The Growing Role of Intracardiac Echo in Congenital Heart Disease Interventions
Abstract
1. Introduction
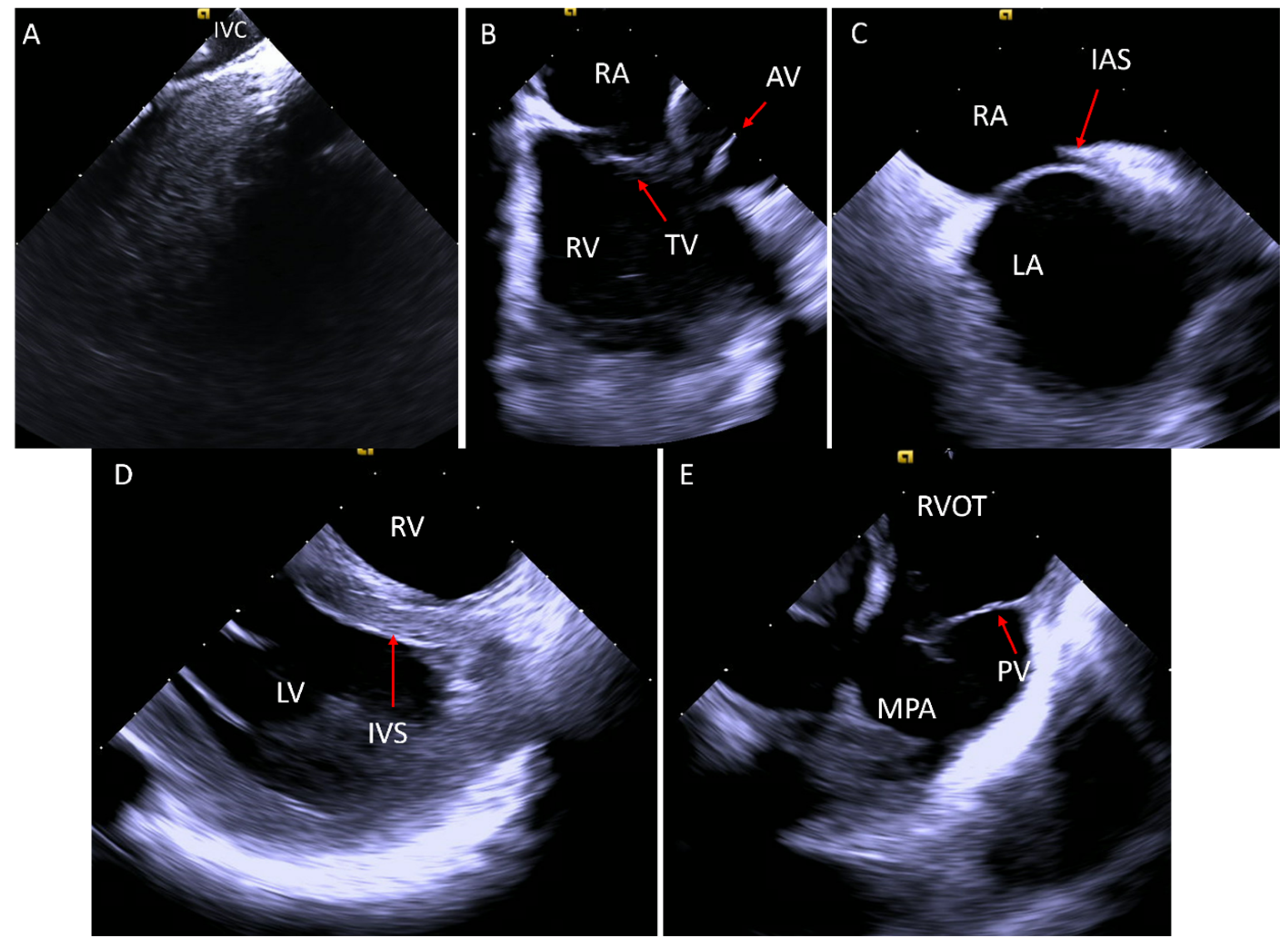
2. Acquiring Baseline Images
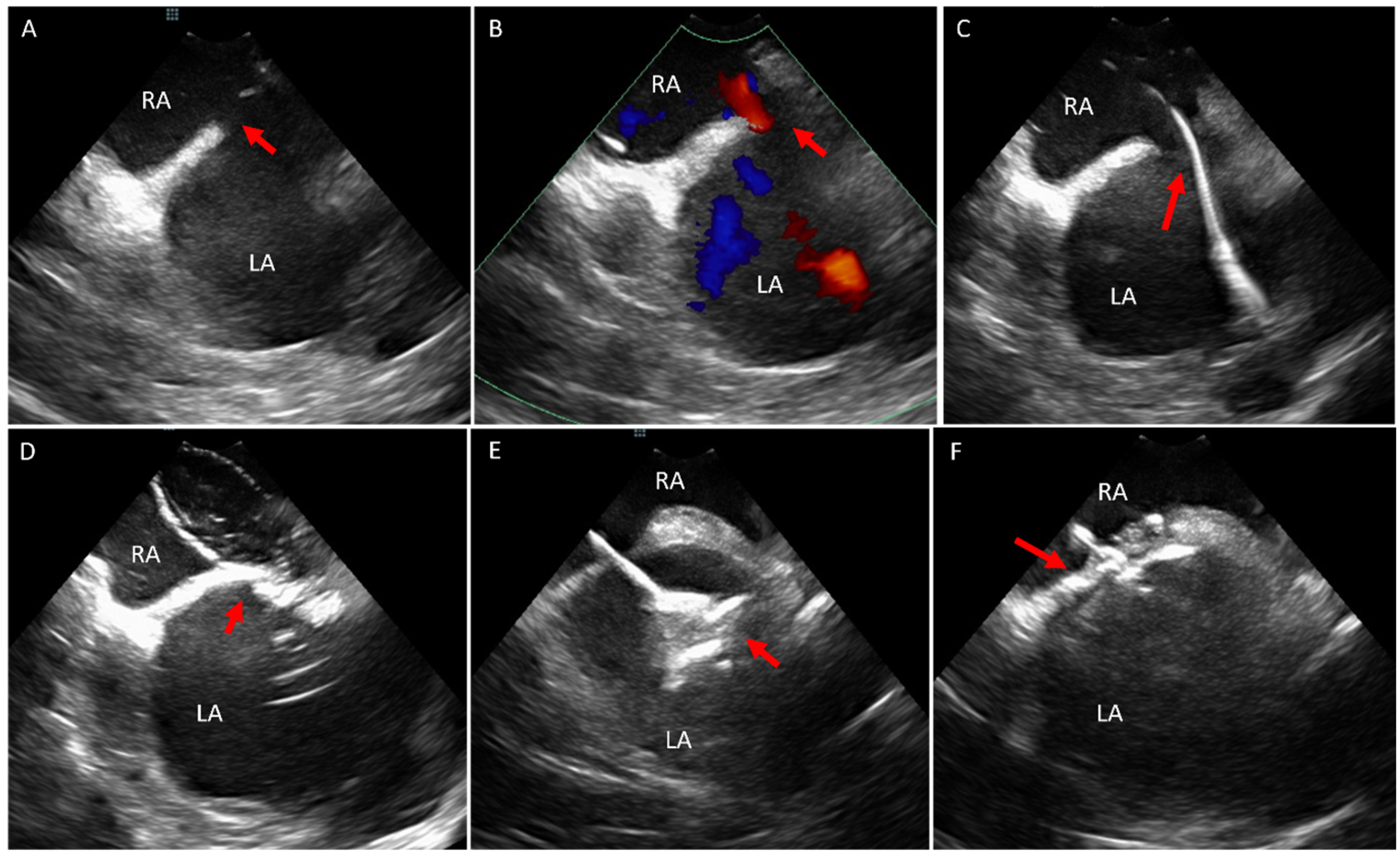
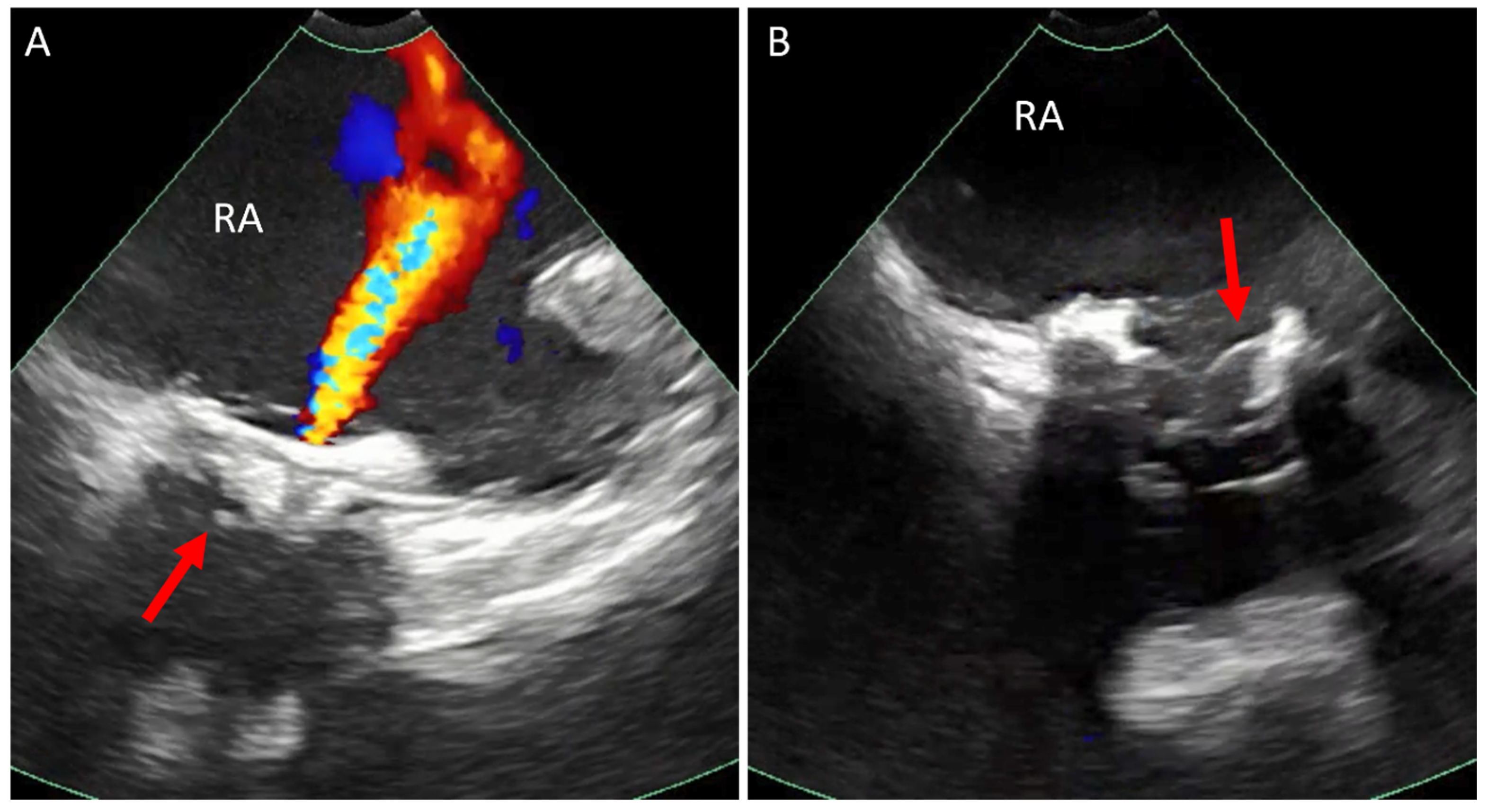
3. Ostium Secundum Atrial Septal Defect (ASD) and Patent Foramen Ovale (PFO) Closure
4. Ventricular Septal Defect (VSD)
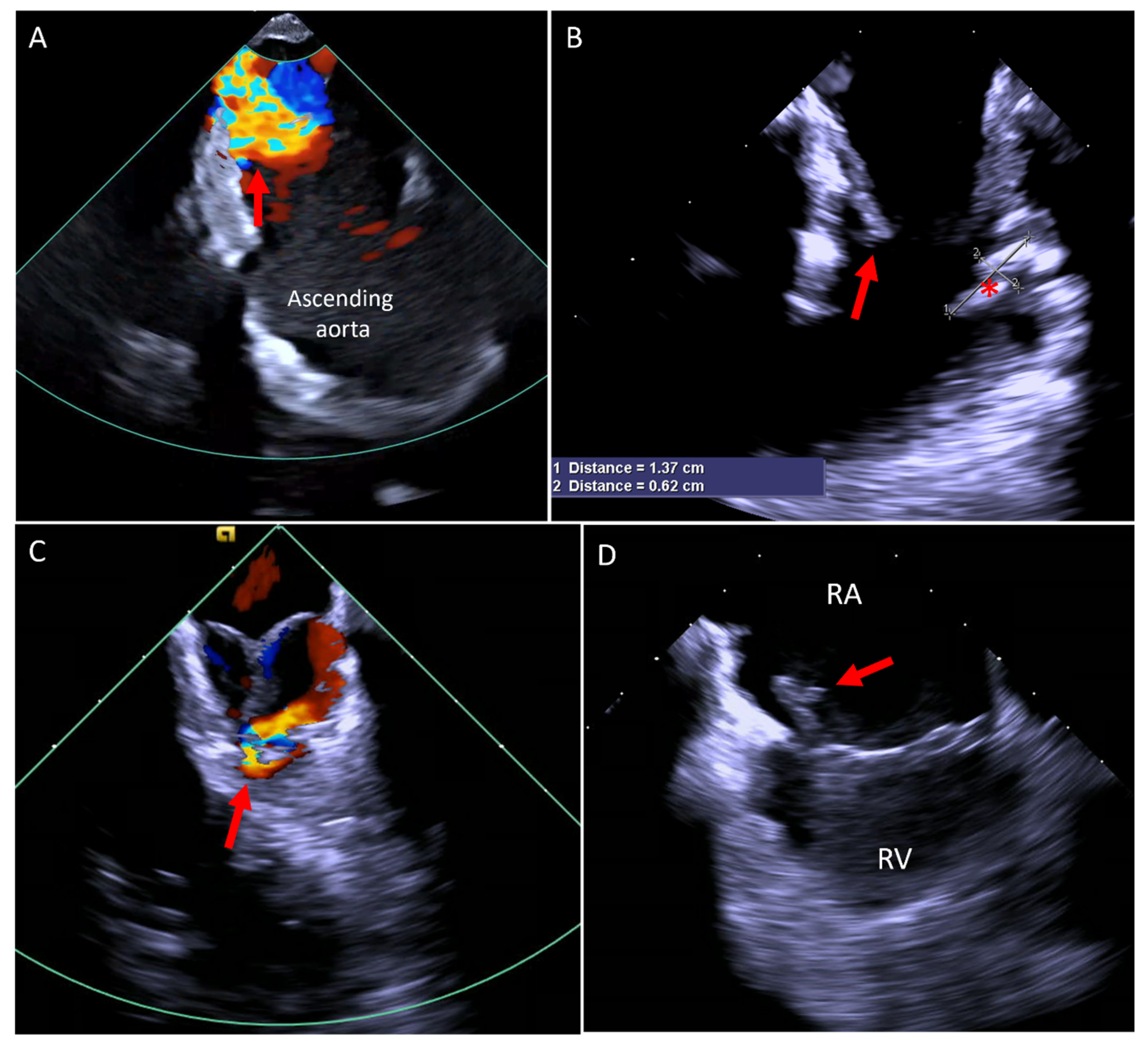
5. Valvular Interventions
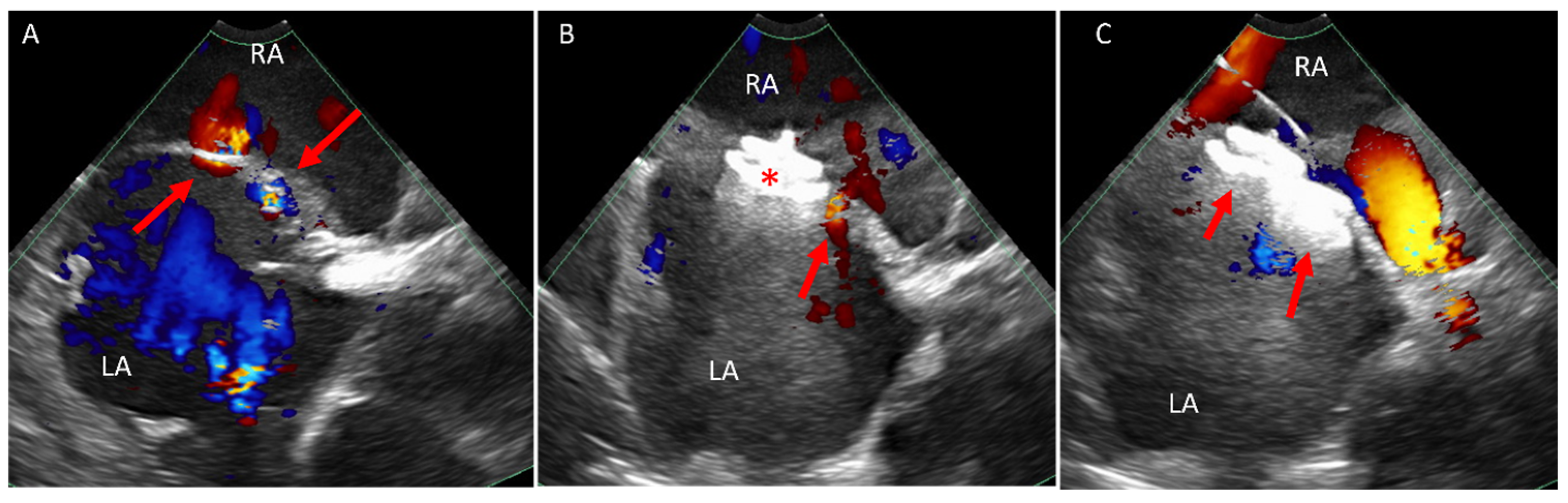
5.1. Paravalve Leak (PVL) Occlusion
5.2. Transcatheter Tricuspid (Right Atrioventricular) Valve in Valve or Valve in Ring Replacement (TVIV/TVIR)
5.3. Transcatheter Pulmonary Valve Replacement (TCPVR)
6. ICE in Specific Congenital Cases
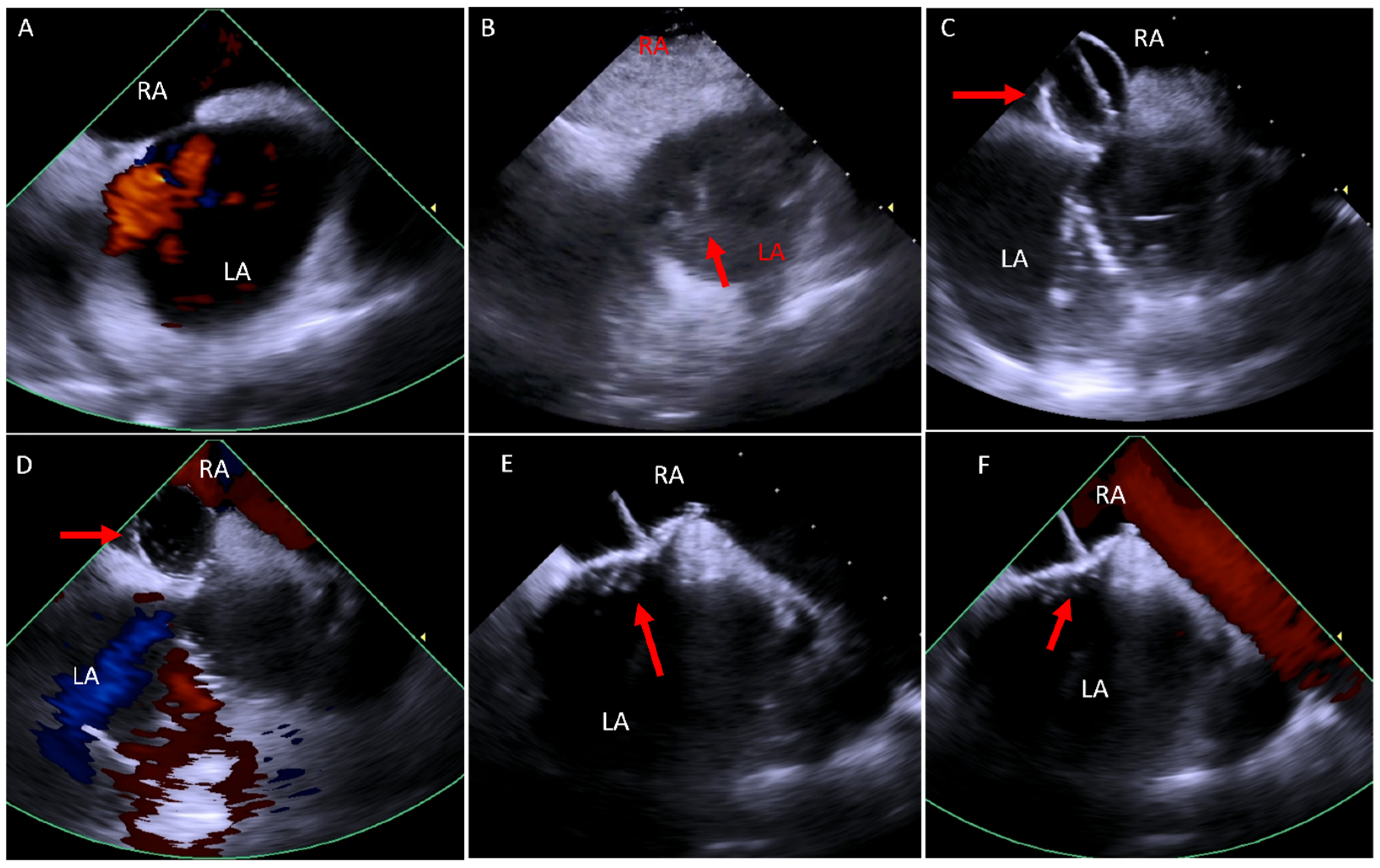
6.1. Baffle Leak Occlusion
6.2. Fontan Fenestration Occlusion
7. ICE as a Diagnostic Tool
8. ICE in the Electrophysiology (EP) Procedures
9. ICE Application in Interventional Cardiology in the Non-Congenital World
10. The Cost Effectiveness of ICE
11. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffman, J.I.E.; Kaplan, S. The Incidence of Congenital Heart Disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Marelli, A.J.; Mackie, A.S.; Ionescu-Ittu, R.; Rahme, E.; Pilote, L. Congenital Heart Disease in the General Population. Circulation 2007, 115, 163–172. [Google Scholar] [CrossRef]
- Liu, A.; Diller, G.-P.; Moons, P.; Daniels, C.J.; Jenkins, K.J.; Marelli, A. Changing Epidemiology of Congenital Heart Disease: Effect on Outcomes and Quality of Care in Adults. Nat. Rev. Cardiol. 2023, 20, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.G.; Pearson, G.D.; Barst, R.J.; Child, J.S.; Del Nido, P.; Gersony, W.M.; Kuehl, K.S.; Landzberg, M.J.; Myerson, M.; Neish, S.R.; et al. Report of the National Heart, Lung, and Blood Institute Working Group on Research in Adult Congenital Heart Disease. J. Am. Coll. Cardiol. 2006, 47, 701–707. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.W.; Cha, H.R.; Huh, J.; Kang, I.-S.; Jun, T.-G.; Yang, J.-H.; Han, M.Y.; Song, J. Long-Term Observational Outcomes after Total Correction of Congenital Heart Disease in Korean Patients with Down Syndrome: A National Cohort Study. Children 2022, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Cieszynski, T. Intracardiac method for the investigation of structure of the heart with the aid of ultrasonics. Arch. Immunol. Ther. Exp. 1960, 8, 551–557. [Google Scholar]
