Remote Monitoring of Patients with Retinal Vein Occlusions Treated with Anti-VEGF: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
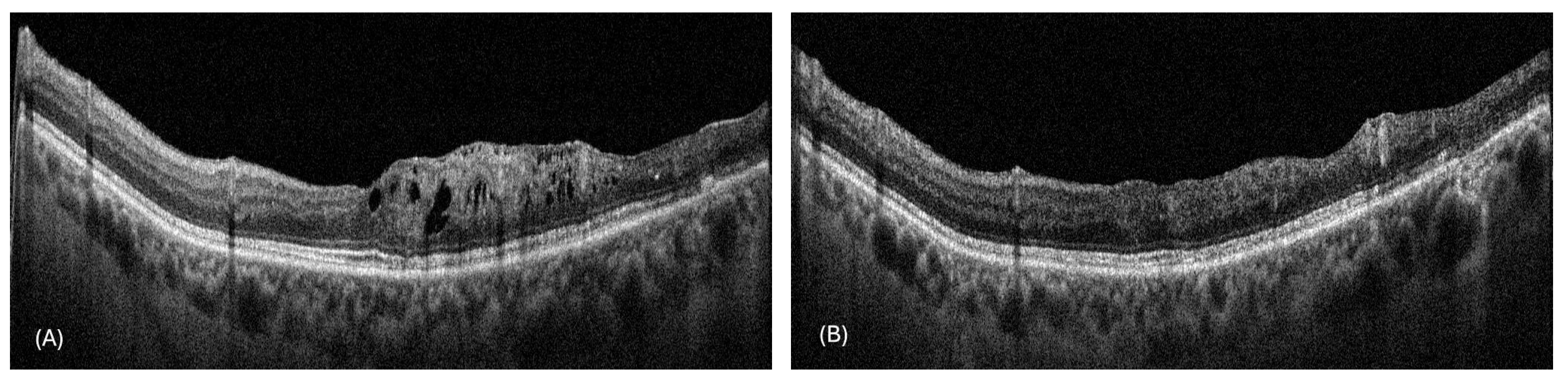
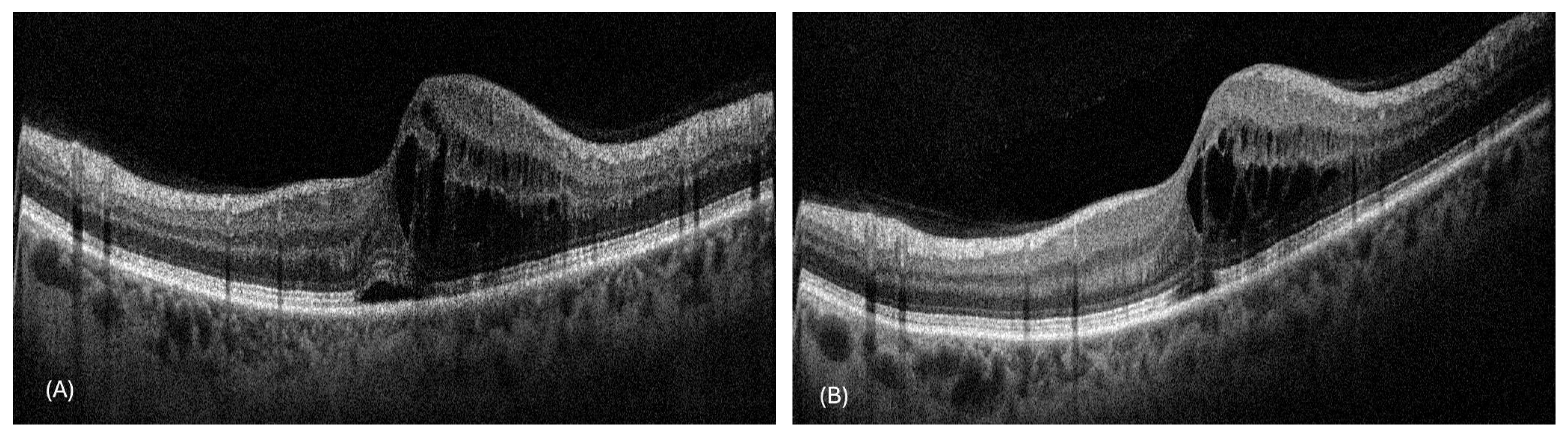
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RVO | Retinal Vein Occlusion |
| BRVO | Branch Retinal Vein Occlusion |
| CRVO | Central Retinal Vein Occlusion |
| VEGF | Vascular Endothelial Growth Factor |
| FA | Fluorescein Angiography |
| OCT | Optical Coherence Tomography |
| BCVA | Best-Corrected Visual Acuity |
| CRT | Central Retinal Thickness |
| DR | Diabetic Retinopathy |
| PRN | Pro-Re-Nata |
References
- Lendzioszek, M.; Bryl, A.; Poppe, E.; Zorena, K.; Mrugacz, M. Retinal Vein Occlusion-Background Knowledge and Foreground Knowledge Prospects-A Review. J. Clin. Med. 2024, 13, 3950. [Google Scholar] [CrossRef]
- Blair, K.; Czyz, C.N. Central Retinal Vein Occlusion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rogers, S.; McIntosh, R.L.; Cheung, N.; Lim, L.; Wang, J.J.; Mitchell, P.; Kowalski, J.W.; Nguyen, H.; Wong, T.Y.; International Eye Disease Consortium. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010, 117, 313–319.e1. [Google Scholar] [CrossRef] [PubMed]
- Muhtaseb, R.E.; Huther, A.; Alwreikat, A.M.; Ramsey, D.J. Optimizing open-angle glaucoma risk assessment in patients with retinal vein occlusions. Eye 2024, 38, 2985–2991. [Google Scholar] [CrossRef]
- La Spina, C.; De Benedetto, U.; Parodi, M.B.; Coscas, G.; Bandello, F. Practical management of retinal vein occlusions. Ophthalmol. Ther. 2012, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.C.Y.; Lip, G.Y.H.; Lip, P.L. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: A systematic review. Eye 2016, 30, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Gerendas, B.S.; Midena, E.; Sivaprasad, S.; Tadayoni, R.; Wolf, S.; Loewenstein, A. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2019, 242, 123–162. [Google Scholar] [CrossRef]
- Nicholson, L.; Talks, S.J.; Amoaku, W.; Talks, K.; Sivaprasad, S. Retinal vein occlusion (RVO) guideline: Executive summary. Eye 2022, 36, 909–912. [Google Scholar] [CrossRef]
- Rezar, S.; Eibenberger, K.; Bühl, W.; Georgopoulos, M.; Schmidt-Erfurth, U.; Sacu, S.; Macula Study Group Vienna. Anti-VEGF treatment in branch retinal vein occlusion: A real-world experience over 4 years. Acta Ophthalmol. 2015, 93, 719–725. [Google Scholar] [CrossRef]
- Stewart, M.W. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin. Proc. 2012, 87, 77–88. [Google Scholar] [CrossRef]
