Cardiac Contractility Modulation Improves Left Ventricular Function, Including Global Longitudinal Strain, in Patients with Chronic Heart Failure
Abstract
1. Background
2. Methods
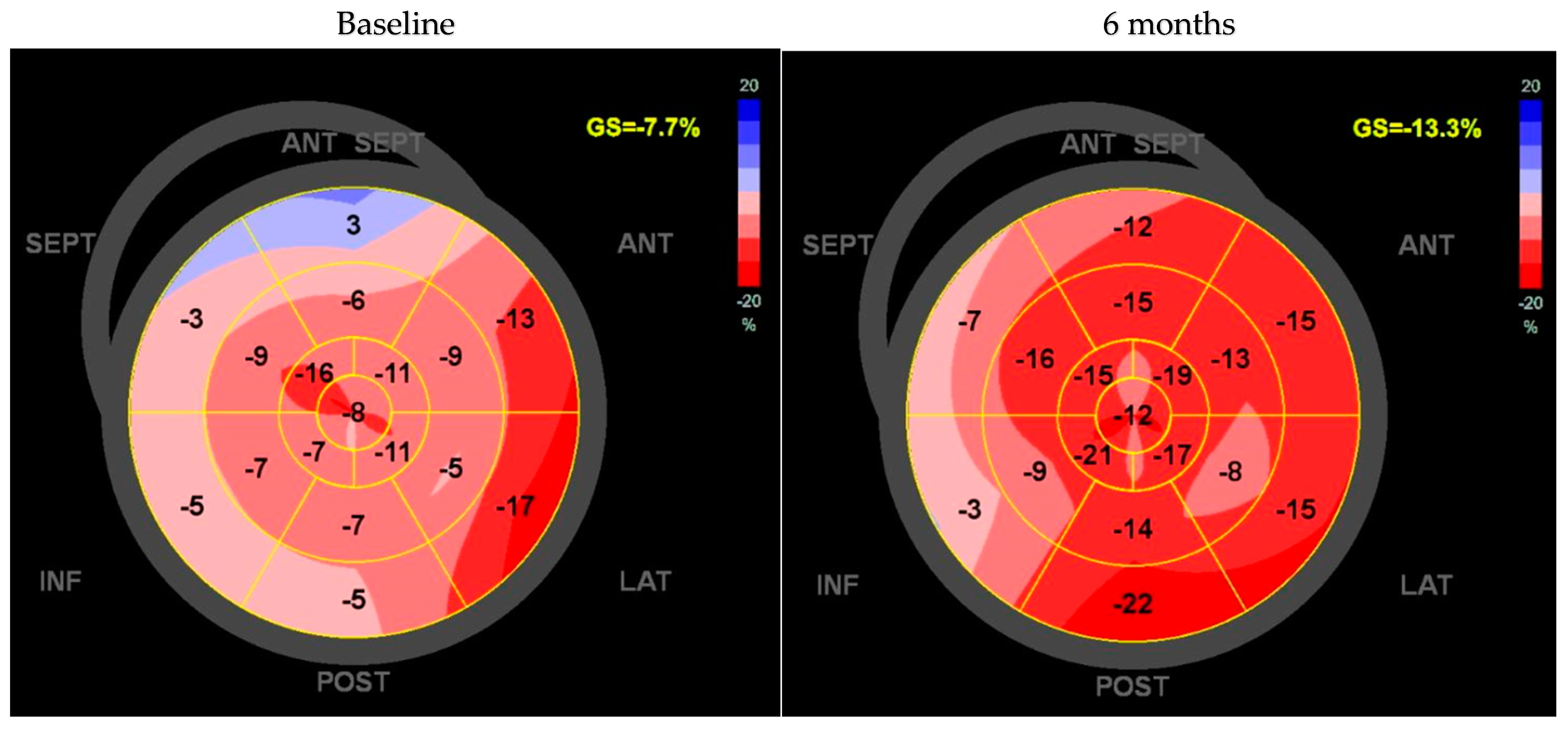
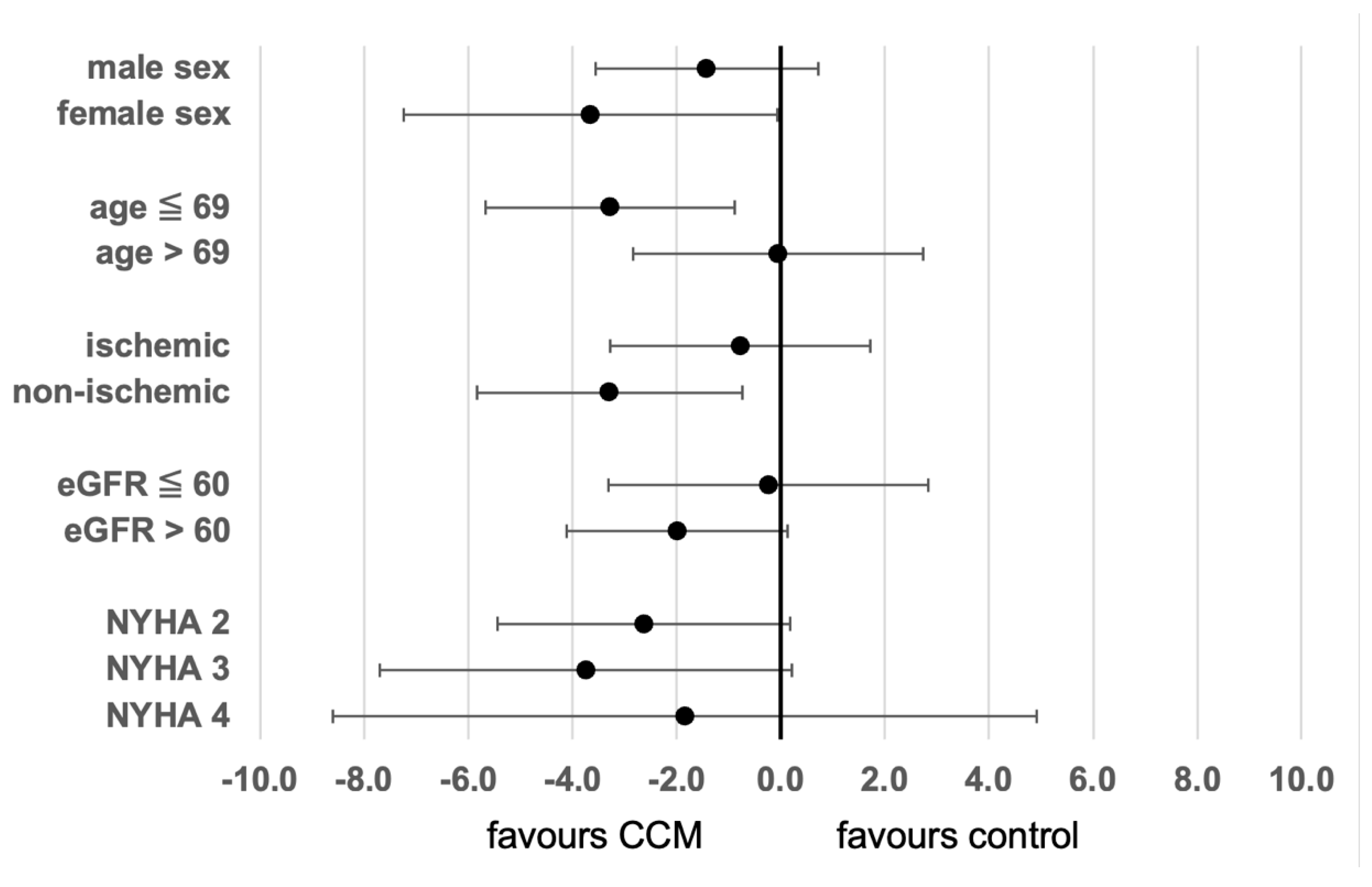
3. Results
Safety Evaluation
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.; Shiino, K.; Obonyo, N.G.; Hanna, J.; Chamberlain, R.; Small, A.; Scalia, I.G.; Scalia, W.; Yamada, A.; Hamilton-Craig, C.R.; et al. Left Ventricular Global Strain Analysis by Two-Dimensional Speckle-Tracking Echocardiography: The Learning Curve. J. Am. Soc. Echocardiogr. 2017, 30, 1081–1090. [Google Scholar] [PubMed]
- Sitia, S.; Tomasoni, L.; Turiel, M. Speckle tracking echocardiography: A new approach to myocardial function. World J. Cardiol. 2010, 2, 1–5. [Google Scholar] [PubMed]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11 Pt 1, 260–274. [Google Scholar]
- Hackstein, J.; Pichler, O.; Teuner, A. Computer-assisted analysis of ultrasound sequences of the left ventricle for determining ejection fraction. Biomed. Tech. 1996, 41, 118–122. [Google Scholar]
- Otterstad, J.E.; Froeland, G.; Sutton, M.S.J.; Holme, I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur. Heart J. 1997, 18, 507–513. [Google Scholar] [PubMed]
- Marwick, T.H. Ejection Fraction Pros and Cons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2360–2379. [Google Scholar]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Bonanomi, A.; Rigamonti, E.; Lombardo, M. Influence of chest wall conformation on reproducibility of main echocardiographic indices of left ventricular systolic function. Minerva Cardiol. Angiol. 2024, 72, 111–124. [Google Scholar]
- Park, J.J.; Park, J.-B.; Park, J.-H.; Cho, G.-Y. Global Longitudinal Strain to Predict Mortality in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1947–1957. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kuschyk, J.; Falk, P.; Demming, T.; Marx, O.; Morley, D.; Rao, I.; Burkhoff, D. Long-term clinical experience with cardiac contractility modulation therapy delivered by the Optimizer Smart system. Eur. J. Heart Fail. 2021, 23, 1160–1169. [Google Scholar]