- Alkhouli, M.; Hijazi, Z.M.; Holmes, D.R.; Rihal, C.S.; Wiegers, S.E. Intracardiac Echocardiography in Structural Heart Disease Interventions. JACC Cardiovasc. Interv. 2018, 11, 2133–2147. [Google Scholar] [CrossRef]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; Garcia, F. Use of Intracardiac Echocardiography in Interventional Cardiology. Circulation 2018, 137, 2278–2294. [Google Scholar] [CrossRef]
- Hijazi, Z.M.; Shivkumar, K.; Sahn, D.J. Intracardiac Echocardiography During Interventional and Electrophysiological Cardiac Catheterization. Circulation 2009, 119, 587–596. [Google Scholar] [CrossRef]
- Cooper, J.M.; Epstein, L.M. Use of Intracardiac Echocardiography to Guide Ablation of Atrial Fibrillation. Circulation 2001, 104, 3010–3013. [Google Scholar] [CrossRef]
- VeriSight ICE Catheter. Available online: https://www.usa.philips.com/healthcare/resources/landing/ice-catheter-verisight (accessed on 22 February 2025).
- ACUSON AcuNav Volume 4D ICE Catheter. Available online: https://www.siemens-healthineers.com/en-us/ultrasound/cardiovascular/acunav-volume-ice (accessed on 22 February 2025).
- Marelli, A.J.; Ionescu-Ittu, R.; Mackie, A.S.; Guo, L.; Dendukuri, N.; Kaouache, M. Lifetime Prevalence of Congenital Heart Disease in the General Population From 2000 to 2010. Circulation 2014, 130, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Van der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e81–e192. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the Management of Adult Congenital Heart Disease: The Task Force for the Management of Adult Congenital Heart Disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD). Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.M.; Wang, Z.; Cao, Q.-L.; Koenig, P.; Waight, D.; Lang, R. Transcatheter Closure of Atrial Septal Defects and Patent Foramen Ovale under Intracardiac Echocardiographic Guidance: Feasibility and Comparison with Transesophageal Echocardiography. Catheter. Cardiovasc. Interv. 2001, 52, 194–199. [Google Scholar] [CrossRef]
