Unraveling Acute Cardiorenal Syndrome: Predictors and Consequences in Acute Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Methodology
2.4.1. Patient Profile and Laboratory Parameters
2.4.2. Laboratory Testing
2.4.3. Imaging Parameters
2.4.4. Endpoints
2.4.5. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Patient Characteristics Based on the Occurrence of Acute Cardiorenal Syndrome (ACRS)
3.3. Predictors of Acute Cardiorenal Syndrome
3.4. ACRS and Mortality
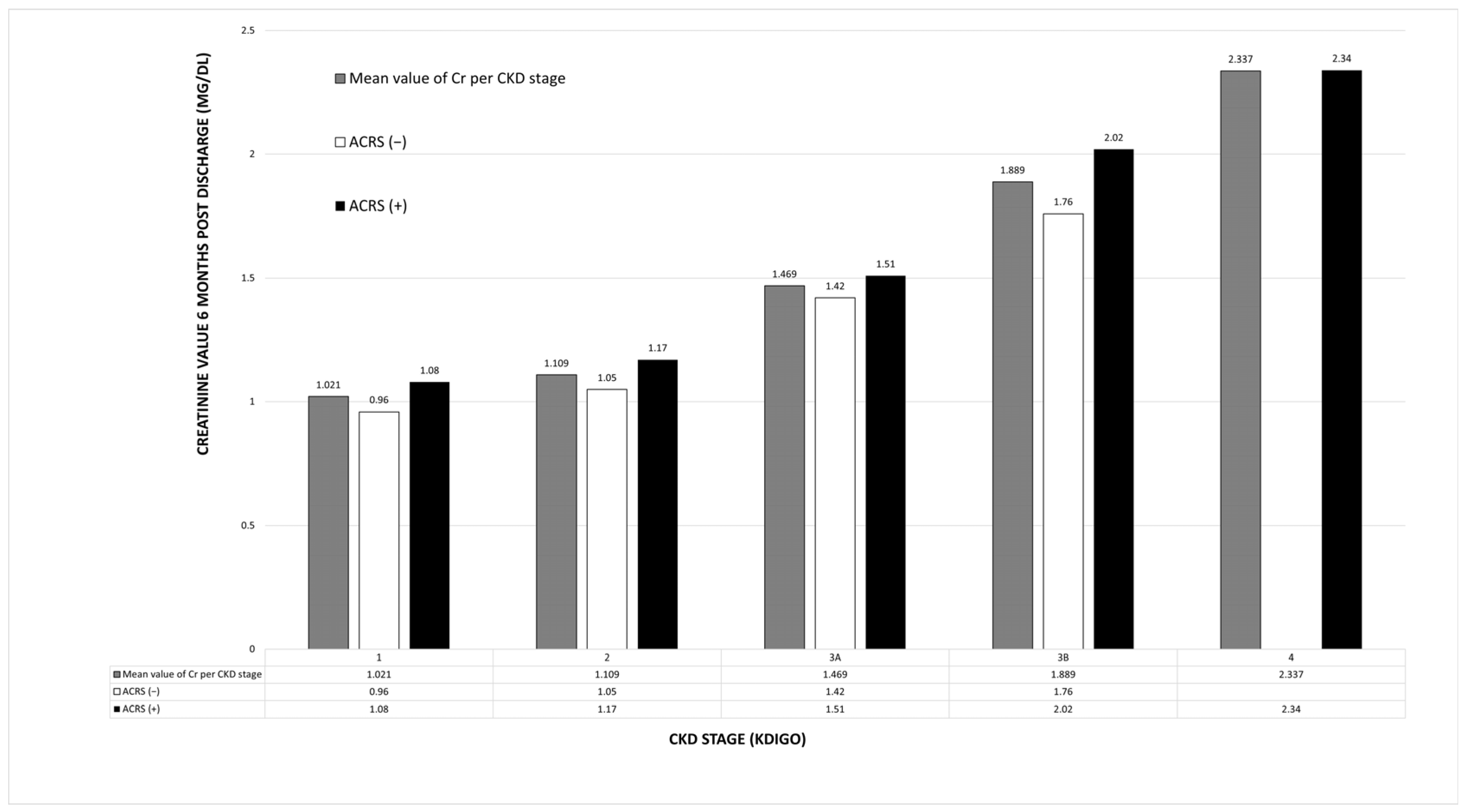
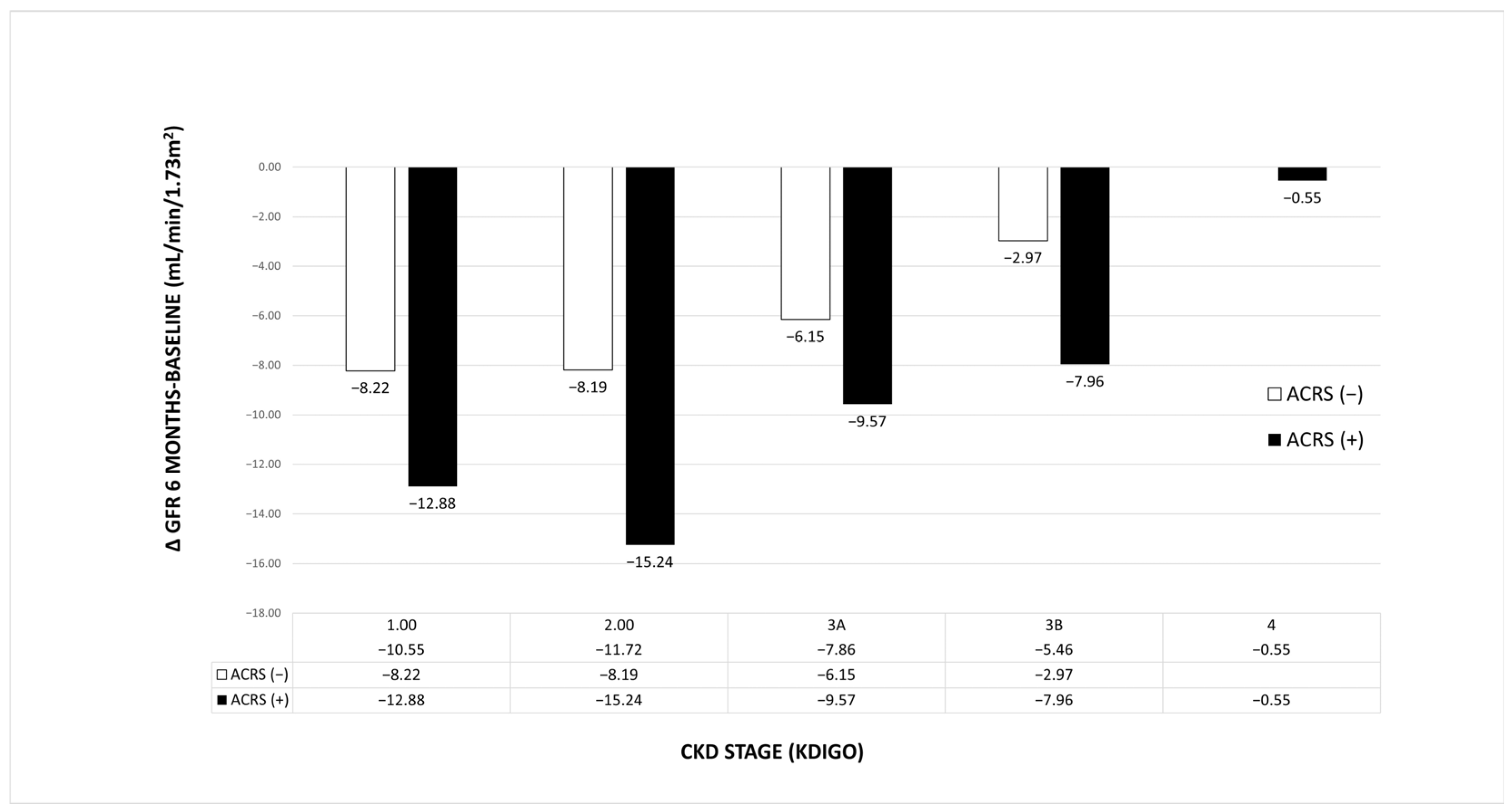
3.5. Effect of ACRS on Renal Function After 6 Months
4. Discussion
4.1. Incidence of ACRS and Epidemiological Data
4.2. Risk Factors for ACRS
4.3. Outcomes
4.4. ACRS and Renal Function
4.5. Limitations
5. Conclusions
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95% CI | 95% Confidence interval |
| ACEi | Angiotensin-converting enzyme inhibitor |
| ACRS | Acute cardiorenal syndrome |
| Admission ΔCr | Difference between admission creatinine value and the minimum creatinine value recorded during hospitalization |
| ADQI | Acute Dialysis Quality Initiative |
| AHF | Acute heart failure |
| AKI | Acute kidney injury |
| ANCOVA | Analysis of covariance |
| ARBs | Angiotensin receptor blocker |
| ARNIs | Angiotensin receptor/neprilysin inhibitor |
| CKD | Chronic kidney disease |
| CRS | Cardiorenal syndrome |
| ED | Emergency department |
| eGFR | Estimated Glomerular filtration rate |
| GFR | Glomerular filtration rate |
| ESC | European Society of Cardiology |
| GDPR | General Data Protection Regulation |
| Hb | Hemoglobin |
| HbA1c | Glycosylated hemoglobin |
| HF | Heart Failure |
| Hs-cTnI | High-sensitive troponin-I |
| IQR | Interquartile range |
| KIM-1 | Kidney injury molecule 1 |
| KDIGO | Kidney Disease Improving Global Outcomes |
| LVEF | Left ventricular ejection fraction |
| MRAs | Mineralocorticoid receptor blockers |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| NYHA | New York Heart Association |
| OR | Odds Ratio |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RRT | Renal replacement therapy |
| SD | Standard deviation |
| SGLT2i | Sodium-glucose cotransporter-2 inhibitors |
| TSH | Thyroid-stimulating hormone |
| TV S′ | Lateral tricuspid annular systolic velocity |
| WRF | Worsening renal function |