- Campbell, C.M.; Kahwash, R.; Abraham, W.T. Optimizer Smart in the treatment of moderate-to-severe chronic heart failure. Future Cardiol. 2020, 16, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Giallauria, F.; Vigorito, C.; Piepoli, M.F.; Coats, A.J.S. Effects of cardiac contractility modulation by non-excitatory electrical stimulation on exercise capacity and quality of life: An individual patient s data meta-analysis of randomized controlled trials. Int. J. Cardiol. 2014, 175, 352–357. [Google Scholar] [CrossRef]
- Borggrefe, M.; Burkhoff, D. Clinical effects of cardiac contractility modulation (CCM) as a treatment for chronic heart failure. Eur. J. Heart Fail. 2012, 14, 703–712. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mishra, S.; Rastogi, S.; Wang, M.; Rousso, B.; Mika, Y.; Remppis, A.; Sabbah, H.N. Ca2+-binding proteins in dogs with heart failure: Effects of cardiac contractility modulation electrical signals. Clin. Transl. Sci. 2009, 2, 211–215. [Google Scholar] [CrossRef]
- Stix, G.; Borggrefe, M.; Wolpert, C.; Hindricks, G.; Kottkamp, H.; Böcker, D.; Wichter, T.; Mika, Y.; Ben-Haim, S.; Burkhoff, D.; et al. Chronic electrical stimulation during the absolute refractory period of the myocardium improves severe heart failure. Eur. Heart J. 2004, 25, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Kuck, K.-H.; Goldsmith, R.L.; Lindenfeld, J.; Reddy, V.Y.; Carson, P.E.; Mann, D.L.; Saville, B.; Parise, H.; Chan, R.; et al. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart Fail. 2018, 6, 874–883. [Google Scholar] [CrossRef]
- Abi-Samra, F.; Gutterman, D. Cardiac contractility modulation: A novel approach for the treatment of heart failure. Heart Fail. Rev. 2016, 21, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Nademanee, K.; Volosin, K.; Krueger, S.; Neelagaru, S.; Raval, N.; Obel, O.; Weiner, S.; Wish, M.; Carson, P.; et al. Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J. Card Fail. 2011, 17, 710–717. [Google Scholar] [CrossRef]