- Mullen, M.J.; Dias, B.F.; Walker, F.; Siu, S.C.; Benson, L.N.; McLaughlin, P.R. Intracardiac Echocardiography Guided Device Closure of Atrial Septal Defects. J. Am. Coll. Cardiol. 2003, 41, 285–292. [Google Scholar] [CrossRef]
- De Cillis, E.; Acquaviva, T.; Ursi, R.; Soldato, N.; Basile, P.; Siena, P.; Carella, M.C.; Baggiano, A.; Mushtaq, S.; Fusini, L.; et al. A Comparison of Intracardiac Echocardiography and Transesophageal Echocardiography for Guiding Device Closure of Ostium Secundum Atrial Septal Defect: A 15-Year Experience. Echocardiography 2024, 41, e15724. [Google Scholar] [CrossRef]
- Koenig, P.R.; Abdulla, R.; Cao, Q.-L.; Hijazi, Z.M. Use of Intracardiac Echocardiography to Guide Catheter Closure of Atrial Communications. Echocardiography 2003, 20, 781–787. [Google Scholar] [CrossRef]
- Morray, B.H. Ventricular Septal Defect Closure Devices, Techniques, and Outcomes. Interv. Cardiol. Clin. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Butera, G.; Carminati, M.; Chessa, M.; Piazza, L.; Micheletti, A.; Negura, D.G.; Abella, R.; Giamberti, A.; Frigiola, A. Transcatheter Closure of Perimembranous Ventricular Septal Defects: Early and Long-Term Results. J. Am. Coll. Cardiol. 2007, 50, 1189–1195. [Google Scholar] [CrossRef]
- Cao, Q.-L.; Zabal, C.; Koenig, P.; Sandhu, S.; Hijazi, Z.M. Initial Clinical Experience with Intracardiac Echocardiography in Guiding Transcatheter Closure of Perimembranous Ventricular Septal Defects: Feasibility and Comparison with Transesophageal Echocardiography. Catheter. Cardiovasc. Interv. 2005, 66, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Bartel, T.; Bonaros, N.; Müller, L.; Friedrich, G.; Grimm, M.; Velik-Salchner, C.; Feuchtner, G.; Pedross, F.; Müller, S. Intracardiac Echocardiography: A New Guiding Tool for Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2011, 24, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Bartel, T.; Edris, A.; Velik-Salchner, C.; Müller, S. Intracardiac Echocardiography for Guidance of Transcatheter Aortic Valve Implantation under Monitored Sedation: A Solution to a Dilemma? Eur. Heart J. —Cardiovasc. Imaging 2016, 17, 1–8. [Google Scholar] [CrossRef][Green Version]