- Spooner, K.L.; Fraser-Bell, S.; Hong, T.; Wong, J.G.; Chang, A.A. Long-term outcomes of anti-VEGF treatment of retinal vein occlusion. Eye 2022, 36, 1194–1201. [Google Scholar] [CrossRef]
- Hang, A.; Feldman, S.; Amin, A.P.; Ochoa, J.A.R.; Park, S.S. Intravitreal Anti-Vascular Endothelial Growth Factor Therapies for Retinal Disorders. Pharmaceuticals 2023, 16, 1140. [Google Scholar] [CrossRef] [PubMed]
- Laouri, M.; Chen, E.; Looman, M.; Gallagher, M. The burden of disease of retinal vein occlusion: Review of the literature. Eye 2011, 25, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Bitar, H.; Alismail, S. The role of eHealth, telehealth, and telemedicine for chronic disease patients during COVID-19 pandemic: A rapid systematic review. Digit Health 2021, 7, 20552076211009396. [Google Scholar] [CrossRef]
- Fekadu, G.; Bekele, F.; Tolossa, T.; Fetensa, G.; Turi, E.; Getachew, M.; Abdisa, E.; Assefa, L.; Afeta, M.; Demisew, W.; et al. Impact of COVID-19 pandemic on chronic diseases care follow-up and current perspectives in low resource settings: A narrative review. Int. J. Physiol. Pathophysiol. Pharmacol. 2021, 13, 86–93. [Google Scholar]
- Yu, S.; Wan, R.; Bai, L.; Zhao, B.; Jiang, Q.; Jiang, J.; Li, Y. Transformation of chronic disease management: Before and after the COVID-19 outbreak. Front. Public Health 2023, 11, 1074364. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Khoshrounejad, F.; Hamednia, M.; Mehrjerd, A.; Pichaghsaz, S.; Jamalirad, H.; Sargolzaei, M.; Hoseini, B.; Aalaei, S. Telehealth-Based Services During the COVID-19 Pandemic: A Systematic Review of Features and Challenges. Front. Public Health 2021, 9, 711762. [Google Scholar] [CrossRef]
- Tan, A.J.; Rusli, K.D.; McKenna, L.; Tan, L.L.; Liaw, S.Y. Telemedicine experiences and perspectives of healthcare providers in long-term care: A scoping review. J. Telemed. Telecare 2024, 30, 230–249. [Google Scholar] [CrossRef]
- Snoswell, C.L.; Chelberg, G.; De Guzman, K.R.; Haydon, H.M.; Thomas, E.E.; Caffery, L.J.; Smith, A.C. The clinical effectiveness of telehealth: A systematic review of meta-analyses from 2010 to 2019. J. Telemed. Telecare 2023, 29, 669–684, Correction in J. Telemed. Telecare 2024, 30, 1667. [Google Scholar] [CrossRef]
- Horton, M.B.; Silva, P.S.; Cavallerano, J.D.; Aiello, L.P. Operational Components of Telemedicine Programs for Diabetic Retinopathy. Curr. Diab. Rep. 2016, 16, 128. [Google Scholar] [CrossRef]
