Evaluation of the Real-Life Efficacy and Safety of the Treatment with Lutetium-177 Dotatate for Metastatic Neuroendocrine Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population
2.3. Data Collection
2.4. Data Analysis
2.5. Ethical Approval
3. Results
3.1. General Description Analysis
3.2. Efficacy Analysis
3.3. Safety Analysis
4. Discussion
4.1. Population Characteristics
4.2. Efficacy of Treatment
4.3. Prognostic Factors
4.4. Safety of Treatment
4.5. Limitations of the Study
4.6. Further Lines of Investigation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 177LU | Lutetium-177 dotatate |
| AEs | Adverse events |
| CEICA | Ethics Committee from the Community of Aragón |
| CEMAE | Evaluation of Drugs and Sanitary Products in Specialized Attention Committee of Aragón |
| CI | Confidence interval |
| GI | Gastrointestinal |
| GEP | Gastroenteropancreatic |
| NECs | Neuroendocrine carcinomas |
| NENs | Neuroendocrine tumors neoplasms |
| NETs | Neuroendocrine tumors |
| ORR | Overall response rate |
| OS | Overall survival |
| pd | Poorly differentiated |
| PFS | Progression free survival |
| PRRT | Peptide receptor radionuclide therapy |
| rSSA | Radiolabeled somatostatin analogs |
| SSAs | Somatostatin analogs |
| SSRs | somatostatin receptors |
| wd | Well differentiated |
| 99Y-PRRT | Yttrium-90 (90Y)-based PRRT |
References
- Clift, A.K.; Kidd, M.; Bodei, L.; Toumpanakis, C.; Baum, R.P.; Oberg, K.; Modlin, I.M.; Frilling, A. Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology 2020, 110, 444–476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Digestive System Tumours. In WHO Classification of Tumours, 5th ed.; IARC Press: Lyon, France, 2019. [Google Scholar]
- Sorbye, H.; Grande, E.; Pavel, M.; Tesselaar, M.; Fazio, N.; Reed, N.S.; Knigge, U.; Christ, E.; Ambrosini, V.; Couvelard, A.; et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J Neuroendocrinol. 2023, 35, e13249. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, M.S.; Lafaro, K.J.; Pawlik, T.M. Role of Locoregional and Systemic Approaches for the Treatment of Patients with Metastatic Neuroendocrine Tumors. J. Gastrointest. Surg. 2015, 19, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.H.; Tameez Ud Din, A.; Khan, A.H. Neuroendocrine Tumor Therapy with Lutetium-177: A Literature Review. Cureus 2019, 11, e3986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haider, M.; Das, S.; Al-Toubah, T.; Pelle, E.; El-Haddad, G.; Strosberg, J. Somatostatin receptor radionuclide therapy in neuroendocrine tumors. Endocr.-Relat. Cancer. 2021, 28, R81–R93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.; Krenning, E.P.; van Essen, M.; Kam, B.L.; Teunissen, J.J.; Kwekkeboom, D.J. Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J. Nucl. Med. 2011, 52, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. 177Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023–1031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; E Pavel, M.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763, Erratum in Lancet Oncol. 2022, 23, e59. [Google Scholar] [CrossRef] [PubMed]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.; Franssen, G.J.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Igarashi, H.; Jensen, R.T. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): Recent insights and advances. J. Gastroenterol. 2012, 47, 941–960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kulkarni, H.R.; Singh, A.; Niepsch, K.; Müller, D.; Baum, R.P. Peptide Receptor Radionuclide Therapy in Grade 3 Neuroendocrine Neoplasms: Safety and Survival Analysis in 69 Patients. J. Nucl. Med. 2019, 60, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Srirajaskanthan, R.; El-Haddad, G.; Wolin, E.M.; Chasen, B.R.; Kulke, M.H.; Bushnell, D.L.; Caplin, M.E.; Baum, R.P.; Hendifar, A.E.; et al. Symptom Diaries of Patients with Midgut Neuroendocrine Tumors Treated with 177Lu-DOTATATE. J. Nucl. Med. 2021, 62, 1712–1718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urso, L.; Panareo, S.; Castello, A.; Ambrosio, M.R.; Zatelli, M.C.; Caracciolo, M.; Tonini, E.; Valpiani, G.; Boschi, A.; Uccelli, L.; et al. Glucose Metabolism Modification Induced by Radioligand Therapy with [177Lu]Lu/[90Y]Y-DOTATOC in Advanced Neuroendocrine Neoplasms: A Prospective Pilot Study within FENET-2016 Trial. Pharmaceutics 2022, 14, 2009. [Google Scholar] [CrossRef]
- Strosberg, J.; Leeuwenkamp, O.; Siddiqui, M.K. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2021, 93, 102141, Erratum in Cancer Treat Rev. 2021, 97, 102203. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Aggarwal, P.; Sood, A.; Chandekar, K.R.; Das, C.K.; Gupta, R.; Khosla, D.; Das, N.; Kapoor, R.; Kumar, R.; et al. 177Lu-DOTATATE Plus Capecitabine Versus 177Lu-DOTATATE Alone in Patients with Advanced Grade 1/2 Gastroenteropancreatic Neuroendocrine Tumors (LuCAP): A Randomized, Phase 2 Trial. J. Nucl. Med. 2025, 66, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Bison, S.M.; Haeck, J.C.; Bol, K.; Koelewijn, S.J.; Groen, H.C.; Melis, M.; Veenland, J.F.; Bernsen, M.R.; de Jong, M. Optimization of combined temozolomide and peptide receptor radionuclide therapy (PRRT) in mice after multimodality molecular imaging studies. EJNMMI Res. 2015, 5, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parghane, R.V.; Bhandare, M.; Chaudhari, V.; Ostwal, V.; Ramaswamy, A.; Talole, S.; Shrikhande, S.V.; Basu, S. Surgical Feasibility, Determinants, and Overall Efficacy of Neoadjuvant 177Lu-DOTATATE PRRT for Locally Advanced Unresectable Gastroenteropancreatic