- Ishizu, K.; Shirai, S.; Miyawaki, N.; Nakano, K.; Fukushima, T.; Ko, E.; Tsuru, Y.; Tashiro, H.; Tabata, H.; Nakamura, M.; et al. Impact of Transjugular Intracardiac Echocardiography-Guided Self-Expandable Transcatheter Aortic Valve Implantation on Reduction of Conduction Disturbances. Circ. Cardiovasc. Interv. 2024, 17, e013094. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Yakubov, S.J.; Singh, G.; Rogers, J.H.; Kander, N.H.; Tang, G.H.L. 4-Dimensional Intracardiac Echocardiography in Transcatheter Mitral Valve Repair with the Mitraclip System. JACC Cardiovasc. Imaging 2021, 14, 2033–2040. [Google Scholar] [CrossRef]
- Dávila-Román, V.G.; Waggoner, A.D.; Kennard, E.D.; Holubkov, R.; Jamieson, W.R.E.; Englberger, L.; Carrel, T.P.; Schaff, H.V. Prevalence and Severity of Paravalvular Regurgitation in the Artificial Valve Endocarditis Reduction Trial (AVERT) Echocardiography Study. J. Am. Coll. Cardiol. 2004, 44, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Millán, X.; Skaf, S.; Joseph, L.; Ruiz, C.; García, E.; Smolka, G.; Noble, S.; Cruz-González, I.; Arzamendi, D.; Serra, A.; et al. Transcatheter Reduction of Paravalvular Leaks: A Systematic Review and Meta-Analysis. Can. J. Cardiol. 2015, 31, 260–269. [Google Scholar] [CrossRef]
- Sorajja, P.; Cabalka, A.K.; Hagler, D.J.; Rihal, C.S. Long-Term Follow-Up of Percutaneous Repair of Paravalvular Prosthetic Regurgitation. J. Am. Coll. Cardiol. 2011, 58, 2218–2224. [Google Scholar] [CrossRef]
- Alkhouli, M.; Sarraf, M.; Maor, E.; Sanon, S.; Cabalka, A.; Eleid, M.F.; Hagler, D.J.; Pollak, P.; Reeder, G.; Rihal, C.S. Techniques and Outcomes of Percutaneous Aortic Paravalvular Leak Closure. JACC Cardiovasc. Interv. 2016, 9, 2416–2426. [Google Scholar] [CrossRef]
- Offen, S.; Playford, D.; Strange, G.; Stewart, S.; Celermajer, D.S. Adverse Prognostic Impact of Even Mild or Moderate Tricuspid Regurgitation: Insights from the National Echocardiography Database of Australia. J. Am. Soc. Echocardiogr. 2022, 35, 810–817. [Google Scholar] [CrossRef]
- Hahn, R.T.; Makkar, R.; Thourani, V.H.; Makar, M.; Sharma, R.P.; Haeffele, C.; Davidson, C.J.; Narang, A.; O’Neill, B.; Lee, J.; et al. Transcatheter Valve Replacement in Severe Tricuspid Regurgitation. N. Engl. J. Med. 2025, 392, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Sanon, S.; Cabalka, A.K.; Babaliaros, V.; Rihal, C.; Gafoor, S.; Webb, J.; Latib, A. Transcatheter Tricuspid Valve-in-Valve and Valve-in-Ring Implantation for Degenerated Surgical Prosthesis. JACC Cardiovasc. Interv. 