A New Approach for Reconstruction of Severe Horizontal Atrophy of the Posterior Mandible Using “The Honeycomb Technique”: A 10–14 Year Follow-Up Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgical Protocol
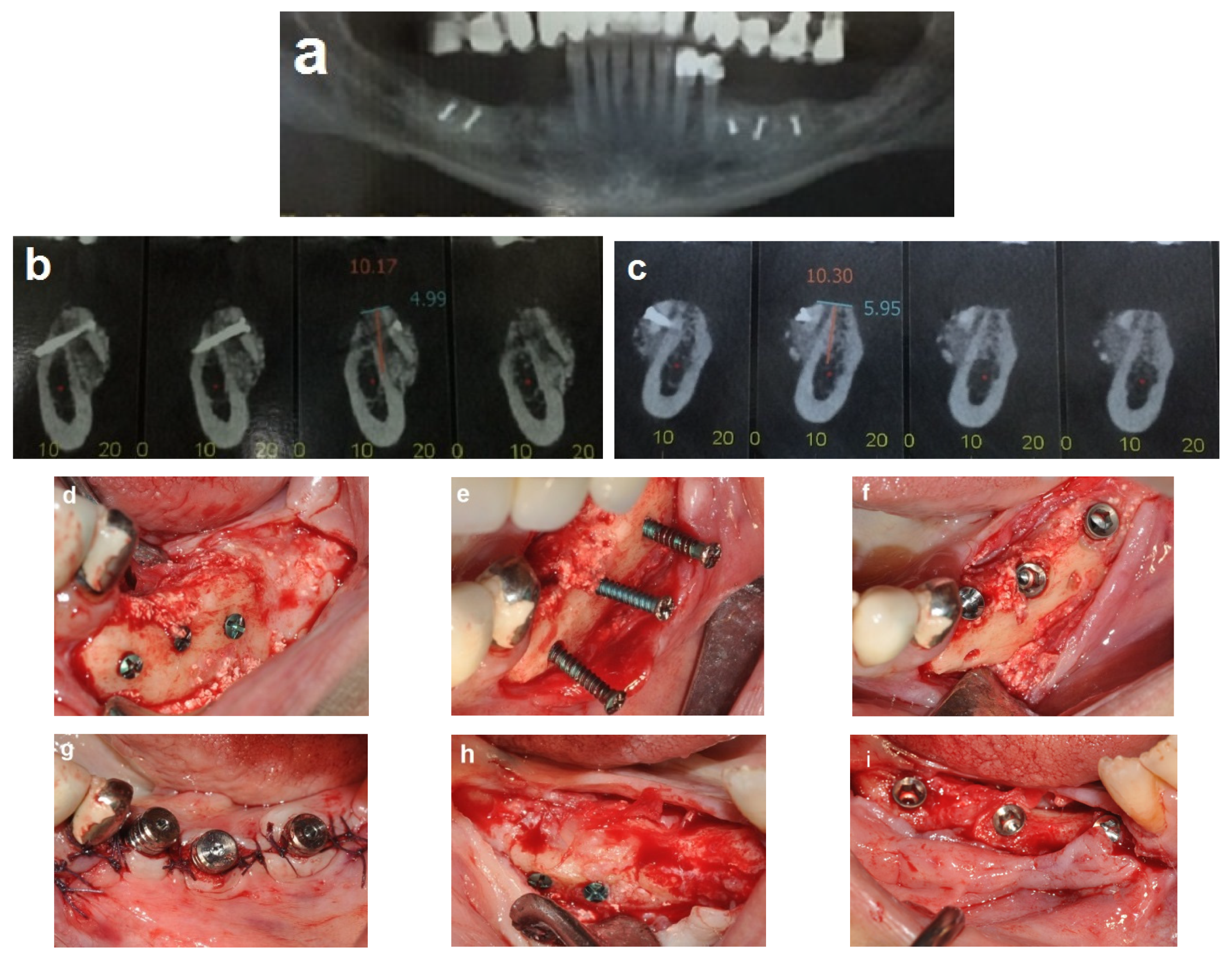
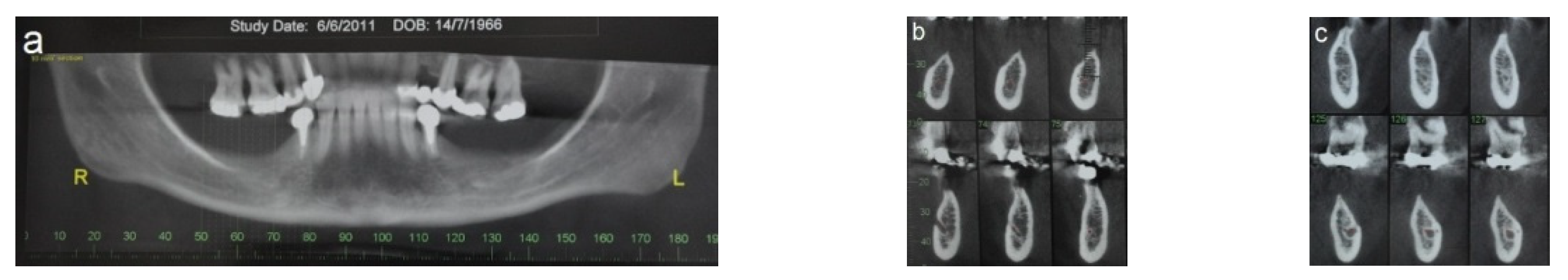
2.3. Illustration Case (Patient No. 21)
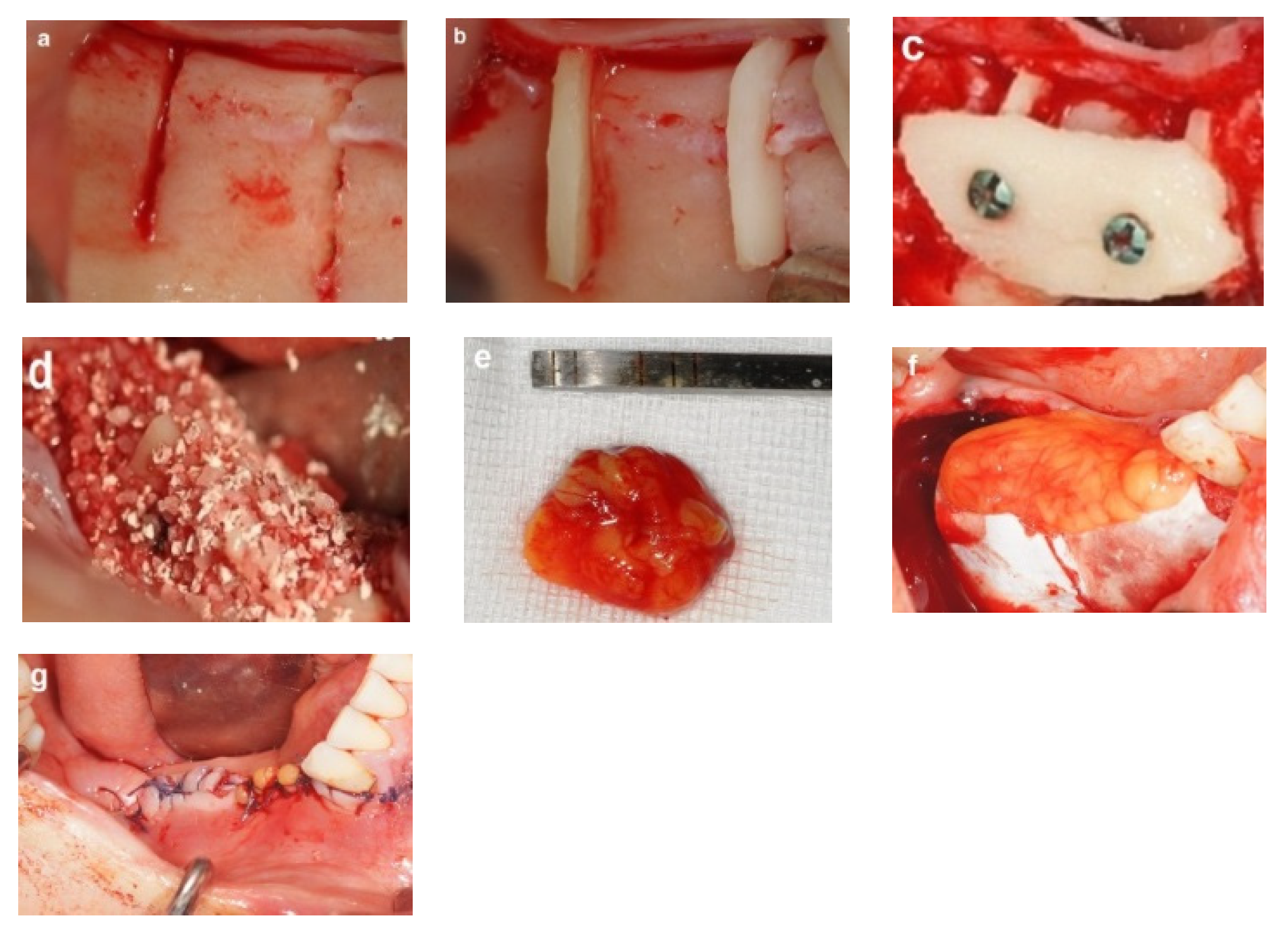
2.4. Honeycomb Technique Procedure
2.4.1. Donor Site Preparation
2.4.2. Bone Block Harvest
2.4.3. Recipient Site Preparation
2.4.4. Augmentation Procedure
- Bone wedges were positioned into the prepared grooves at the recipient site, ensuring that each wedge fitted securely within its respective groove. Once properly adapted, each wedge was gently tapped into place using a flat-edged cylindrical instrument and a hammer. Stability was verified by attempting to dislodge each wedge, and any unstable wedge should be replaced (Figure 3b).
- A thin cortical bone plate was secured laterally over the bone wedges using screws, creating multiple bone compartments resembling a honeycomb structure (Figure 3c). Sharp edges were trimmed to avoid soft tissue trauma. The horizontal dimensions of the augmented site are determined by the combined dimensions of the bone wedges and the laterally fixed cortical bone block.