Neuroendocrine Tumors. J. Nucl. Med. 2021, 62, 1558–1563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Partelli, S.; Landoni, L.; Bartolomei, M.; Zerbi, A.; Grana, C.M.; Boggi, U.; Butturini, G.; Casadei, R.; Salvia, R.; Falconi, M. Neoadjuvant 177Lu-DOTATATE for non-functioning pancreatic neuroendocrine tumours (NEOLUPANET): Multicentre phase II study. Br. J. Surg. 2024, 111, znae178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Characteristic | Overall (n = 68) | Cumulative % |
|---|---|---|
| Gender | ||
| Male | 44 (64.7%) | 64.7% |
| Female | 24 (35.3%) | 100% |
| Primary tumor location | ||
| GI tract | 17 (25.0%) | 25.0% |
| Pancreas | 31 (45.6%) | 70.6% |
| Lung | 8 (11.8%) | 82.4% |
| Others | 12 (17.6%) | 100% |
| Metastatic location | ||
| Liver | 62 (91%) | - |
| Lungs | 5 (7.4%) | - |
| Peritoneum | 14 (20.6%) | - |
| Bone | 26 (38.2%) | - |
| Previous treatments | ||
| ≤1 | 33 (48.5%) | 48.5% |
| >1 | 35 (51.5%) | 100% |
| Tumor differentiation | ||
| Well differentiated | 59 (86.8%) | 86.8% |
| Moderately differentiated | 2 (2.9%) | 89.7% |
| Poorly differentiated | 4 (5.9%) | 95.6% |
| Unknown | 3 (4.4%) | 100% |
| Grade | ||
| G1 | 20 (29.4%) | 29.4% |
| G2 | 34 (50.0%) | 79.4% |
| G3 | 6 (8.8%) | 88.2% |
| Unknown | 8 (11.8%) | 100% |
| Location | Frequency | % from Total | Cumulative % | |
|---|---|---|---|---|
| Complete response | GI | 0 | 0.0% | 0.0% |
| Pancreas | 1 | 1.5% | 1.5% | |
| Lung | 0 | 0.0% | 1.5% | |
| Other | 0 | 0.0% | 1.5% | |
| Stabilization of the disease | GI | 12 | 17.6% | 19.1% |
| Pancreas | 14 | 20.6% | 39.7% | |
| Lung | 5 | 7.4% | 47.1% | |
| Other | 7 | 10.3% | 57.4% | |
| Partial response | GI | 2 | 2.9% | 60.3% |
| Pancreas | 11 | 16.2% | 76.5% | |
| Lung | 2 | 2.9% | 79.4% | |
| Other | 5 | 7.4% | 86.8% | |
| Progressive disease | GI | 1 | 1.5% | 88.2% |
| Pancreas | 6 | 8.8% | 97.1% | |
| Lung | 1 | 1.5% | 98.5% | |
| Other | 0 | 0.0% | 98.5% | |
| Unknown | GI | 0 | 0.0% | 98.5% |
| Pancreas | 1 | 1.5% | 100.0% | |
| Lung | 0 | 0.0% | 100.0% | |
| Other | 0 | 0.0% | 100.0% |
| Independent Variable | Category | Yes (n) | No (n) | OR (95%CI) | p Value |
|---|---|---|---|---|---|
| Histological grade | G1 | 4 | 16 | Reference value | Reference value |
| G2 | 21 | 13 | 6.46 (1.90–26.58) | p = 0.005 | |
| G3 | 4 | 2 | 8.0 (1.16–75.78) | p = 0.044 | |
| Unknown | 2 | 6 | 1.33 (0.16–8.94) | p = 0.771 | |
| Previous treatments | ≤1 | 9 | 24 | Reference value | Reference value |
| >1 | 22 | 13 | 4.45 (1.66–13.14) | p = 0.004 | |
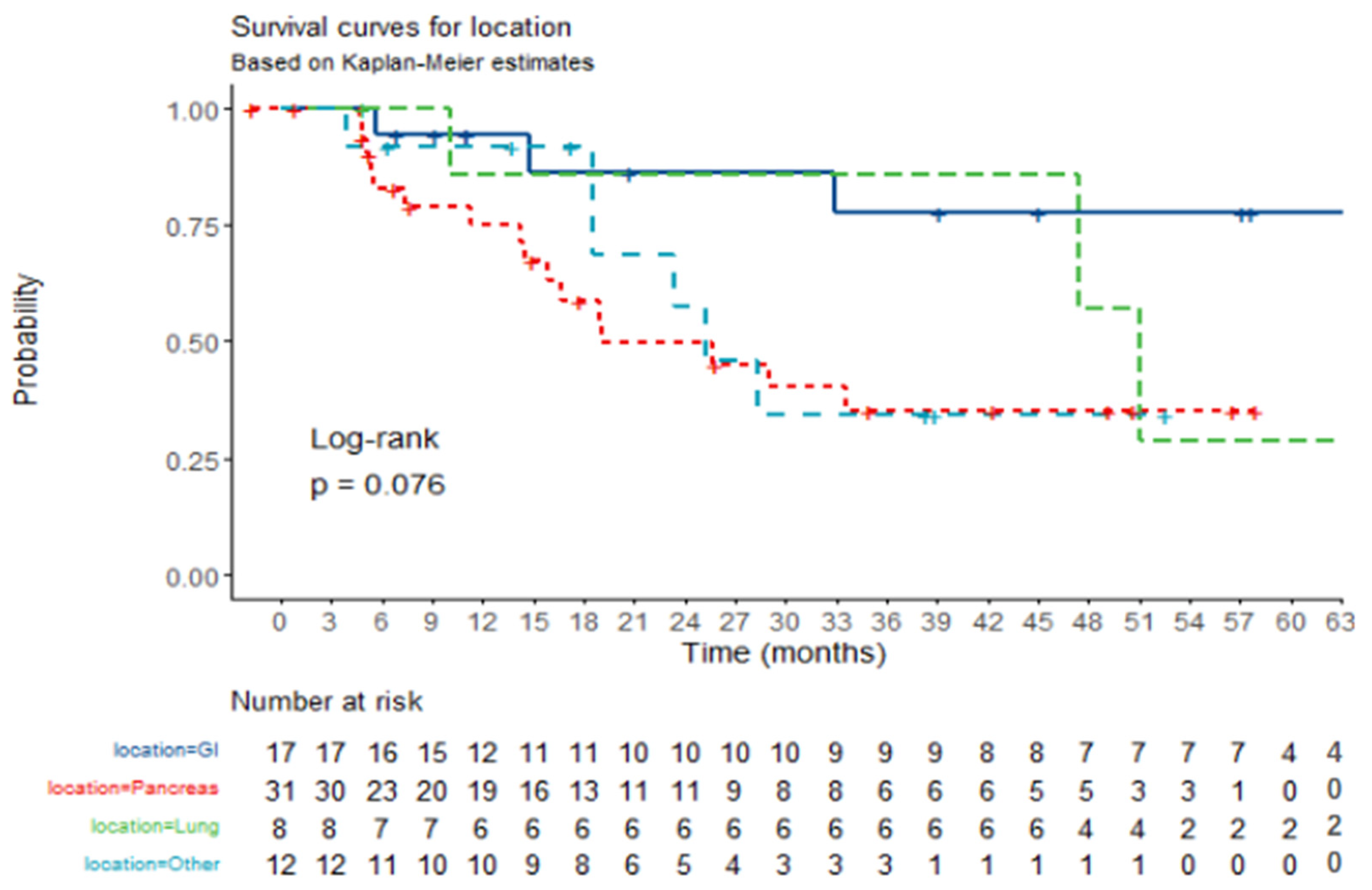
| Location | GI | 5 | 12 | Reference value | Reference value |
| Pancreas | 16 | 15 | 3.0 (0.84–12.58) | p = 0.105 | |
| Lung | 5 | 3 | 5.0 (0.85–35.46) | p = 0.084 | |
| Other | 6 | 6 | 3.0 (0.62–16.11) | p = 0.178 |