2019, 12, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Merckx, J.; Aggoun, Y.; Bonnet, D.; Acar, P.; Le Bidois, J.; Sidi, D.; Kachaner, J. Percutaneous Replacement of Pulmonary Valve in a Right-Ventricle to Pulmonary-Artery Prosthetic Conduit with Valve Dysfunction. Lancet 2000, 356, 1403–1405. [Google Scholar] [CrossRef]
- Khambadkone, S.; Bonhoeffer, P. Nonsurgical Pulmonary Valve Replacement: Why, When, and How? Catheter. Cardiovasc. Interv. 2004, 62, 401–408. [Google Scholar] [CrossRef]
- Georgiev, S.; Ewert, P.; Tanase, D.; Hess, J.; Hager, A.; Cleuziou, J.; Meierhofer, C.; Eicken, A. A Low Residual Pressure Gradient Yields Excellent Long-Term Outcome After Percutaneous Pulmonary Valve Implantation. JACC Cardiovasc. Interv. 2019, 12, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Eicken, A.; Ewert, P.; Hager, A.; Peters, B.; Fratz, S.; Kuehne, T.; Busch, R.; Hess, J.; Berger, F. Percutaneous Pulmonary Valve Implantation: Two-Centre Experience with More than 100 Patients. Eur. Heart J. 2011, 32, 1260–1265. [Google Scholar] [CrossRef]
- Sinha, S.; Aboulhosn, J.; Asnes, J.; Bocks, M.; Zahn, E.; Goldstein, B.H.; Zampi, J.; Hellenbrand, W.; Salem, M.; Levi, D. Initial Results from the Off-Label Use of the SAPIEN S3 Valve for Percutaneous Transcatheter Pulmonary Valve Replacement: A Multi-Institutional Experience. Catheter. Cardiovasc. Interv. 2019, 93, 455–463. [Google Scholar] [CrossRef]
- Ren, J.-F.; Chen, S.; Callans, D.J.; Jiang, C.; Marchlinski, F.E. Transcatheter Pulmonary Valve Replacement Needs Better Imaging Technique with Intracardiac Echocardiography. JACC Cardiovasc. Interv. 2019, 12, 2558–2559. [Google Scholar] [CrossRef]
- Alba, C.G.d; Zablah, J.E.; Burkett, D.; Jone, P.-N.; Rodriguez, S.A.; Morgan, G.J. Use of Three-Dimensional Intracardiac Echocardiography Catheter in the Evaluation of Prosthetic Pulmonary Valves after Transcatheter Replacement. J. Am. Soc. Echocardiogr. 2024, 37, 226–236. [Google Scholar] [CrossRef]
- Mustard, W.T.; Keith, J.D.; Trusler, G.A.; Fowler, R.; Kidd, L. Management of transposition of the great vessels. J. Thorac. Cardiovasc. Surg. 1964, 48, 953–958. [Google Scholar]
- Senning, A. Surgical Correction of Transposition of the Great Vessels. Surgery 1959, 45, 966–980. [Google Scholar] [PubMed]