| ΔCr | Difference between maximum creatinine and minimum creatinine value during hospitalization |
| ΔCr1 | Difference between admission creatinine value and baseline creatinine value |
| ΔGFR | Difference between maximum glomerular filtration rate and minimum glomerular filtration rate value during hospitalization |
| ΔGFR (6M-BASELINE) | Difference between glomerular filtration rate 6 months post hospitalization and baseline glomerular filtration rate value |
Appendix A
Appendix A.1. Definition and Classification of Acute and Chronic Kidney Disease
| GFR Categories | GFR (mL/min/1.73 m2) | Description |
|---|---|---|
| G1 | >90 | Normal or high |
| G2 | 60–89 | Mildly decreased |
| G3a | 45–59 | Mildly to moderately decreased |
| G3b | 30–44 | Moderately to severely decreased |
| G4 | 15–29 | Severy decreased |
| G5 | <15 | Kidney failure |
- An increase in serum creatinine of 0.3 mg/dL or more within 48 h, or
- An increase in serum creatinine to more than 1.5 times the baseline value, known or
- Presumed to have occurred within the preceding seven days, or
- Urine output of less than 0.5 mL/kg/hour for a duration of six hours
| AKI Stage | Serum Creatinine Criteria | Urine Output Criteria |
|---|---|---|
| 1 | Serum Cr increase ≥ 0.3 mg/dL within 48 h or Serum Cr increase ≥ 1.5–2 fold from baseline | <0.5 mL/kg/h for 6 consecutive hours |
| 2 | Serum Cr increase ≥ 2–3 fold from baseline | <0.5 mL/kg/h for 12 h |
| 3 | Serum Cr increase ≥ 3 fold from baseline or Serum creatinine increase ≥ 4 mg/dL or Initiated on RRT (irrespective of stage at time of initiation) | <0.3 mL/kg/h for 24 h or anuria for 12 h |
References
- Mitsas, A.C.; Elzawawi, M.; Mavrogeni, S.; Boekels, M.; Khan, A.; Eldawy, M.; Stamatakis, I.; Kouris, D.; Daboul, B.; Gunkel, O.; et al. Heart Failure and Cardiorenal Syndrome: A Narrative Review on Pathophysiology, Diagnostic and Therapeutic Regimens—From a Cardiologist’s View. J. Clin. Med. 2022, 11, 7041. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Saha, S.; Bahl, A.; Mittal, A.; Basak, T. A comprehensive review of acute cardio-renal syndrome: Need for novel biomarkers. Front. Pharmacol. 2023, 14, 1152055. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of kidney function throughout the heart failure trajectory—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased Central Venous Pressure Is Associated with Impaired Renal Function and Mortality in a Broad Spectrum of Patients with Cardiovascular Disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.L.; Kovesdy, C.P.; Ronco, C. Contemporary laboratory assessment of acute cardiorenal syndrome for early diagnosis: A call for action. Am. Heart J. 2023, 261, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Łagosz, P.; Biegus, J.; Urban, S.; Zymliński, R. Renal Assessment in Acute Cardiorenal Syndrome. Biomolecules 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Lisa, A.; Carbone, F.; Liberale, L.; Montecucco, F. The Need to Identify Novel Markers for Early Renal Injury in Cardiorenal Syndrome. Cells 2024, 13, 1283. [Google Scholar] [CrossRef] [PubMed]
- Seckinger, D.; Ritter, O.; Patschan, D. Risk factors and outcome variables of cardiorenal syndrome type 1 from the nephrologist’s perspective. Int. Urol. Nephrol. 2022, 54, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Lewis, R.D.; Morgan, A.R.; Whyte, M.B.; Hanif, W.; Bain, S.C.; Davies, S.; Dashora, U.; Yousef, Z.; Patel, D.C.; et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv. Ther. 2022, 39, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef]
- Li, P.K.T.; Burdmann, E.A.; Mehta, R.L. Acute kidney injury: Global health alert. Kidney Int. 2013, 83, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Peveri, G.; Cani, D.; Latta, F.; Bonelli, A.; Tomasoni, D.; Sbolli, M.; Ravera, A.; Carubelli, V.; Saccani, N.; et al. In-hospital and long-term mortality for acute heart failure: Analysis at the time of admission to the emergency department. ESC Heart Fail. 2020, 7, 2650–2661. [Google Scholar] [CrossRef]
- Hu, W.; He, W.; Liu, W.; Fang, X.; Wu, Y.; Yu, F.; Hao, W. Risk Factors and Prognosis of Cardiorenal Syndrome Type 1 in Elderly Chinese Patients: A Retrospective Observational Cohort Study. Kidney Blood Press. Res. 2016, 41, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Liang, Y.; Fang, Y.; Jin, Y.; Su, W.; Zhang, G.; Wang, J.; Xiong, H.; Shang, D.; Chai, Y.; et al. Predictors of acute kidney injury in patients with acute decompensated heart failure in emergency departments in China. J. Int. Med. Res. 2021, 49, 03000605211016208. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Liu, M.; Huang, J.; Chen, L.; Xu, Y. Impact of anemia on clinical outcomes in patients with acute heart failure: A systematic review and meta-analysis. Clin. Cardiol. 