| Independent Variable | Category | All | HR (95% CI) | p Value |
|---|---|---|---|---|
| Grade | G1 | 20 | Reference value | Reference value |
| G2 | 34 | 3.41 (1.15–10.07) | p = 0.027 | |
| G3 | 6 | 7.83 (1.80–34.13) | p = 0.006 | |
| Unknown | 8 | 0.82 (0.16–4.62) | p = 0.823 | |
| Previous treatments | ≤1 | 33 | Reference value | Reference value |
| >1 | 35 | 2.22 (0.97–5.07) | p = 0.058 |
| Adverse Event | Frequency/Overall (%) | Cumulative % |
|---|---|---|
| Any adverse event | ||
| Yes | 29/68 (42.6%) | 42.6% |
| No | 3/68 (57.4%) | 100% |
| Digestive | ||
| Nausea | 12/68 (17.6%) | - |
| Grade 1 | 11/12 (91.7%) | 91.7% |
| Grade 2 | 1/12 (8.3%) | 100.0% |
| Vomiting | 9/68 (13.2%) | - |
| Grade 1 | 8/9 (88.9%) | 88.9% |
| Grade 2 | 1/9 (11.1%) | 100.0% |
| Diarrhea | 9/68 (13.2%) | - |
| Grade 1 | 9/9 (100%) | 100% |
| Hematologic | ||
| WCC decrease | 8/68 (11.8%) | - |
| Grade 1 Grade 4 | 7/8 (87.5%) 1/8 (12.5%) | 87.5% 100.0% |
| PC decrease | 4/68 (5.9%) | - |
| Grade 2 Grade 3 Grade 4 | 2/4 (50.0%) 1/4 (25.0%) 1/4 (25.0%) | 50.0% 75.0% 100.0% |
| General | ||
| Asthenia | 4 (5.9%) | - |
| Grade 1 | 4/4 (100%) | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos Ramírez, S.E.; Andrés García, A.; Blanco Abad, C.; Gomila Pons, P.; Gómez Mugarza, P.; Ruffini Egea, S.E.; Gallart Caballero, L.; Polo Marques, E.; Alonso Orduña, V. Evaluation of the Real-Life Efficacy and Safety of the Treatment with Lutetium-177 Dotatate for Metastatic Neuroendocrine Tumors. J. Clin. Med. 2025, 14, 2384. https://doi.org/10.3390/jcm14072384
Campos Ramírez SE, Andrés García A, Blanco Abad C, Gomila Pons P, Gómez Mugarza P, Ruffini Egea SE, Gallart Caballero L, Polo Marques E, Alonso Orduña V. Evaluation of the Real-Life Efficacy and Safety of the Treatment with Lutetium-177 Dotatate for Metastatic Neuroendocrine Tumors. Journal of Clinical Medicine. 2025; 14(7):2384. https://doi.org/10.3390/jcm14072384
Chicago/Turabian StyleCampos Ramírez, Sara Elena, Alejandro Andrés García, Carmen Blanco Abad, Paula Gomila Pons, Pablo Gómez Mugarza, Sofía Elena Ruffini Egea, Luis Gallart Caballero, Eduardo Polo Marques, and Vicente Alonso Orduña. 2025. "Evaluation of the Real-Life Efficacy and Safety of the Treatment with Lutetium-177 Dotatate for Metastatic Neuroendocrine Tumors" Journal of Clinical Medicine 14, no. 7: 2384. https://doi.org/10.3390/jcm14072384
APA StyleCampos Ramírez, S. E., Andrés García, A., Blanco Abad, C., Gomila Pons, P., Gómez Mugarza, P., Ruffini Egea, S. E., Gallart Caballero, L., Polo Marques, E., & Alonso Orduña, V. (2025). Evaluation of the Real-Life Efficacy and Safety of the Treatment with Lutetium-177 Dotatate for Metastatic Neuroendocrine Tumors. Journal of Clinical Medicine, 14(7), 2384. https://doi.org/10.3390/jcm14072384