- Brandão-de-Resende, C.; Alcântara, L.A.R.; Vasconcelos-Santos, D.V.; Diniz-Filho, A. Glaucoma and Telemedicine. J. Glaucoma 2023, 32, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, D.; Borselli, M.; Gratteri, S.; Zampogna, G.; Feola, A.; Della Corte, M.; Guarna, F.; Scorcia, V.; Giannaccare, G. Applications and Current Medico-Legal Challenges of Telemedicine in Ophthalmology. Int. J. Environ. Res. Public Health 2022, 19, 5614. [Google Scholar] [CrossRef]
- Leal, J.; Luengo-Fernandez, R.; Stratton, I.M.; Dale, A.; Ivanova, K.; Scanlon, P.H. Cost-effectiveness of digital surveillance clinics with optical coherence tomography versus hospital eye service follow-up for patients with screen-positive maculopathy. Eye 2019, 33, 640–647. [Google Scholar] [CrossRef]
- Schuster, A.K.; Wolfram, C.; Hudde, T.; Klatt, A.; Schnegelsberg, B.; Midani-Oezkan, H.; Ross, M.; Ziemssen, F.; Pfeiffer, N. Impact of Routinely Performed Optical Coherence Tomography Examinations on Quality of Life in Patients with Retinal Diseases-Results from the ALBATROS Data Collection. J. Clin. Med. 2023, 12, 3881. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.W.; Miller, J.B. Telemedicine for the Diagnosis and Management of Age-Related Macular Degeneration: A Review. J. Clin. Med. 2022, 11, 835. [Google Scholar] [CrossRef]
- Starr, M.R.; Barkmeier, A.J.; Engman, S.J.; Kitzmann, A.; Bakri, S.J. Telemedicine in the Management of Exudative Age-Related Macular Degeneration within an Integrated health care System. Am. J. Ophthalmol. 2019, 208, 206–210. [Google Scholar] [CrossRef]
- Scott, I.U.; Ip, M.S.; VanVeldhuisen, P.C.; Oden, N.L.; Blodi, B.A.; Fisher, M.; Chan, C.K.; Gonzalez, V.H.; Singerman, L.J.; Tolentino, M.; et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch. Ophthalmol. 2009, 127, 1115–1128, Correction in Arch Ophthalmol. 2009, 127, 1655. [Google Scholar] [CrossRef]
- Ip, M.S.; Scott, I.U.; VanVeldhuisen, P.C.; Oden, N.L.; A Blodi, B.; Fisher, M.; Singerman, L.J.; Tolentino, M.; Chan, C.K.; Gonzalez, V.H.; et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch. Ophthalmol. 2009, 127, 1101–1114, Correction in Arch. Ophthalmol. 2009, 127, 1648. [Google Scholar] [CrossRef]
- Brown, D.M.; Campochiaro, P.A.; Singh, R.P.; Li, Z.; Gray, S.; Saroj, N.; Rundle, A.C.; Rubio, R.G.; Murahashi, W.Y. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology 2010, 117, 1124–1133.e1. [Google Scholar] [CrossRef]
- Haller, J.A.; Bandello, F.; Belfort, R., Jr.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.-H.; Jacques, M.-L.; Jiao, J.; et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010, 117, 1134–1146.e3. [Google Scholar] [CrossRef]