2024, 47, e24228. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Fan, J.; Wan, X.; Liu, H.; Shi, Y.; Shu, H.; Liu, Y.; Lu, T.; Gong, Z.; Gu, L. The association between blood albumin level and cardiovascular complications and mortality risk in ICU patients with CKD. BMC Cardiovasc. Disord. 2022, 22, 322. [Google Scholar] [CrossRef]
- Chao, P.; Cui, X.; Wang, S.; Zhang, L.; Ma, Q.; Zhang, X. Serum albumin and the short-term mortality in individuals with congestive heart failure in intensive care unit: An analysis of MIMIC. Sci. Rep. 2022, 12, 16251. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Guerrero, G.; Ronco, C.; Reis, T. Cardiorenal Syndrome and Inflammation: A Forgotten Frontier Resolved by Sorbents? Cardiorenal Med. 2024, 14, 454–458. [Google Scholar] [CrossRef] [PubMed]
| Phenotype | Description | Clinical Examples |
|---|---|---|
| Type 1–Acute cardiorenal syndrome (ACRS) | Acute heart failure (AHF) resulting in acute kidney injury (AKI) | Acute myocardial infarction, Cardiogenic shock, Acute decompensated heart failure |
| Type 2–Chronic cardiorenal syndrome | Chronic heart failure resulting in chronic kidney disease (CKD) | Chronic heart failure irrespective of its underlying cause |
| Type 3–Acute renocardiac syndrome | Acute kidney injury resulting in acute heart failure | Volume overload, Metabolic disturbances in uremia, Inflammatory surge |
| Type 4–Chronic renocardiac syndrome | Chronic kidney disease resulting in chronic heart failure | Left ventricular hypertrophy, CKD associated cardiomyopathy |
| Τype 5–Secondary CRS | Systematic process resulting in HF and kidney failure | Amyloidosis, Sepsis, Cirrhosis |
| Parameter | Total n = 218 | ACRS(+) n = 112 | ACRS (−) n = 106 | p-Value |
|---|---|---|---|---|
| Demographics: | ||||
| Male sex | 116 (53.2%) | 62 (55.4%) | 54 (50.9%) | 0.514 |
| Age > 75 years | 161 (73.9%) | 90 (80.4%) | 71 (67%) | 0.025 |
| Age (years) | 82 (74–86) | 82 (78–87) | 79 (71–86) | 0.014 |
| Comorbidities: | ||||
| Atrial fibrillation | 133 (61%) | 67 (59.8%) | 66 (62.3%) | 0.712 |
| Diabetes mellitus | 98 (45%) | 54 (48.2%) | 44 (41.5%) | 0.320 |
| Arterial hypertension | 194 (89%) | 99 (88.4%) | 95 (89.6%) | 0.772 |
| Airway disease | 68 (31.2%) | 36 (32.1%) | 32 (30.2%) | 0.756 |
| Implantable cardiac defibrillator | 9 (4.1%) | 5 (4.5%) | 4 (3.8%) | 0.798 |
