
| [55,57,58,59,60] | Appetite | miR-33, miR-103 | let-7a, miR-7a, miR-9, miR-30e, miR-100, miR-132, miR-141, miR-145, miR-200a, miR-218, miR-342, miR-383, miR-384-3p, miR-429, miR-488 | |
| [55,61] | Neuropeptide Y expression | let7b, miR-29b, miR-33, miR-140- miR-143, miR-503 | miR-708, miR-2137 | |
| [39,60] | Leptin tolerance | miR-15a, miR-16, miR-33, miR-200a, miR-200b, miR-223, miR-363, miR-429, miR-532 | let7a, miR-9, miR-30e, miR-132, miR-145, miR-218, miR-342 | |
| [62] | Orexin expression | miR-137, miR-637, miR-654, miR-665 | N/D | |
| [63] | Testosterone expression | miR-150 | miR-15a, miR-320 | |
| [56] | Thyroid hormones expression | miR-27, miR-155, miR-181, miR-200a, miR-221, miR-224, miR-246, miR-383, miR-425 | miR-21, miR-146, miR-214 | |
| [64] | Parathyroid hormone expression | miR-24 | miR-27b, miR-136b, miR-146b, miR-503 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shnayder, N.A.; Pekarets, N.A.; Pekarets, N.I.; Dmitrenko, D.V.; Grechkina, V.V.; Petrova, M.M.; Al-Zamil, M.; Nasyrova, R.F. MicroRNAs as Epigenetic Biomarkers of Pathogenetic Mechanisms of the Metabolic Syndrome Induced by Antiseizure Medications: Systematic Review. J. Clin. Med. 2025, 14, 2432. https://doi.org/10.3390/jcm14072432
Shnayder NA, Pekarets NA, Pekarets NI, Dmitrenko DV, Grechkina VV, Petrova MM, Al-Zamil M, Nasyrova RF. MicroRNAs as Epigenetic Biomarkers of Pathogenetic Mechanisms of the Metabolic Syndrome Induced by Antiseizure Medications: Systematic Review. Journal of Clinical Medicine. 2025; 14(7):2432. https://doi.org/10.3390/jcm14072432
Chicago/Turabian StyleShnayder, Natalia A., Nikolai A. Pekarets, Natalia I. Pekarets, Diana V. Dmitrenko, Violetta V. Grechkina, Marina M. Petrova, Mustafa Al-Zamil, and Regina F. Nasyrova. 2025. "MicroRNAs as Epigenetic Biomarkers of Pathogenetic Mechanisms of the Metabolic Syndrome Induced by Antiseizure Medications: Systematic Review" Journal of Clinical Medicine 14, no. 7: 2432. https://doi.org/10.3390/jcm14072432
APA StyleShnayder, N. A., Pekarets, N. A., Pekarets, N. I., Dmitrenko, D. V., Grechkina, V. V., Petrova, M. M., Al-Zamil, M., & Nasyrova, R. F. (2025). MicroRNAs as Epigenetic Biomarkers of Pathogenetic Mechanisms of the Metabolic Syndrome Induced by Antiseizure Medications: Systematic Review. Journal of Clinical Medicine, 14(7), 2432. https://doi.org/10.3390/jcm14072432