- Kloppe, A.; Mijic, D.; Schiedat, F.; Bogossian, H.; Mügge, A.; Rousso, B.; Lemke, B. A randomized comparison of 5 versus 12 hours per day of cardiac contractility modulation treatment for heart failure patients: A preliminary report. Cardiol. J. 2016, 23, 114–119. [Google Scholar] [CrossRef]
- Kadish, A.; Nademanee, K.; Volosin, K.; Krueger, S.; Neelagaru, S.; Raval, N.; Obel, O.; Weiner, S.; Wish, M.; Carson, P.; et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am. Heart. J. 2011, 161, 329–337.e1–2. [Google Scholar]
- Masarone, D.; Kittleson, M.M.; De Vivo, S.; D’Onofrio, A.; Ammendola, E.; Nigro, G.; Contaldi, C.; Martucci, M.L.; Errigo, V.; Pacileo, G.; et al. The Effects of Device-Based Cardiac Contractility Modulation Therapy on Left Ventricle Global Longitudinal Strain and Myocardial Mechano-Energetic Efficiency in Patients with Heart Failure with Reduced Ejection Fraction. J. Clin. Med. 2022, 11, 5866. [Google Scholar] [CrossRef]
- Pierucci, N.; La Fazia, V.M.; Gianni, C.; Mohanty, S.; Lavalle, C.; Cishek, M.B.; Canby, R.C.; Natale, A. Cardiac contractility modulation in a patient with refractory systolic heart failure following orthotopic heart transplant. Hear. Case Rep. 2024, 10, 33–37. [Google Scholar]
- Pouleur, A.C.; Knappe, D.; Shah, A.M.; Uno, H.; Bourgoun, M.; Foster, E.; McNitt, S.; Hall, W.J.; Zareba, W.; Goldenberg, I.; et al. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: The MADIT-CRT trial. Eur. Heart J. 2011, 32, 1720–1729. [Google Scholar] [PubMed]
- Delgado, V.; Ypenburg, C.; Zhang, Q.; Mollema, S.A.; Fung, J.W.-H.; Schalij, M.J.; Yu, C.-M.; Bax, J.J. Changes in global left ventricular function by multidirectional strain assessment in heart failure patients undergoing cardiac resynchronization therapy. J. Am. Soc. Echocardiogr. 2009, 22, 688–694. [Google Scholar] [PubMed]
- Sidiropoulos, G.; Karakasis, P.; Antoniadis, A.; Saplaouras, A.; Karamitsos, T.; Fragakis, N. The Effect of Cardiac Resynchronization Therapy on Right Ventricular Function: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4173. [Google Scholar] [CrossRef] [PubMed]