- Heier, J.S.; Clark, W.L.; Boyer, D.S.; Brown, D.M.; Vitti, R.; Berliner, A.J.; Kazmi, H.; Ma, Y.; Stemper, B.; Zeitz, O.; et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: Two-year results from the COPERNICUS study. Ophthalmology 2014, 121, 1414–1420.e1, Correction in Ophthalmology 2014, 121, 2293. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.U.; VanVeldhuisen, P.C.; Ip, M.S.; Blodi, B.A.; Oden, N.L.; Awh, C.C.; Kunimoto, D.Y.; Marcus, D.M.; Wroblewski, J.J.; King, J. Effect of Bevacizumab vs Aflibercept on Visual Acuity Among Patients With Macular Edema Due to Central Retinal Vein Occlusion: The SCORE2 Randomized Clinical Trial. JAMA 2017, 317, 2072–2087. [Google Scholar] [CrossRef]
- Wong, T.Y.; Larsen, E.K.; Klein, R.; Mitchell, P.; Couper, D.; Klein, B.; Hubbard, L.; Siscovick, D.; Sharrett, A. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: The Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology 2005, 112, 540–547. [Google Scholar] [CrossRef]
- Bertelsen, M.; Linneberg, A.; Rosenberg, T.; Christoffersen, N.; Vorum, H.; Gade, E.; Larsen, M. Comorbidity in patients with branch retinal vein occlusion: Case-control study. BMJ 2012, 345, e7885. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, B.; McCarthy, M.J.; Podhajsky, P. Systemic diseases associated with various types of retinal vein occlusion. Am. J. Ophthalmol. 2001, 131, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Magargal, L.E.; Schachat, A.; Shah, H. Neovascular glaucoma. Etiologic considerations. Ophthalmology 1984, 91, 315–320. [Google Scholar] [CrossRef]
- Dumbrăveanu, L.; Cușnir, V.; Bobescu, D. A review of neovascular glaucoma. Etiopathogenesis and treatment. Rom. J. Ophthalmol. 2021, 65, 315–329. [Google Scholar] [CrossRef]
- Clark, W.L.; Boyer, D.S.; Heier, J.S.; Brown, D.M.; Haller, J.A.; Vitti, R.; Kazmi, H.; Berliner, A.J.; Erickson, K.; Chu, K.W.; et al. Intravitreal Aflibercept for Macular Edema Following Branch Retinal Vein Occlusion: 52-Week Results of the VIBRANT Study. Ophthalmology 2016, 123, 330–336. [Google Scholar] [CrossRef]
- Chatziralli, I.; Theodossiadis, G.; Moschos, M.M.; Mitropoulos, P.; Theodossiadis, P. Ranibizumab versus aflibercept for macular edema due to central retinal vein occlusion: 18-month results in real-life data. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1093–1100. [Google Scholar] [CrossRef]