- The compartments were filled with allograft particulate bone (Figure 3d), covered with a collagen membrane (Geistlich Bio-Gide, Geistlich Pharma, Switzerland), and covered with a free buccal fat pad graft that was secured with sutures (Figure 3e,f). The free buccal fat pad graft enhances the flap closure and provides soft tissue augmentation.
- The flap was closed using tension-free sutures (Figure 3g).
2.4.5. Postoperative Management
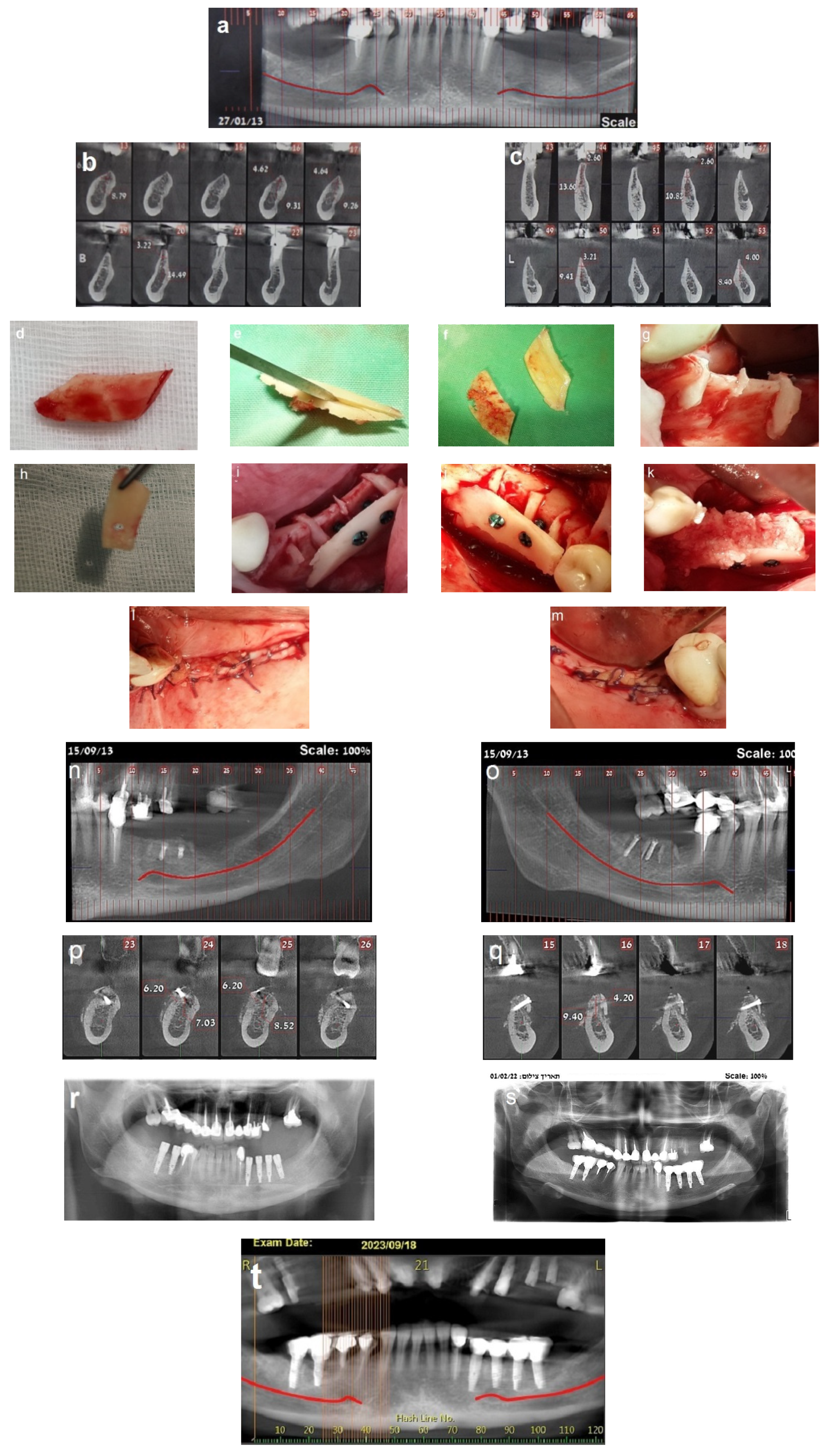
2.5. Case Presentation
2.5.1. Case 1 (Patient No. 20)
2.5.2. Case 2 (Patient No. 12)
3. Results
3.1. The Healing Process
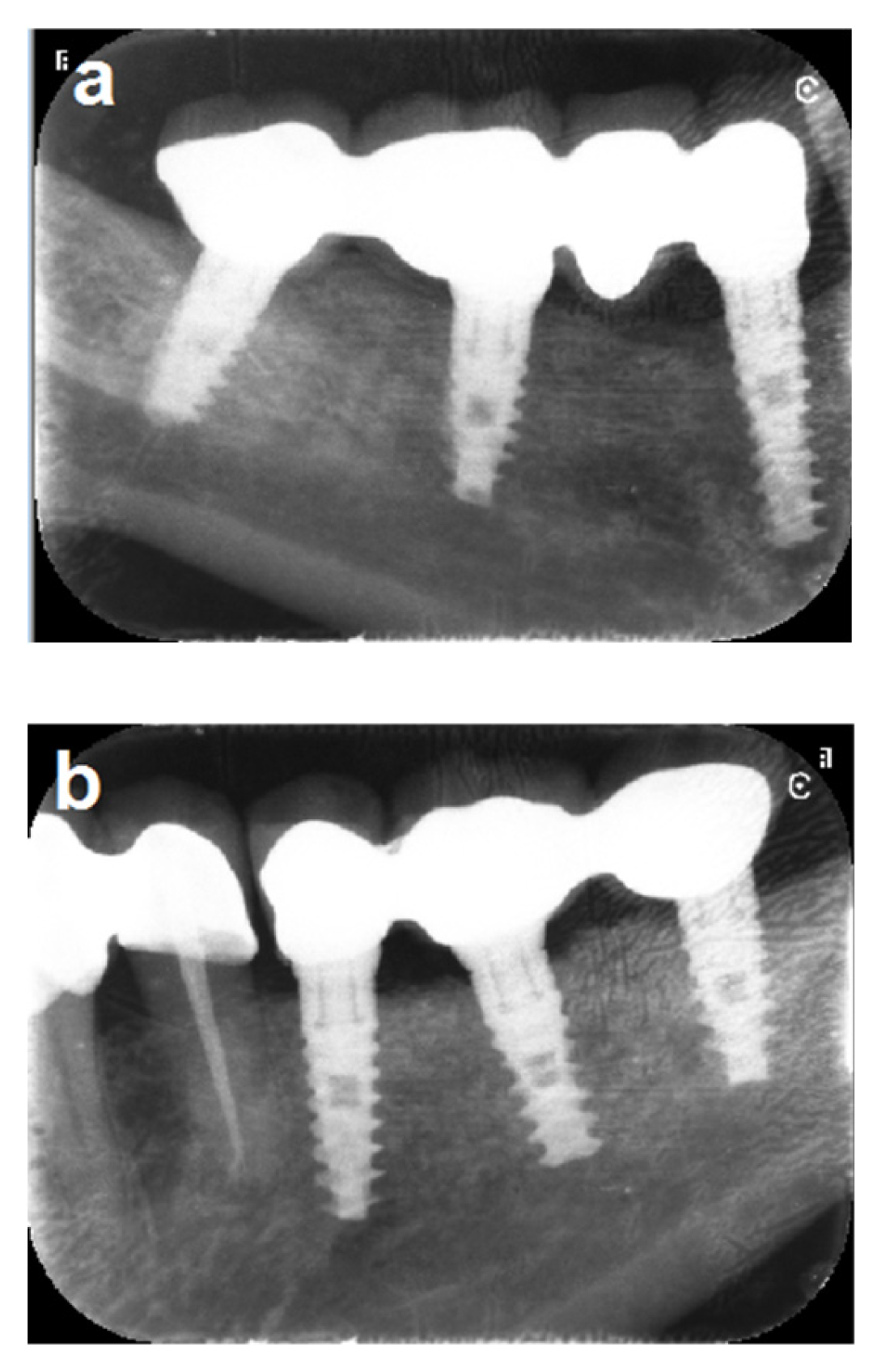
3.2. Bone Gain and Implant Placement
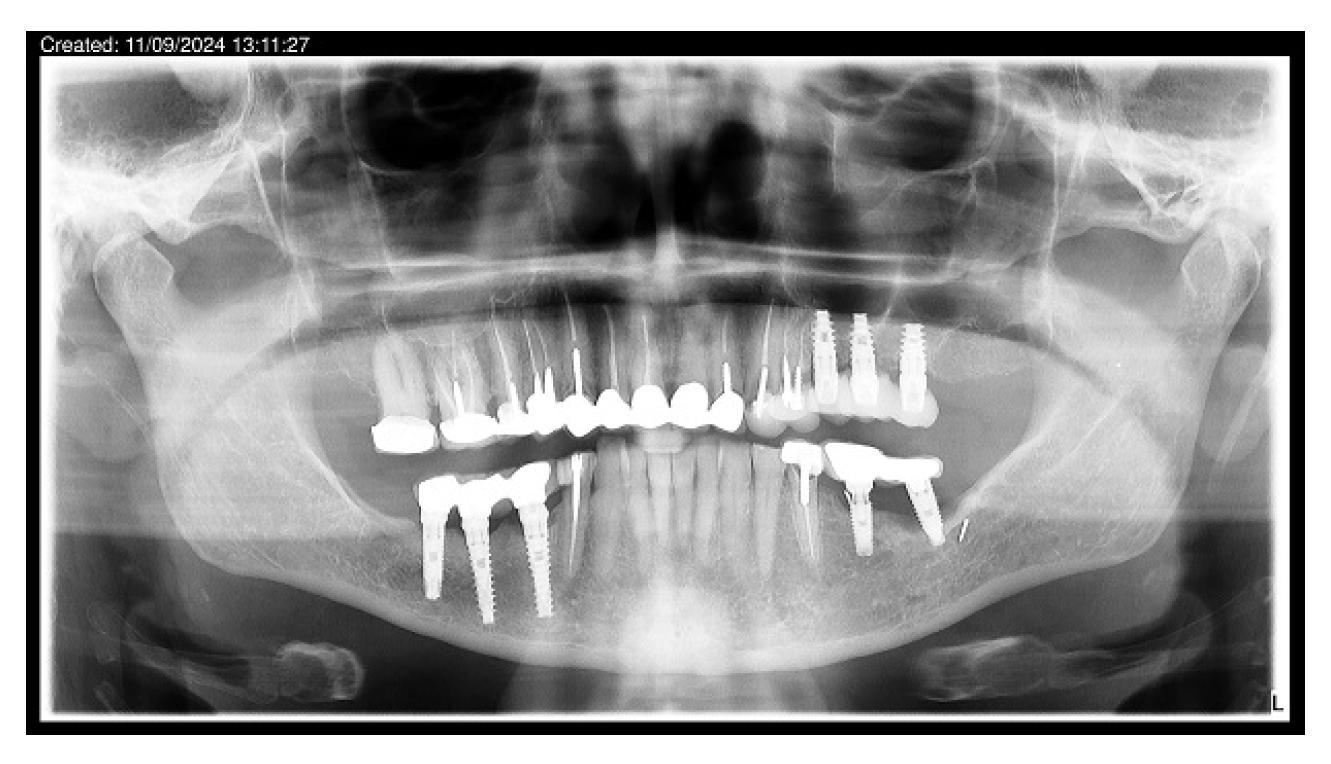
3.3. Implant Placement and Follow-Up
3.4. Implant Success Rate
3.5. Implant Survival Rate
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Baumer, D.; Ozga, A.N.; Korner, G.; Baumer, A. Patient satisfaction and oral health-related quality of life 10 years after implant placement. BMC Oral Health 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babu, R.S.A.; Ogle, O. Tissue response: Biomaterials, dental implants, and compromised osseous tissue. Dent. Clin. N. Am. 2015, 59, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extraction alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Zucchelli, G.; Cannizzaro, G.; Barausse, C.; Diazzi, M.; Trullenque-Eriksson, A.; Esposito, M. Immediate, immediate-delayed (6 weeks) and delayed (4 months) post-extractive single implants: 4-month post-loading data from a randomized controlled trial. Eur. J. Oral Implantol. 2016, 9, 233–247. [Google Scholar] [PubMed]
- Alenazi, A.; Alotaibi, A.A.; Aljaeide, Y.M.; Alqhtani, N.R. The need for socket preservation: A systematic review. J. Med. Life 2022, 15, 309–312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasi, A.S.; Kalsi, J.S.; Bassi, S. Alveolar ridge preservation: Why, when and how. Br. Dent. J. 2019, 227, 264–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bahat, O. Treatment planning and placement of implants in the posterior maxillae: Report 0f 732 consecutive Nobelpharma implants. Int. J. Oral Maxillofac. Implant. 1993, 8, 151–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanz-Sanchez, I.; Ortiz-Vigon, A.; Sanz-Martin, I.; Figuero, E.; Sanz, M. Effectiveness of lateral bone augmentation on the alveolar crest dimension: A systematic review a meta-analysis. J. Dent. Res. 2015, 94 (Suppl. S9), 128S–142S. [Google Scholar] [CrossRef] [PubMed]