- Borggrefe, M.M.; Lawo, T.; Butter, C.; Schmidinger, H.; Lunati, M.; Pieske, B.; Misier, A.R.; Curnis, A.; Böcker, D.; Remppis, A.; et al. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur. Heart J. 2008, 29, 1019–1028. [Google Scholar]
- Linde, C.; Grabowski, M.; Ponikowski, P.; Rao, I.; Stagg, A.; Tschöpe, C. Cardiac contractility modulation therapy improves health status in patients with heart failure with preserved ejection fraction: A pilot study (CCM-HFpEF). Eur. J. Heart Fail. 2022, 24, 2275–2284. [Google Scholar]
- Greene, S.J.; Butler, J.; Spertus, J.A.; Hellkamp, A.S.; Vaduganathan, M.; DeVore, A.D.; Albert, N.M.; Duffy, C.I.; Patterson, J.H.; Thomas, L.; et al. Comparison of New York Heart Association Class and Patient-Reported Outcomes for Heart Failure with Reduced Ejection Fraction. JAMA Cardiol. 2021, 6, 522–531. [Google Scholar]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age < 18 years | Planned interventional or surgical procedures other than CCM implantation within the next 3 months |
| Chronic heart failure NYHA II, III, or IV | Inability to assess heart function via echocardiography |
| LVEF 25–45% | Lack of consent to participate in the study |
| Guideline-based pharmacological treatment | Inability to attend follow-up visits |
| Clinical indication for CCM therapy | |
| QRS complex < 130 ms | |
| Written informed consent |
| Parameter | Mean (All Patients) [N = 22] |
|---|---|
| Male sex | 16 (73%) |
| Age [years] | 70 ± 6 |
| Height [cm] | 170 ± 10 |
| Weight [kg] | 96 ± 22 |
| GFR [mL/min∗1.73 m2] | 62 ± 17 |
| NT-pro BP [pg/mL] | 2669 ± 3716 |
| Etiology of heart failure | |
| Ischemic cardiomyopathy | 11 (50%) |
| Non-ischemic cardiomyopathy | 11 (50%) |
| NYHA stage | |
| NYHA I | 0 (0%) |
| NYHA II | 4 (18%) |
| NYHA III | 12 (55%) |
| NYHA IV | 6 (27%) |
| Hypertension | 18 (82%) |
| Diabetes mellitus | 14 (64%) |
| Coronary heart disease | 11 (50%) |
| History of PCI | 11 (50%) |
| History of CABG | 1 (5%) |
| History of heart value surgery | 6 (27%) |
| Antiplatelet therapy (APT) | |
| Single APT | 9 (41%) |
| Dual APT | 2 (9%) |
| None | 11 (50%) |
| (D)OAC | 11 (50%) |
| ß-blockers | 19 (86%) |
| ACE inhibitors | 3 (14%) |
| ARB | 1 (5%) |
| Sacubitril/Valsartan | 18 (82%) |