| Active smoking | 25 (11.5%) | 13 (11.6%) | 12 (11.3%) | 0.947 |
| LVEF: ≤40% 41–49% ≥50% | 85 (39%) 27 (12.4%) 106 (48.6%) | 48 (42.9%) 10 (8.9%) 54 (48.2%) | 37 (34.9%) 17 (16%) 52 (49.1%) | 0.229 0.149 0.901 |
| Chronic kidney disease | 121 (55.5%) | 78 (69.6%) | 43 (40.6%) | <0.001 |
| CKD stage (KDIGO) 1 2 3A 3Β 4 | 20 (9.2%) 78 (35.8%) 64 (29.3%) 44 (20.2%) 12 (5.5%) | 6 (5.35%) 28 (25%) 35 (31.25%) 32 (28.6%) 11 (9.8%) | 14 (13.2%) 50 (47.2%) 29 (27.4%) 12 (11.3%) 1 (0.9%) | <0.001 |
| NYHA stage 2 3 4 | 45 (20.6%) 139 (63.8%) 34 (15.6%) | 14 (12.5%) 73 (65.2%) 25 (22.3%) | 31 (29.2%) 66 (62.3%) 9 (8.5%) | <0.001 |
| Chronic HF medication: | ||||
| ARNI | 9 (5.7%) | 3 (2.7%) | 6 (5.7%) | 0.269 |
| ACEi/ARB | 107 (49.1%) | 57 (50.9%) | 50 (47.2%) | 0.583 |
| Β-blocker | 133 (61%) | 69 (61.6%) | 64 (60.4%) | 0.852 |
| MRA | 51 (23.4%) | 29 (25.9%) | 22 (20.8%) | 0.370 |
| SGLT2i | 34 (15.6%) | 16 (14.3%) | 18 (17%) | 0.584 |
| Furosemide | 115 (52.8%) | 61 (54.5%) | 54 (50.9%) | 0.603 |
| Furosemide dose (mg) | 20 (0–40) | 30 (0–55) | 20 (0–40) | 0.345 |
| In-hospital complications and mortality up to 6 months: | ||||
| Need for inotropes/vasopressors | 39 (17.9%) | 34 (30.4%) | 5 (4.7%) | <0.001 |
| AKI stages (KDIGO) 0 1 2 3 | 106 (48.6%) 94 (43.1%) 13 (6%) 5 (2.3%) | 0 (0%) 94 (83.9%) 13 (11.6%) 5 (4.5%) | 106 (100%) 0 (0%) 0 (0%) 0 (0%) | <0.001 |
| Total hospital days (n = 206) | 7 (5–10) | 8 (6–12) | 6 (5–8) | <0.001 |
| Need for RRT | 4 (1.8%) | 4 (3.6%) | 0 (0%) | 0.05 |
| Death (all-cause) | 12 (5.5%) | 10 (8.9%) | 2 (1.9%) | 0.023 |
| Cardiovascular death | 8 (3.7%) | 6 (5.4%) | 2 (1.9%) | 0.173 |
| 6-month mortality | 44 (20.2%) | 32 (28.6%) | 12 (11.3%) | 0.002 |
| Parameter | Total (n = 218) | ACRS (+) (n = 112) | ACRS (−) (n = 106) | p-Value |
|---|---|---|---|---|
| Laboratory evaluation on admission: | ||||
| GFR (mL/min/1.73 m2) | 57 ± 21.5 | 48.3 ± 20 | 66 ± 19 | <0.001 |
| Cr (mg/dL) | 1.14 (0.94–1.49) | 1.36 (0.86–1.78) | 1.02 (0.86–1.22) | <0.001 |
| Baseline Cr (mg/dL) | 1.1 (0.9–1.4) | 1.2 (1–1.6) | 1 (0.9–1.22) | <0.001 |
| Baseline GFR (mL/min/1.73 m2) | 56 (44–78) | 50 (39–64.75) | 66.5 (54–80) | <0.001 |
| Urea (mg/dL) | 56.5 (44–85) | 68.5 (50 -103.5) | 48 (40–63) | <0.001 |
| NT-proBNP (pg/mL) | 5972 (3126–11,345) | 7625 (3662–14,898) | 5237 (2797–8974) | 0.003 |