- Grandi, T.; Svezia, L.; Grandi, G. Narrow implants (2.75–3.25 mm diameter) supporting a fixed splinted prosthesis in posterior regions of mandible: One-year results from a prospective cohort study. Int. J. Implant Dent. 2017, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Barausse, C.; Pistilli, R.; Bellini, P.; Buti, J.; Felice, P. Immediate loading of 3 mm-diameter implants as an alternative to horizontal bone augmentation for placing 4 mm-diameter implants: One-year post-loading results from multicenter randomized controlled trail. Clin. Trials Dent. 2020, 2, 61–76. [Google Scholar] [CrossRef]
- Agabiti, I.; Botticelli, D. Two-Stage Ridge Split at Narrow Alveolar Mandibular Bone Ridges. J. Oral Maxillofac. Surg. 2017, 75, 2115.e1–2115.e12. [Google Scholar] [CrossRef] [PubMed]
- Holtzclaw, D.J.; Toscano, N.J.; Rosen, P.S. Reconstruction of posterior mandibular alveolar ridge deficiencies with the piezoelectric hinge-assisted ridge split technique: A retrospective observational report. J. Periodontol. 2010, 81, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Hanser, T. Mandibular bone block harvesting from the retromolar region: A 10-year prospective clinical study. Int. J. Oral Maxillofac. Implant. 2015, 30, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Matthies, L.; Windisch, P.; Gosau, M.; Jung, R.; Brodala, N.; Stefanini, M.; Kleinheinz, J.; Payer, M.; Henningsen, A.; et al. Horizontal augmentation techniques in the mandible: A systematic review. Int. J. Implant Dent. 2022, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Ersanli, S.; Arısan, V.; Bedeloğlu, E. Evaluation of the autogenous bone block transfer for dental implant placement: Symphysal or ramus harvesting? BMC Oral Health 2016, 16, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Misch, C.M. Ridge augmentation using mandibular ramus bone grafts for the placement of dental implants: Presentation of a technique. Pract. Periodontics Aesthet. Dent. 1996, 8, 127–135. [Google Scholar] [PubMed]
- Mish, C.M. Comparison of intraoral donor site for inlay grafting prior to implant placement. Int. J. Oral Maxillofac. Implant. 1997, 12, 767–776. [Google Scholar] [PubMed]
- Daoud, S.; Zoabi, A.; Kasem, A.; Totry, A.; Oren, D.; Redenski, I.; Srouji, S.; Kablan, F. Computer-Assisted Evaluation Confirms Spontaneous Healing of Donor Site One Year following Bone Block Harvesting from Mandibular Retromolar Region—A Cohort Study. Diagnostics 2024, 14, 504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009, 24, 237–259. [Google Scholar] [PubMed]