| MRA | 15 (68%) |
| SGLT2 inhibitors | 14 (64%) |
| Diuretic LVDd [mm] LVDs [mm] LVVd [mL] LVVs [mL] LV-EF [%] Global longitudinal strain [%] KCCQ [points] | 19 (82%) 58.3 ± 8.6 46.6 ± 9.8 167.7 ± 54.8 107.1 ± 48.2 34.4 ± 8.1 −9.2 ± 3.2 31 ± 16.8 |
| Parameter | Active CCM (N = 22) | No Active CCM (N = 39) | p-Value |
|---|---|---|---|
| LVDd [mm] | 56.1 ± 11.0 | 60.4 ± 11.0 | 0.193 |
| LVDs [mm] | 45.9 ± 13.6 | 49.3 ± 11.3 | 0.592 |
| LVVd [mL] | 172.4 ± 69.2 | 174.7 ± 61.6 | 0.859 |
| LVVs [mL] | 99.2 ± 59.4 | 111.7 ± 46.9 | 0.377 |
| LV-EF [%] | 41.7 ± 10.1 | 35.5 ± 7.6 | <0.05 |
| GLS [%] | −10.3 ± 3.7 | −8.3 ± 3.2 | <0.05 |
| KCCQ [points] | 62.0 ± 21.3 | 35.1 ± 20.1 | <0.05 |
| Parameter | Value (N = 22) |
|---|---|
| ACS | 0 (0%) |
| Stroke | 0 (0%) |
| Hospitalization for heart failure | 6 (27%) |
| Cardiac arrest | 1 (4%) |
| Hospitalization for other causes | 8 (36%) |
| Cardiovascular procedures | 5 (23%) |
| Death | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raab, C.; Roehl, P.; Wiora, M.; Ebelt, H. Cardiac Contractility Modulation Improves Left Ventricular Function, Including Global Longitudinal Strain, in Patients with Chronic Heart Failure. J. Clin. Med. 2025, 14, 2251. https://doi.org/10.3390/jcm14072251
Raab C, Roehl P, Wiora M, Ebelt H. Cardiac Contractility Modulation Improves Left Ventricular Function, Including Global Longitudinal Strain, in Patients with Chronic Heart Failure. Journal of Clinical Medicine. 2025; 14(7):2251. https://doi.org/10.3390/jcm14072251
Chicago/Turabian StyleRaab, Cornelia, Peter Roehl, Matthias Wiora, and Henning Ebelt. 2025. "Cardiac Contractility Modulation Improves Left Ventricular Function, Including Global Longitudinal Strain, in Patients with Chronic Heart Failure" Journal of Clinical Medicine 14, no. 7: 2251. https://doi.org/10.3390/jcm14072251
APA StyleRaab, C., Roehl, P., Wiora, M., & Ebelt, H. (2025). Cardiac Contractility Modulation Improves Left Ventricular Function, Including Global Longitudinal Strain, in Patients with Chronic Heart Failure. Journal of Clinical Medicine, 14(7), 2251. https://doi.org/10.3390/jcm14072251