- Gaur, L.; Cedars, A.; Diller, G.P.; Kutty, S.; Orwat, S. Management Considerations in the Adult with Surgically Modified D-Transposition of the Great Arteries. Heart 2021, 107, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Landzberg, M.J.; Lambert, J.; O’Donnell, C.P. Long-Term Outcomes after the Atrial Switch for Surgical Correction of Transposition: A Meta-Analysis Comparing the Mustard and Senning Procedures. Cardiol. Young 2004, 14, 284–292. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, G.; Bonassin Tempesta, F.; Lopes, B.S.; Babic, D.; Oxenius, A.; Seeliger, T.; Gruner, C.; Tanner, F.C.; Biaggi, P.; Attenhofer Jost, C.; et al. High Prevalence of Baffle Leaks in Adults after Atrial Switch Operations for Transposition of the Great Arteries. Eur. Heart J.—Cardiovasc. Imaging 2017, 18, 531–535. [Google Scholar] [CrossRef]
- Ehrin, J.; Armstrong, M.D. Intracardiac Echocardiography to Guide Percutaneous Closure of Atrial Baffle Defects. J. Invasive Cardiol. 2012, 24, 473–476. [Google Scholar]
- Kuppahally, S.S.; Litwin, S.E.; Green, L.S.; Ishihara, S.M.; Freedman, R.A.; Michaels, A.D. Utility of Intracardiac Echocardiography for Atrial Baffle Leak Closure in Repaired Transposition of the Great Arteries. Echocardiography 2010, 27, E90–E93. [Google Scholar] [CrossRef]
- Neijenhuis, R.M.L.; Regeer, M.V.; Van der Kley, F.; Vliegen, H.W.; Jongbloed, M.R.M.; Kiès, P.; Schalij, M.J.; Jukema, J.W.; Egorova, A.D. Contemporary Management Strategies of Baffle Leaks in Adults with a Failing Systemic Right Ventricle Late after Atrial Switch: A Case Series and Literature Overview. J. Cardiovasc. Dev. Dis. 2023, 10, 129. [Google Scholar] [CrossRef]
- Devanagondi, R.; Leonard, G. Transcatheter Fontan Fenestration Closure: Sustained Improvements in Oxygen Saturation with Minimal Morbidity and Mortality. Pediatr. Cardiol. 2023, 44, 922–926. [Google Scholar] [CrossRef]
- Reynolds, K.P.; Jone, P.-N.; Morgan, G.J.; Zablah, J.E. Fontan Fenestration Reduction with an Occlutech Atrial Flow Regulator: Unique Views from Three-Dimensional Intracardiac Echocardiography. Pediatr. Cardiol. 2024, 45, 213–215. [Google Scholar] [CrossRef]