- Rabelo, G.D.; de Paula, P.M.; Rocha, F.S.; Jorao Silva, C.; Zanetta-Barbosa, D. Retrospective study of bone grafting procedures before implants placement. Implant Dent. 2010, 19, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Hashemipoor, M.; Asghari, N.; Mohammadi, M.; Kalantari, M.; Arabsolghar, M.; Ranjbar, H. Radiological and histological evaluation of horizontal ridge augmentation using corticocancellous freeze-dried bone allograft with and without autogenous bone: A randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2020, 22, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Azpur, G.; de la Fuente, A.; Chavez, E.; Valdivia, E.; Khouly, I. Horizontal ridge augmentation with guided bone regeneration using particulate xenogenic bone substitutes with or without autogenous block grafts: A randomized controlled trial. Clin. Implant Dent. Relat. Res. 2019, 21, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Behnia, H.; Shayesteh, Y.S.; Morad, G.; Alikhasi, M. Localized bone augmentation with cortical bone blocks tended over different particulate bone substitutes: A retrospective study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1481–1493. [Google Scholar] [PubMed]
- Khairnar, M.S.; Khairnar, D.; Bakshi, K. Modified ridge splitting and bone expansion osteotomy for placement of dental implant in esthetic zone. Contemp. Clin. Dent. 2014, 5, 110–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implantol. 2014, 7 (Suppl. S2), S203–S217. [Google Scholar] [PubMed]
- Pikos, M.A. Mandibular block autografts for alveolar ridge augmentation. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2005, 13, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Antoun, A.; Missika, P. Bone Augmentation in Oral Implantology; Quintessenz: Berlin, Germany; London, UK, 2007. [Google Scholar]
- Khoury, F.; Hanser, T. 3D vertical alveolar crest augmentation in the posterior mandible using the tunnel technique: A 10-year clinical study. Int. J. Oral Implantol. 2022, 15, 111–126. [Google Scholar] [PubMed]
- Kablan, F. Wedge Technique. In Academy of Osseointegration; Clinical Innovation Committee: Orlando, FL, USA, 2010; 25 Annual Meeting. [Google Scholar]
- Kablan, F. The Use of Cortical Bone Wedges from the Mandibular Ramus “Wedge Technique” for 3-Dimensional Bone Augmentation of the Atrophic Ridges. In Current Concepts in Dental Implantology. From Science to Clinical Research; IntechOpen: London, UK, 2022; Chapter 10; pp. 177–195. [Google Scholar] [CrossRef]
- Acocella, A.; Bertolai, R.; Colafranceschi, M.; Sacco, R. Clinical, histological and histomorphometric evaluation of the healing of mandibular ramus bone block grafts for alveolar ridge augmentation before implant placement. J. Craniomaxillofac. Surg. 2010, 38, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. Periodontal regeneration of human infrabony defects. II. Re-entry procedures and bone measures. J. Periodont. 1993, 64, 261–268. [Google Scholar] [PubMed]