| Hs-cTnΙ (pg/mL) | 26.6 (13.13–68) | 31.29 (14.77–94.73) | 24.7 (12.53–51.67) | 0.164 |
| Sodium (mEq/L) | 139 (137–141) | 139 (136–141) | 139 (138–141) | 0.057 |
| Potassium (mEq/L) | 4.53 ± 0.59 | 4.56 ± 0.61 | 4.5 ± 0.58 | 0.476 |
| In hospital laboratory evaluation: | ||||
| HbA1c (%) | 6 (5.6–6.8) | 6.1 (5.6–6.9) | 5.9 (5.6–6.62) | 0.246 |
| Hb (g/dL) | 11.6 ± 1.8 | 11.3 ± 1.8 | 11.8 ± 1.8 | 0.019 |
| Albumin (g/dL) | 4 (3.8–4.2) | 4 (3.8–4.3) | 4 (3.8–4.2) | 0.949 |
| Total protein (g/dL) | 6.7 (6.4–7.2) | 6.7 (6.3–7.2) | 6.8 (6.4–7.2) | 0.770 |
| TSH (mU/L) | 1.24 (0.72–2.16) | 1.42 (0.72–2.22) | 1.17 (0.72–2.09) | 0.567 |
| Echocardiographic parameters: | ||||
| LVEF (%) | 45 (30–55) | 45 (25–55) | 45 (30–55) | 0.276 |
| ePASP (mmHg) | 40 (35–50) | 40 (35–50) | 40 (35–55) | 0.437 |
| E/E′ | 15 (12–20) | 15 (12–20) | 14.5 (12–20.5) | 0.846 |
| TV S′ (cm/s) | 10.5 (9–12) | 11 (9–12) | 10.5 (9–12) | 0.807 |
| Tricuspid regurgitation: No/mild Moderate Severe | 128 (58.7%) 52 (23.9%) 38 (17.4%) | 69 (61.6%) 27 (24.1%) 16 (14.3%) | 59 (55.7%) 25 (23.6%) 22 (20.8%) | 0.440 |
| Mitral regurgitation: No/mild Moderate Severe | 136 (62.4%) 66 (30.3%) 16 (7.3%) | 70 (62.5%) 36 (32.1%) 6 (5.4%) | 66 (62.3%) 30 (28.3%) 10 (9.4%) | 0.473 |
| Mitral stenosis No/mild Moderate Severe | 211 (96.8%) 4 (1.8%) 3 (1.4%) | 108 (96.4%) 3 (2.7%) 1 (0.9%) | 103 (97.2%) 1 (0.9%) 2 (1.9%) | 0.512 |
| Aortic regurgitation: No/mild Moderate Severe | 195 (89.4%) 21 (9.7%) 2 (0.9%) | 99 (88.4%) 12 (10.7%) 1 (0.9%) | 96 (90.6%) 9 (8.5%) 1 (0.9%) | 0.846 |
| Severe aortic stenosis | 28 (12.8%) | 13 (11.6%) | 15 (14.2%) | 0.575 |
| Outcomes for Survivors at 6 Months | ||||
|---|---|---|---|---|
| Parameter: | Total (n = 174) | ACRS (+) (n = 80) | ACRS (−) (n = 94) | p-Value |
| 6 months | ||||
| Emergency department visit | 97 (55.7%) | 50 (62.5%) | 47 (50%) | 0.098 |
| Readmission (all-cause) | 69 (39.7%) | 41 (51.2%) | 28 (29.8%) | 0.004 |
| Number of readmissions (all-cause) | 0 (0-1) | 0.5 (0–2) | 0 (0–1) | 0.005 |
| Readmission due to AHF | 35 (20.1%) | 17 (21.3%) | 18 (19.1%) | 0.730 |
| CKD stage (KDIGO) 1 2 3A 3B 4 5 | 12 (6.9%) 49 (28.2%) 43 (24.7%) 49 (28.2%) 16 (9.2%) 5 (2.8%) | 3 (3.75%) 13 (16.25%) 15 (18.75%) 30 (37.5%) 14 (17.5%) 5 (6.25%) | 9 (9.6%) 36 (38.3%) 28 (29.8%) 19 (20.2%) 2 (2.1%) 0 (0%) | <0.001 |