- Nobre Cardoso, J.; Keane, P.A.; Sim, D.A.; Bradley, P.; Agrawal, R.; Addison, P.K.; Egan, C.; Tufail, A. Systematic Evaluation of Optical Coherence Tomography Angiography in Retinal Vein Occlusion. Am. J. Ophthalmol. 2016, 163, 93–107.e6. [Google Scholar] [CrossRef]
| Number of Patients Who Completed Follow-Up (12 Months) | n | 34 |
|---|---|---|
| Mean age | years | 67.56 ± 8.04 |
| Females | n (%) | 14 (41.2%) |
| Males | n (%) | 20 (58.8%) |
| Number of eyes affected by CRVO | n (%) | 10 (29.4%) |
| Number of eyes affected by BRVO | n (%) | 24 (70.6%) |
| Patients who underwent cataract surgery during follow-up | n (%) | 2 (5.9%) |
| Mean gain in BCVA | letters | 11.47 ± 5.56 |
| Number of visits to a peripheral center per patient | mean | 5.71 ± 1.14 |
| Mean number of injections | mean | 5.26 ± 1.29 |
| Number of visits to our center for fluorescein angiography | mean | 2.1 ± 0.8 |
| Number of eyes that underwent laser treatment at our center | n (%) | 12 (35.3%) 34 admissions |
| Number of OCT scans evaluated via remote monitoring | n | 194 |
| Number of visits to our center because OCT in the peripheral center was inconclusive | n | 14 (7.2%) |
| Number of visits to our hospital for other specialist consultations (internist, cardiologist) | mean | 2.4 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellino, N.; Cappellani, F.; Dammino, E.; Rubegni, G.; Scollo, D.; Russo, A.; Avitabile, T.; Longo, A. Remote Monitoring of Patients with Retinal Vein Occlusions Treated with Anti-VEGF: A Pilot Study. J. Clin. Med. 2025, 14, 2330. https://doi.org/10.3390/jcm14072330
Castellino N, Cappellani F, Dammino E, Rubegni G, Scollo D, Russo A, Avitabile T, Longo A. Remote Monitoring of Patients with Retinal Vein Occlusions Treated with Anti-VEGF: A Pilot Study. Journal of Clinical Medicine. 2025; 14(7):2330. https://doi.org/10.3390/jcm14072330
Chicago/Turabian StyleCastellino, Niccolò, Francesco Cappellani, Edoardo Dammino, Giovanni Rubegni, Davide Scollo, Andrea Russo, Teresio Avitabile, and Antonio Longo. 2025. "Remote Monitoring of Patients with Retinal Vein Occlusions Treated with Anti-VEGF: A Pilot Study" Journal of Clinical Medicine 14, no. 7: 2330. https://doi.org/10.3390/jcm14072330
APA StyleCastellino, N., Cappellani, F., Dammino, E., Rubegni, G., Scollo, D., Russo, A., Avitabile, T., & Longo, A. (2025). Remote Monitoring of Patients with Retinal Vein Occlusions Treated with Anti-VEGF: A Pilot Study. Journal of Clinical Medicine, 14(7), 2330. https://doi.org/10.3390/jcm14072330