- Maiorana, C.; Beretta, M.; Salina, S.; Santoro, F. Reduction of autogenous bone graft resorption by means of bio-oss coverage: A prospective study. Int. J. Periodontics Restor. Dent. 2005, 25, 19–25. [Google Scholar] [PubMed]
- Mertens, C.; Braun, S.; Krisam, J.; Hoffmann, J. The influence of wound closure on graft stability: An in vitro comparison of different bone grafting techniques for the treatment of one-wall horizontal bone defects. Clin. Implant Dent. Relat. Res. 2019, 21, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Wui, H.; Jung, R.E.; Hammerle, C.H.; Benic, G.I. Influence of blinded wound closure on the volume stability of different GBR materials: An in vitro cone-beam computed tomographic examination. Clin. Oral Implant. Res. 2016, 27, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kablan, F. The Use of Free Tissue Fat Transfer to Improve Soft Tissue-Thickness at Recipient Sites of Bone Augmentations; Academy of Osseointegration: Washington, DC, USA, 2011; Clinical Innovation Committee 26 Annual Meeting. [Google Scholar]
- Kablan, F.; Laster, Z. The use of free fat tissue transfer from the buccal fat pad to obtain and maintain primary closure and to improve soft tissue thickness at bone-augmented sites: Technique presentation and report of case series. Int. J. Oral Maxillofac. Implant. 2014, 29, e220–e231. [Google Scholar] [CrossRef] [PubMed]
- Kablan, F. The use of Buccal fat pad free graft in regenerative treatment of peri-implantitis: A new and predictable technique. Ann. Maxillofac. Surg. 2015, 5, 179–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Castro, C.H.; de Souza, L.N.; Fernandes Santos Melo, M. Use of the buccal fat pad as free graft for closure of oronasal fistula in a cleft palate patient. J. Craniofac. Surg. 2015, 26, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Kablan, F. The use of buccal fat pad free graft in closure of soft-tissue defects and dehiscence in the hard palate. Ann. Maxillofac. Surg. 2016, 6, 241–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.T.; Sasidaran, R. Buccal Fat Pad: An Effective Option for Facial Reconstruction and Aesthetic Augmentation. Aesthet. Plast. Surg. 2017, 6, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Kablan, F.; Subeh, A.A.; Moses, O. The use of a buccal free fat tissue graft to enhance primary soft tissue closure during socket preservation and improve ridge contour at the extraction site: Presentation of the Technique, and Report of Case Series. Dent. Res. Oral Health 2023, 6, 40–51. [Google Scholar] [CrossRef]