| Renal function deterioration (CKD stage) | 57 (32.8%) | 34 (42.5%) | 23 (24.5%) | 0.012 |
| New baseline Cr (mg/dL) | 1.39 ± 0.4 | 1.64 ± 0.77 | 1.19 ± 0.39 | <0.001 |
| New baseline GFR (mL/min/1.73 m2) | 50 (36–66) | 41 (30–56.75) | 59 (45–74) | <0.001 |
| ΔGFR (6M–BASELINE) | −8 ± 11 | −10 ± 13 | −7 ± 9 | 0.03 |
| RRT | 10 (5.7%) | 7 (8.8%) | 3 (3.2%) | 0.116 |
| In-Hospital Renal Function: | ||||
|---|---|---|---|---|
| Parameter: | Total (n = 218) | ACRS (+) (n = 112) | ACRS (−) (n = 106) | p-Value |
| Maximum Cr (mg/dL) | 1.5 (1.15–2) | 1.92 (1.58–2.53) | 1.17 (1–1.4) | <0.001 |
| Minimum eGFR (mL/min/1.73 m2) | 42 (28–57.25) | 29.5 (23–38.75) | 55 (46–65.25) | <0.001 |
| Minimum Cr (mg/dL) | 1.05 (0.87–1.35) | 1.18 (0.93–1.55) | 0.96 (0.8–1.14) | <0.001 |
| Maximum eGFR (mL/min/1.73 m2) | 63.5 ± 22 | 56 ± 21.8 | 71 ± 19.5 | <0.001 |
| ΔCr (mg/dL) | 0.37 (0.21–0.66) | 0.64 (0.44–0.98) | 0.21 (0.14–0.29) | <0.001 |
| ΔGFR (mL/min/1.73 m2) | 17 (10–26) | 22 (13–33) | 12.5 (7–20) | <0.001 |
| ΔCr1 [admission-baseline] (mg/dL) | 0.03 (−0.042–0.18) | 0.09 (−0.02–0.315) | −0.01 (−0.08–0.08) | <0.001 |
| Admission ΔCr [Admission Cr − Min Cr ≥ 0.3 mg/dL] | 37 (17%) | 31 (27.7%) | 6 (5.7%) | <0.001 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Parameter | OR | 95% CI | p | OR | 95% CI | p |
| Age (1 year) | 1.04 | 1.01–1.07 | 0.020 | - | - | - |
| CKD stage (1 stage) | 2.10 | 1.56–2.83 | 0.000 | 2.30 | 1.64–3.23 | 0.000 |
| ΝYHA class (1 class) | 2.47 | 1.52–4.03 | 0.000 | |||
| ΔCr1 [ADMISSION − BASΕLINE] (1 SD) | 2.898 | 1.81–4.63 | 0.000 | 3.53 | 2.02–6.18 | 0.000 |
| ΝΤ-proBNP (pg/mL) (1 SD) | 1.71 | 1.23–2.37 | 0.001 | |||
| Admission Cr (1 mg/dL) | 11.37 | 4.72–23.84 | 0.000 | |||
| Hb (1 g/dL) | 0.84 | 0.72–0.97 | 0.020 | - | - | - |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Parameter | OR | 95% CI | p | OR | 95% CI | p |
| Age (years) | 1.12 | 1.02–1.23 | 0.017 | - | - | - |
| ACRS (yes = 1, no = 0) | 5.10 | 1.09–23.84 | 0.038 | - | - | - |
| ΝYHA (per 1 class) | 3.20 | 1.16–48.87 | 0.025 | - | - | - |
| AKI (per 1 stage) | 3.14 | 1.58–6.26 | 0.001 | - | - | - |
| Inotropes/Vasopressors (yes = 1, no = 0) | 30.52 | 6.36–146.6 | 0.000 | 10.67 | 1.52–74.96 | 0.017 |
| Troponin I (pg/mL) (1 SD) | 2.75 | 1.67–4.52 | 0.000 | 2.452 | 1.17–5.14 | 0.018 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Parameter | OR | 95% CI | p | OR | 95% CI | p |