- Daoud, E.G.; Kalbfletsch, S.J.; Hummel, J.D. Intracardiac Echocardiography to Guide Transseptal Left Heart Catheterization for Radiofrequency Catheter Ablation. J. Cardiovasc. Electrophysiol. 1999, 10, 358–363. [Google Scholar] [CrossRef]
- Anter, E.; Silverstein, J.; Tschabrunn, C.M.; Shvilkin, A.; Haffajee, C.I.; Zimetbaum, P.J.; Buxton, A.E.; Josephson, M.E.; Gelfand, E.; Manning, W.J. Comparison of Intracardiac Echocardiography and Transesophageal Echocardiography for Imaging of the Right and Left Atrial Appendages. Heart Rhythm. 2014, 11, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Nölker, G.; Heintze, J.; Gutleben, K.-J.; Muntean, B.; Pütz, V.; Yalda, A.; Vogt, J.; Horstkotte, D. Cryoballoon Pulmonary Vein Isolation Supported by Intracardiac Echocardiography: Integration of a Nonfluoroscopic Imaging Technique in Atrial Fibrillation Ablation. J. Cardiovasc. Electrophysiol. 2010, 21, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Martin, D.O.; Wazni, O.; Gillinov, A.M.; Klein, A.; Bhargava, M.; Saad, E.; Bash, D.; Yamada, H.; Jaber, W.; et al. Phased-Array Intracardiac Echocardiography Monitoring During Pulmonary Vein Isolation in Patients with Atrial Fibrillation. Circulation 2003, 107, 2710–2716. [Google Scholar] [CrossRef]
- Pierucci, N.; La Fazia, V.M.; Mohanty, S.; Schiavone, M.; Doty, B.; Gabrah, K.; Della Rocca, D.G.; Burkhardt, J.D.; Al-Ahmad, A.; Di Biase, L.; et al. Results of ICE-Guided Isolation of the Superior Vena Cava with Pulsed Field Ablation. JACC Clin. Electrophysiol. 2025, in press. [Google Scholar] [CrossRef]
- Uhm, J.-S.; Kim, N.K.; Kim, T.-H.; Joung, B.; Pak, H.-N.; Lee, M.-H. How to Perform Transconduit and Transbaffle Puncture in Patients Who Have Previously Undergone the Fontan or Mustard Operation. Heart Rhythm. 2018, 15, 145–150. [Google Scholar] [CrossRef]
- Eleid, M.F.; Alkhouli, M.; Thaden, J.J.; Zahr, F.; Chadderdon, S.; Guerrero, M.; Reeder, G.S.; Rihal, C.S. Utility of Intracardiac Echocardiography in the Early Experience of Transcatheter Edge to Edge Tricuspid Valve Repair. Circ. Cardiovasc. Interv. 2021, 14, e011118. [Google Scholar] [CrossRef]
- Isath, A.; Padmanabhan, D.; Haider, S.W.; Siroky, G.; Perimbeti, S.; Correa, A.; Chahal, C.A.A.; Shenthar, J.; Asirvatham, S.; Mehta, D. Does the Use of Intracardiac Echocardiography during Atrial Fibrillation Catheter Ablation Improve Outcomes and Cost? A Nationwide 14-Year Analysis from 2001 to 2014. J. Interv. Card. Electrophysiol. 2021, 61, 461–468. [Google Scholar] [CrossRef]