- Cheng, J.W.; Hsiao, K.Y.; Chen, M.Y.; Huang, T.T.; Chen, K.C. Free buccal fat pad graft for the bone defect filling of medication-related osteonecrosis of the jaws: A novel surgical approach. J. Dent. Sci. 2024, 19, 1846–1849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbu, H.M.; Andreescu, C.F.; Lorean, A.; Kolerman, R.; Moraru, L.; Mortellaro, C.; Mijiritsky, E. Comparison of Two Techniques for Lateral Ridge Augmentation in Mandible with Ramus Block Graft. J. Craniofac. Surg. 2016, 27, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Soehardi, A.; Meijer, G.J.; Strooband, V.F.; de Koning, M.; Stoelinga, P.J. The potential of the horizontal ramus of the mandible as a donor site for block and particular grafts in pre-implant surgery. Int. J. Oral Maxillofac. Surg. 2009, 38, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Kaus, T. Ridge augmentation using mandibular block bone grafts: Preliminary results of an ongoing prospective study. Int. J. Oral Maxillofac. Implant. 2001, 16, 378–388. [Google Scholar] [PubMed]
- Clementini, M.; Morlupi, A.; Agrestini, C.; Ottria, L. Success rate of dental implants inserted in autologous bone graft regenerated areas: A systematic review. Oral Implantol. 2012, 4, 3–10. [Google Scholar] [PubMed] [PubMed Central]









| Patient No | Site No | Age | Gender | Augmentation Site | Available Bone (mm) | Donor Site | Follow-Up in Years Since Implant Loading |
|---|---|---|---|---|---|---|---|
| 1 | 1 2 | 53 | F | 35–37 45–47 | 1.5.0–2.0 2.5–3.5 | RT | 14 |
| 2 | 3 4 | 57 | M | 34–37 43–47 | 2.0–3.5 1.5–2.0 | RT & LT | 14 |
| 3 | 5 6 | 52 | F | 36–37 46–47 | 2.0–3.0 2.0–3.5 | RT | 14 |
| 4 | 7 | 46 | F | 44–47 | 1.5–3.0 | RT | 14 |
| 5 | 8 9 | 38 | F | 32–37 45–46 | 2.5–3.0 2.0–4.0 | RT & LT | 14 |
| 6 | 10 | 46 | F | 36–37 | 1.5–3.0 | LT | 13.5 |
| 7 | 11 12 | 44 | F | 34–37 44–47 | 1.0–3.0 1.0–3.0 | RT & LT * | 13 |
| 8 | 13 | 43 | F | 45–47 | 2.0–3.5 | RT | 13 |
| 9 | 14 15 | 53 | M | 35–37 45–47 | 1.5–3.0 1.5–3.0 | RT | 13 |
| 10 | 16 17 | 61 | M | 34–37 45–47 | 1.5–3.5 1.5–3.0 | RT | 13 |
| 11 | 18 | 28 | F | 36–37 | 2.0–3.0 | LT | 13 |
| 12 | 19 20 | 47 | F | 36–37 46–47 | 2.0–3.0 1.5–3.0 | RT | 13 |
| 13 | 21 | 62 | F | 35–37 | 1.5–3.5 | LT | 13 |
| 14 | 22 23 | 53 | F | 35–37 46–47 | 1.5–3.0 1.5–3.0 | LT | - |
| 15 | 24 25 | 56 | F | 36–37 46–47 | 2.0–2.5 1.5–2.5 | LT | 12.5 |
| 16 | 26 27 | 62 | F | 34–37 46–47 | 2.0–4.0 2.5–4.0 | RT | 12.4 |
| 17 | 28 29 | 56 | M | 34–37 44–47 | 1.5–2.0 1.5–3.0 | RT | 12 |
| 18 | 30 31 | 47 | M | 35–37 45–47 | 1.5–3.0 1.5–2.5 | RT | 11.8 |
| 19 | 32 33 | 37 | F | 35–37 45–47 | 2.0–3.0 2.0–3.0 | RT | 11 |
| 20 | 34 35 | 36 | F | 34–37 46–47 | 2.0–3.0 2.0–3.0 | RT | 11 |
| 21 | 36 37 | 42 | F | 36–37 44–47 | 1.5–3.0 1.5–3.0 | RT | 10 |
| 22 | 38 | 28 | M | 44–46 | 1.5–3.0 | RT | 10 |
| 23 | 39 | 42 | F | 44–47 | 1.5–3.0 | RT | 10 |