| Age (1 year) | 1.05 | 1.01–1.10 | 0.015 | - | - | - |
| ACRS (yes = 1, no = 0) | 3.13 | 1.51–6.48 | 0.002 | 2.22 | 1.01–4.89 | 0.0047 |
| CKD stage (KDIGO) (1 stage) | 2.10 | 1.56–2.83 | 0.000 | - | - | |
| AKI stage (1 stage) | 1.90 | 1.21–2.99 | 0.000 | |||
| ΝYHA (1 stage) | 2.53 | 1.40–4.55 | 0.002 | - | - | - |
| NT-proBNP (pg/mL) (1 SD) | 1.75 | 1.30–2.37 | 0.000 | 1.41 | 1.01–1.99 | 0.048 |
| Admission Cr (1 mg/dL) | 11.37 | 4.72–23.84 | 0.000 | |||
| Albumin (1g/dL) | 0.29 | 0.12–0.70 | 0.006 | 0.30 | 0.11–0.79 | 0.015 |
| Maximum Cr (1 mg/dL) | 1.94 | 1.25–3.01 | 0.003 | - | - | - |
| Troponin-I (pg/mL) (1 SD) | 1.77 | 1.15–2.72 | 0.009 | 1.74 | 1.06–2.66 | 0.028 |
| Hb (1 g/dL) | 0.75 | 0.62–0.92 | 0.006 | 0.85 | 0.65–1 | 0.050 |
| Inotropes/Vasopressors (yes = 1, no = 0) | 3.75 | 1.76–7.98 | 0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aletras, G.; Bachlitzanaki, M.; Stratinaki, M.; Lamprogiannakis, E.; Panagoutsos, S.; Kantartzi, K.; Georgopoulou, T.; Petrakis, I.; Foukarakis, E.; Pantazis, Y.; et al. Unraveling Acute Cardiorenal Syndrome: Predictors and Consequences in Acute Heart Failure. J. Clin. Med. 2025, 14, 2270. https://doi.org/10.3390/jcm14072270
Aletras G, Bachlitzanaki M, Stratinaki M, Lamprogiannakis E, Panagoutsos S, Kantartzi K, Georgopoulou T, Petrakis I, Foukarakis E, Pantazis Y, et al. Unraveling Acute Cardiorenal Syndrome: Predictors and Consequences in Acute Heart Failure. Journal of Clinical Medicine. 2025; 14(7):2270. https://doi.org/10.3390/jcm14072270
Chicago/Turabian StyleAletras, Georgios, Maria Bachlitzanaki, Maria Stratinaki, Emmanuel Lamprogiannakis, Stylianos Panagoutsos, Konstantia Kantartzi, Theodora Georgopoulou, Ioannis Petrakis, Emmanuel Foukarakis, Yannis Pantazis, and et al. 2025. "Unraveling Acute Cardiorenal Syndrome: Predictors and Consequences in Acute Heart Failure" Journal of Clinical Medicine 14, no. 7: 2270. https://doi.org/10.3390/jcm14072270
APA StyleAletras, G., Bachlitzanaki, M., Stratinaki, M., Lamprogiannakis, E., Panagoutsos, S., Kantartzi, K., Georgopoulou, T., Petrakis, I., Foukarakis, E., Pantazis, Y., Hamilos, M., & Stylianou, K. (2025). Unraveling Acute Cardiorenal Syndrome: Predictors and Consequences in Acute Heart Failure. Journal of Clinical Medicine, 14(7), 2270. https://doi.org/10.3390/jcm14072270