- Zahid, S.; Gowda, S.; Hashem, A.; Khan, M.Z.; Ullah, W.; Kaur, G.; Nasir, U.; Rai, D.; Faza, N.N.; Little, S.H.; et al. Feasibility and Safety of Intracardiac Echocardiography Use in Transcatheter Left Atrial Appendage Closure Procedures. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100510. [Google Scholar] [CrossRef] [PubMed]
- Hemam, M.E.; Kuroki, K.; Schurmann, P.A.; Dave, A.S.; Rodríguez, D.A.; Sáenz, L.C.; Reddy, V.Y.; Valderrábano, M. Left Atrial Appendage Closure with the Watchman Device Using Intracardiac vs Transesophageal Echocardiography: Procedural and Cost Considerations. Heart Rhythm. 2019, 16, 334–342. [Google Scholar] [CrossRef]
- Akerman, A.P.; Porumb, M.; Scott, C.G.; Beqiri, A.; Chartsias, A.; Ryu, A.J.; Hawkes, W.; Huntley, G.D.; Arystan, A.Z.; Kane, G.C.; et al. Automated Echocardiographic Detection of Heart Failure with Preserved Ejection Fraction Using Artificial Intelligence. JACC Adv. 2023, 2, 100452. [Google Scholar] [CrossRef]
- Lang, R.M.; Addetia, K.; Miyoshi, T.; Kebed, K.; Blitz, A.; Schreckenberg, M.; Hitschrich, N.; Mor-Avi, V.; Asch, F.M. Use of Machine Learning to Improve Echocardiographic Image Interpretation Workflow: A Disruptive Paradigm Change? J. Am. Soc. Echocardiogr. 2021, 34, 443–445. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghantous, E.; Aboulhosn, J.A. The Growing Role of Intracardiac Echo in Congenital Heart Disease Interventions. J. Clin. Med. 2025, 14, 2414. https://doi.org/10.3390/jcm14072414
Ghantous E, Aboulhosn JA. The Growing Role of Intracardiac Echo in Congenital Heart Disease Interventions. Journal of Clinical Medicine. 2025; 14(7):2414. https://doi.org/10.3390/jcm14072414
Chicago/Turabian StyleGhantous, Eihab, and Jamil A. Aboulhosn. 2025. "The Growing Role of Intracardiac Echo in Congenital Heart Disease Interventions" Journal of Clinical Medicine 14, no. 7: 2414. https://doi.org/10.3390/jcm14072414
APA StyleGhantous, E., & Aboulhosn, J. A. (2025). The Growing Role of Intracardiac Echo in Congenital Heart Disease Interventions. Journal of Clinical Medicine, 14(7), 2414. https://doi.org/10.3390/jcm14072414