| Patient No. | Site No | Horizontal Bone Gain | Implants | |||
|---|---|---|---|---|---|---|
| Right Side | Left Side | No. | Length | Diameter | ||
| 1 | 1, 2 | 3.0–5.0 | 4.0–6.0 | 6 | 10–11.5 | 3.3–4.2 |
| 2 | 3, 4 | 3.0–5.5 | 4.0–5.0 | 7 | 10–13 | 3.3–3.75 |
| 3 | 5, 6 | 3.0–4.5 | 3.0–5.0 | 2 | 11.5 | 3.75 |
| 4 | 7 | 4.0–6.0 | - | 3 | 10–13 | 4.2–3.74 |
| 5 | 8, 9 | 5.0–6.0 | 3.5–5.0 | 6 | 11.5–13 | 4.2–3.75 |
| 6 | 10 | - | 4.0–6.0 | 3 | 10–11.5 | 3.75 |
| 7 | 11,12 | 5.0–8.0 | 5.0–8.0 | 6 | 10–13 | 3.73–4.2 |
| 8 | 13 | 3.0–4.0 | - | 3 | 11.5 | 3.75 |
| 9 | 14, 15 | 4.0–6.0 | 4.0–8.0 | 6 | 11.5–13 | 3.75–4.2 |
| 10 | 16, 17 | 3.0–5.0 | 3.0–5.0 | 6 | 13 | 3.75 |
| 11 | 18 | - | 3.5–4.5 | 2 | 11.5 | 3.75 |
| 12 | 19, 20 | 4.0–6.0 | 4.0–6.0 | 4 | 11.5 | 3.75 |
| 13 | 21 | - | 3.0–6.0 | 3 | 11.5 | 3.75 |
| 14 * | 22, 23 | - | - | - | - | - |
| 15 | 24, 25 | 3.0–5.0 | 4.0–8.0 | 4 | 10–11.5 | 3.75–4.2 |
| 16 | 26, 27 | 3.0–5.0 | 4.0–6.0 | 6 | 11.5–13 | 3.75–4.2 |
| 17 | 28, 29 | 4.0–6.0 | 3.0–6.0 | 7 | 10–13 | 3.75 |
| 18 | 30, 31 | 3.0–5.0 | 3.0–6.0 | 6 | 10–13 | 3.75 |
| 19 | 32, 33 | 4.0–6.0 | 3.0–4.0 | 6 | 10–11.5 | 3.75 |
| 20 | 34, 35 | 5.0–8.0 | 4.0–7.0 | 6 | 11.5–13 | 3.75–4.2 |
| 21 | 36, 37 | 3.0–7.0 | 4.0–8.0 | 6 | 11.5–13 | 3.75–4.2 |
| 22 | 38 | 4.0–7.0 | - | 2 | 11.5 | 4.2 |
| 23 | 39 | - | 3.0–6.0 | 3 | 11.5 | 3.75 |
| Total number of placed implants: 103 Total number of failed implants: 3/103: Implant survival rate: 97% Total number of implants with marginal bone loss of 30%: 5/103. Implant success rate: 7/103: 93.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kablan, F. A New Approach for Reconstruction of Severe Horizontal Atrophy of the Posterior Mandible Using “The Honeycomb Technique”: A 10–14 Year Follow-Up Retrospective Study. J. Clin. Med. 2025, 14, 2246. https://doi.org/10.3390/jcm14072246
Kablan F. A New Approach for Reconstruction of Severe Horizontal Atrophy of the Posterior Mandible Using “The Honeycomb Technique”: A 10–14 Year Follow-Up Retrospective Study. Journal of Clinical Medicine. 2025; 14(7):2246. https://doi.org/10.3390/jcm14072246
Chicago/Turabian StyleKablan, Fares. 2025. "A New Approach for Reconstruction of Severe Horizontal Atrophy of the Posterior Mandible Using “The Honeycomb Technique”: A 10–14 Year Follow-Up Retrospective Study" Journal of Clinical Medicine 14, no. 7: 2246. https://doi.org/10.3390/jcm14072246
APA StyleKablan, F. (2025). A New Approach for Reconstruction of Severe Horizontal Atrophy of the Posterior Mandible Using “The Honeycomb Technique”: A 10–14 Year Follow-Up Retrospective Study. Journal of Clinical Medicine, 14(7), 2246. https://doi.org/10.3390/jcm14072246





